Abstract
In the present study, we characterized the expression and role of forkhead box O (FoxO3a) in kainic acid (KA)-induced hippocampal neuronal cell death. FoxO3a and pFoxO3a expression in the CA1, CA2, and dentate gyrus regions in the hippocampus increased 0.5 and 1 h after intracerebroventricular administration of KA. In addition, both FoxO3a and pFoxO3a expression in the hippocampal CA3 region increased significantly and equally for 1 h but decreased gradually for 24 h after KA administration. In particular, the KA-induced increases in FoxO3a and pFoxO3a expression in the hippocampal CA3 region were inhibited by pretreatment with the N-methyl-d-aspartate (NMDA) receptor antagonist (MK-801, dizocilpine, 1 µg/5 µl) or a non-NMDA receptor antagonist (CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione, 0.5 µg/5 µl). Furthermore, dizocilpine and CNQX produced a neuroprotective effect against KA-induced neuronal death in the CA3 region of the hippocampus. Our results suggest that FoxO3a and pFoxO3 expression is upregulated by KA. Both FoxO3a and pFoxO3a expression appear to be responsible for KA-induced neuronal death in the CA3 region of the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kainic acid (KA) is a non-degradable glutamate analog that is 30-fold more neurotoxic than glutamate. This neuroexcitant binds to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/KA receptors, which are a subtype of ionotropic glutamate receptors in the brain (Bleakman and Lodge 1998). Activation of the KA receptor elicits cellular events, including increased in intracellular Ca2+, production of reactive oxygen species (ROS), and other biochemical events leading to neuronal cell death (Sun et al. 1992; Candelario-Jalil et al. 2001; Milatovic et al. 2002). Neuronal cell death due to KA elicits severe status epilepeticus in the pyramidal layer of the hippocampal CA3 region (Sperk 1994) when KA is administered intracerebroventricularly (i.c.v.). Neuronal death is one of the most prominent features in several diseases of the central nervous system, such as Alzheimer’s disease, Parkinson’s disease, stroke, epilepsy, and spinal cord injury (Benveniste et al. 1984; Simon et al. 1984; Perry et al. 2002; Arzimanoglou et al. 2002; Sapolsky 2003) possibly because of excess activation of neurons by excitatory neurotransmitters (e.g., glutamate), which are massively released as a consequence of energy depletion and result in excitotoxic neuron death (Rothman and Olney 1986; Beal 1992).
Forkhead box O (FOXO) is a transcription factor in the large forkhead family of proteins that contains a conserved DNA-binding domain termed the forkhead box (FOX) (Kaestner et al. 2000). These transcription factors are involved in regulating metabolism, cell proliferation, stress resistance, the immune system, and apoptosis (Horst and Burgering 2007). Four members of the mammalian FoxO-family have been identified, such as FoxO1, FoxO3a, FoxO4 and FoxO6 (Jacobs et al. 2003). FoxO1 is detected mainly in the dentate gyrus (DG), the striatum, and the piriform cortex of the adult murine brain. Furthermore, FoxO3a is expressed in the hippocampal formation (DG and CA 1–3 regions), the neocortex, and the cerebellar granule cells. FoxO6 is found mainly in the hippocampal formation (DG and CA 1–3 regions) and the nucleus accumbens (Hoekman et al. 2006). In situ hybridization experiments of the mouse brain showed that FoxO1 is strongly expressed in the striatum and neuronal subsets of the hippocampus (DG and ventral/posterior part of the CA regions), whereas FoxO3 is more diffusely expressed throughout the brain, including all hippocampal areas, the cortex, and cerebellum, suggesting a complementary role for this FoxO protein to control cognitive and motor function (Maiese et al. 2009). Moreover, FoxO6 is expressed in various parts of the adult mouse brain, including the entire hippocampus, the amygdalohippocampal area, and the shell of the nucleus accumbens (Hoekman et al. 2006). The FoxO family appears to be activated in the central nervous system in response to various stress stimuli, such as epileptic seizure and oxidative stress, where it eliminates damaged neurons by apoptosis. Epileptic brain injury in rats leads to activation of FoxO1 and FoxO3a in hippocampal neurons and upregulation of the proapoptotic gene Bim, leading to neuronal apoptosis (Shinoda et al. 2004). Similarly, ultraviolet damage to Drosophila retinal nervous tissue induces apoptosis via FoxO by inducing the proapoptotic gene Hid (Luo et al. 2007). The induction of apoptosis by FoxO in the fly retina requires activation of the Jun amino-terminal kinase (JNK) pathway, further supporting the idea that JNK activates FoxO factors (Essers et al. 2004; Wang et al. 2005). Finally, oxidative stress induced by hydrogen peroxide treatment promotes apoptosis in rat primary cerebellar neurons by activating FoxO factors (Lehtinen et al. 2006).
The functions of the FoxO family in peripheral and central nervous systems have been studied extensively; however, their expression and function in the hippocampus have not been well characterized. Thus, we examined the effect of i.c.v. administration of KA on temporal expression of FoxO in the hippocampus and the possible involvement of N-methyl-d-aspartate (NMDA) and non-NMDA receptors in KA-induced FoxO expression in the CA3 region of the hippocampus.
Materials and methods
These experiments were approved by the Hallym University Animal Care and Use Committee (registration number: Hallym 2014-47). All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health.
Experimental animals
Male ICR mice (MJ Co., Seoul, Korea; weight, 23–25 g) were used for all experiments. Animals were housed five per cage in a room maintained at 22 ± 0.5 °C with an alternating 12 h light–dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 weeks before testing. Experiments were performed during the light phase of the cycle (10:00–17:00).
Intracerebroventricular (i.c.v.) injection of drugs
The i.c.v. injection volume was 5 µl, and the injection sites were verified by injecting a similar volume of 1 % methylene blue solution and determining the distribution of the injected dye in the ventricular space. The success rate of prior injections with this technique was >95 %. The i.c.v. administration method followed that described by Haley and McCormick (1957). Each unanesthetized mouse was grasped firmly by the loose skin behind the head, and the skin was pulled taut. A 26-gauge needle attached to a 50 µl Hamilton syringe was inserted perpendicularly through the skull into the brain, and the solution was injected. The injection site was 2.4 mm either side of the midline.
Immunocytochemistry
The hippocampal area (Bregma, −3.24 mm) was sectioned coronally with a cryostat at a thickness of 45 µm. Immunohistochemical staining was performed with the Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA). Sections were first rinsed with 0.1 M PBS three times for 10 min each and pre-incubated in 0.1 M PBS containing 1 % bovine serum albumin (BSA) and 0.2 % triton X-100 for 30 min. After rinsing twice with 0.1 M PBS containing 0.5 % BSA for 10–15 min each, the sections were incubated with antibody against FoxO3a (1:200, Ab Frontier, Deajeon, Korea) and pFoxO3a (1:200, Ab Frontier) diluted with 0.1 M PBS containing 0.5 % BSA and 0.05 % sodium azide at 4 °C. After overnight incubation, the sections were rinsed and incubated with 1:200 biotinylated secondary antibody in 0.1 M PBS containing 0.5 % BSA for 1 h at room temperature. After rinsing, the sections were incubated with 1:200 ABC reagent diluted in PBS for 1 h at room temperature and then rinsed with PBS followed with 0.1 M phosphate buffer (PB). Finally, the sections were incubated in the Sigma Fast DAB kit (Sigma, St. Louis, MO, USA) until the desired stain intensity developed. We standardized the lengths of DAB reaction time (10 min for all brain sections) to allow for uniform staining intensity across the experimental groups. Sections were rinsed with 0.1 M PB, mounted on gelatin-coated slides, and dehydrated through an alcohol and xylene series. We counted the numbers of pFoxO3a and FoxO3a-positive cells in each section. Eight animals per group were used for immunostaining.
Cresyl violet staining
After injecting KA, all mice were transcardially perfused for 30 min (5 mg/kg phenobarbital was administered i.p. to anesthetize the mice before perfusion) and post-fixed for 4 h in 4 % paraformaldehyde 1 day later. The brains were cryoprotected in 30 % sucrose, the hippocampal area (Bregma, −3.24 mm) was sectioned coronally (45 µm) on a frozen microtome, and collected in cryoprotectant for storage at −20 °C until processed. Sections were rinsed 3 × 10 min in phosphate-buffered saline (PBS) to remove the cryoprotectant. The sections were mounted on microscope slides (Fisher Scientific, Pittsburgh, PA, USA) and dried in air. The slide-mounted brain sections were soaked in a cresyl violet working solution (0.02 % in a buffer solution of 0.2 % sodium acetate and 0.3 % glacial acetic acid) for 2 min. Then, the slides were dehydrated through a graded ethanol series, cleared in histoclear, and coverslipped using permount (Fisher).
Drugs
KA was purchased from Sigma Chemical Co. KA was prepared in PBS as a vehicle. MK-801 (RBI, Natick, MA, USA), and CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) (RBI) were prepared in saline as a vehicle.
Statistical analysis
The statistical analysis was carried out using the t test or one-way analysis of variance with a Bonferroni post hoc test using GraphPad Prism ver. 4.0 for Windows software (GraphPad Software, La Jolla, CA, USA). p values <0.05 were considered significant. All values are expressed as the mean ± standard error.
Results
Effect of KA on expression of hippocampal FoxO3a and pFoxO3a
Immunohistochemistry was performed to determine hippocampal expression of FoxO3a and pFoxO3a after KA administration. As shown in Fig. 1d, FoxO3a expression increased at 0.5 and 1 h after i.c.v. administration of KA into the hippocampal CA1–3 and DG regions but returned to the control level after 3 and 24 h. Similar to FoxO3a, expression of pFoxO3a increased 0.5 and 1 h after i.c.v. adminstration of KA in hippocampal CA1–3 and DG, but returned to the control level at 3 and 24 h (Fig. 2a–d).
Expression of FoxO3a after administration of kainic acid (KA) (i.c.v.) in the hippocampal CA1–3 and dentate gyrus (DG) regions. An immunohistochemical study of FoxO3a in the hippocampal CA1–3 and DG regions (a–c) was carried out. FoxO3a-positive cells in hippocampal CA1 (a, b), CA2 (d, e), CA3 (f, g), and DG (a, c) regions were counted referencing to the mouse atlas at 0.5, 1, 3, 6 and 24 h after KA administration. Vertical bars in the column graph indicate standard errors. Eight animals were used per group. (**p < 0.01, ***p < 0.001 control vs. other groups)
Expression of pFoxO3a after kainic acid (KA) administration (i.c.v.) in the hippocampal CA1–3 and dentate gyrus (DG) regions. An immunohistochemical study of FoxO3a in hippocampal CA1–3 and DG regions (a, b, and c) was carried out. pFoxO3a-positive cells in hippocampal CA1 (a, b), CA2 (d, e), CA3 (f, g), and DG (a, c) regions were counted referencing to the mouse atlas at 0.5, 1, 3, 6, and 24 h after KA administration. Vertical bars in the column graph indicate the standard errors. Eight animals were used per group. (*p < 0.05, **p < 0.01, ***p < 0.001 control vs. other groups)
Effect of MK-801 and CNQX on KA-induced neuronal cell death and expressions of FoxO3a and pFoxO3a in the CA3 region of the hippocampus
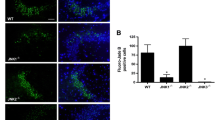
The mice were pretreated with MK-801 (1 µg/5 µl) or CNQX (0.5 µg/5 µl) for 10 min to examine the effect of MK-801 and CNQX on KA-induced neuronal cell death and expression of FoxO3a, and pFoxO3a in the CA3 region of the hippocampus, and KA was administered i.c.v. Cell death was measured by immunocytochemistry. As shown in Fig. 3a, i.c.v. pretreatment with MK-801 or CNQX attenuated KA-induced neuronal cell death in the CA3 region. In addition, the KA-induced increases in FoxO3a and pFoxO3a in the CA3 region were attenuated by MK-801 and CNQX (Fig. 3b, c).
Effect of MK-801 or CNQX on kainic acid (KA)-induced neuronal cell death and expression of FoxO3a and pFoxO3a in the hippocampal CA3 region. Mice were pretreated i.c.v. with MK-801 (1 µg/5 µl) or CNQX (0.5 µg/5 µl) for 10 min. Then KA (0.1 µg/5 µl) was administered i.c.v. a Hippocampal neuronal death was measured 24 h after KA administration using cresyl-violet staining. An immunohistochemical study of FoxO3a and pFoxO3a in the hippocampal CA3 region was carried out. The numbers of FoxO3a and pFoxO3a-positive cells in the hippocampal CA3 region were counted referencing to the mouse atlas at 0.5 h after KA administration (b and c respectively). Vertical bars in the column graph indicate standard errors. Four animals were per group. (***p < 0.001 control vs. saline + KA; +p < 0.05, ++p < 0.01, +++p < 0.001 saline + KA vs. other groups)
Discussion
In this study, the roles of FoxO3a and pFoxO3a in the regulation of hippocampal neuronal cell death induced by KA were explored using NMDA or non-NMDA receptor antagonists. The major findings were (1) increased phosphorylation of FoxO3a protein by KA, (2) increased FoxO3a and pFoxO3a expression in hippocampal CA1–3 and the DG DG by KA, and (3) protection against KA-induced hippocampal CA3 cell death by MK-801 and CNQX. Taken together, these results suggest that KA administered supraspinally causes neuronal cell death in the CA3 region of the hippocampus by activating pFoxO3a and pFoxO3a expression through activation of NMDA and non-NMDA receptors.
FoxO3a and pFoxO3a expression increased in hippocampal CA1, CA2, and the DG in response to KA. In addition, FoxO3a and pFoxO3a expression in the hippocampal CA3 region increased significantly for 1 h and then decreased gradually for 24 h after KA administration. A previous study demonstrated that i.c.v. injected KA causes specific hippocampal CA3 cell death (Lee et al. 2002). In other studies, KA has been implicated in oxidative stress in the hippocampus (Wang et al. 2004). Under conditions of oxidative stress, The FoxO3a factor is phosphorylated by other protein kinases, including AMP-dependent kinase, JNK, mammalian Ste20-like kinase, and the CREB-binding protein (Salih and Brunet 2008). This phosphorylation causes translocation of FoxO3a from the nucleus and appears to result in cell cycle arrest, apoptosis, and detoxification of ROS. Thus, KA-mediated oxidative stress may induce the expression of phosphorylated FoxO3a in the hippocampus.
FoxO3a may play a significant role in injuries involving cerebral ischemia and oxidative stress (Chong et al. 2005a, b). FoxO3a transcription is induced by cellular hypoxia via direct binding of hypoxia-inducible factor (HIF) to the FoxO3 promoter (Bakker et al. 2007). The increased expression of FoxO3a results in enhanced cellular survival by attenuating HIF-induced apoptosis. Inhibiting or knockdown of FoxO3a gene activity in a neuronal cell culture model mediates the ischemic protective effects of metabotropic glutamate receptors (Chong et al. 2006). In contrast, expression of constitutively nuclear FoxO3a promotes death of purified motor neurons and cerebellar granule cells and has been linked to FasL expression (Brunet et al. 1999). As a close homolog to Akt, serum- and glucocorticoid-inducible protein kinase increases cell survival by inhibiting FoxO3a (Leong et al. 2003). Moreover, some studies have demonstrated the neuroprotective effects of MK-801 and CNQX (Kaku et al. 1991; Lee et al. 2002; Zagrean et al. 2014). In our previous study, we found the involvement of both NMDA and non-NMDA receptors in KA-induced pyramidal cell death in the CA3 region of the hippocampus and that phosphorylation of extracellular regulated kinase and dephosphorylation of CREB proteins may play important functional roles in the CA3 pyramidal cell death induced by KA (Lee et al. 2002). However, the role of FoxO3a has not been demonstrated in KA-induced hippocampal cell death. Thus, to investigate the role of FoxO3a in KA-induced cell death, the effect of pretreatment with MK-801 (NMDA receptor antagonist) or CNQX (non-NMDA receptor antagonist) on KA-induced hippocampal neuronal cell death and expression of FoxO were examined. The results showed that MK-801 or CNQX pretreatment exerted a neuroprotective effect against KA-induced hippocampal CA3 neuronal cell death. Furthermore, both MK-801 and CNQX reduced KA-induced enhanced of FoxO3a and pFoxO3a expression in the hippocampal CA3 region. These findings suggest that both FoxO3a and pFoxO3a are activated by NMDA and non-NMDA receptors and play an important role in KA-induced hippocampal CA3 neuronal death. Kim et al. (2014), in part, supports our findings, as they found that a systemic injection increases hippocampal FoxO3a, suggesting that FoxO3a plays an important role in KA-induced hippocampal neuronal cell death.
We conclude that KA administered supraspinally caused neuronal cell death in the CA3 region of the hippocampus by activating FoxO3a and pFoxO3a expression through activation of NMDA and non-NMDA receptors.
References
Arzimanoglou A, Hirsch E, Nehlig A, Castelnau P, Gressens P, Pereira de Vasconcelos A (2002) Epilepsy and neuroprotection: an illustrated review. Epileptic Disord 4:173–182
Bakker WJ, Harris IS, Mak TW (2007) FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell 28:941–953
Beal MF (1992) Mechanisms of excitotoxicity in neurologic diseases. FASEB J 6:3338–3344
Benveniste H, Drejer J, Schousboe A, Diemer NH (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 43:1369–1374
Bleakman D, Lodge D (1998) Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 37:1187–1204
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857–868
Candelario-Jalil E, Al-Dalain SM, Castillo R, Martinez G, Fernandez OS (2001) Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J Appl Toxicol 21:403–407
Chong ZZ, Li F, Maiese K (2005a) Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol 20:299–315
Chong ZZ, Li F, Maiese K (2005b) Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol 75:207–246
Chong ZZ, Li F, Maiese K (2006) Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress. Curr Neurovasc Res 3:107–117
Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Haley TJ, McCormick WG (1957) Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother 12:12–15
Hoekman MF, Jacobs FM, Smidt MP, Burbach JP (2006) Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 6:134–140
Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP (2003) FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 278:35959–35967
Kaestner KH, Knochel W, Martinez DE (2000) Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev 14:142–146
Kaku DA, Goldberg MP, Choi DW (1991) Antagonism of non-NMDA receptors augments the neuroprotective effect of NMDA receptor blockade in cortical cultures subjected to prolonged deprivation of oxygen and glucose. Brain Res 554:344–347
Kim CH, Park SH, Sim YB, Sharma N, Kim SS, Lim SM, Jung JS, Suh HW (2014) Effect of pertussis and cholera toxins administered supraspinally on CA3 hippocampal neuronal cell death and the blood glucose level induced by kainic acid in mice. Neurosci Res 89:31–36
Lee JK, Choi SS, Lee HK, Han KJ, Han EJ, Suh HW (2002) Effects of MK-801 and CNQX on various neurotoxic responses induced by kainic acid in mice. Mol Cells 14:339–347
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125:987–1001
Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL (2003) Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem 278:5871–5882
Luo X, Puig O, Hyun J, Bohmann D, Jasper H (2007) Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J 26:380–390
Maiese K, Hou J, Chong ZZ, Shang YC (2009) A fork in the path: developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev 2:119–129
Milatovic D, Gupta RC, Dettbarn WD (2002) Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res 957:330–337
Perry G, Taddeo MA, Nunomura A, Zhu X, Zenteno-Savin T, Drew KL, Shimohama S, Avila J, Castellani RJ, Smith MA (2002) Comparative biology and pathology of oxidative stress in Alzheimer and other neurodegenerative diseases: beyond damage and response. Comp Biochem Physiol C 133:507–513
Rothman SM, Olney JW (1986) Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol 19:105–111
Salih DA, Brunet A (2008) FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126–136
Sapolsky RM (2003) Neuroprotective gene therapy against acute neurological insults. Nat Rev Neurosci 4:61–69
Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, Lan JQ, Taki W, Simon RP, Henshall DC (2004) Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest 113:1059–1068
Simon RP, Griffiths T, Evans MC, Swan JH, Meldrum BS (1984) Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J Cereb Blood Flow Metab 4:350–361
Sperk G (1994) Kainic acid seizures in the rat. Prog Neurobiol 42:1–32
Sun AY, Cheng Y, Sun GY (1992) Kainic acid-induced excitotoxicity in neurons and glial cells. Prog Brain Res 94:271–280
van der Horst A, Burgering BM (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8:440–450
Wang Q, Yu S, Simonyi A, Rottinghaus G, Sun GY, Sun AY (2004) Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res 29:2105–2112
Wang MC, Bohmann D, Jasper H (2005) JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121:115–125
Zagrean AM, Spataru A, Ceanga M, Zagrean L (2014) The single versus combinatorial effects of MK-801, CNQX, nifedipine and AP-3 on primary cultures of cerebellar granule cells in an oxygen-glucose deprivation model. Rom J Morphol Embryol 55:811–816
Acknowledgments
This research was supported by Priority Research Centers (NRF-2009-0094071 and NRF-2014R1A1A1006791) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology and Hallym University Specialization Fund (HRF-S-52).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Rights and permissions
About this article
Cite this article
Park, SH., Sim, YB., Lee, JK. et al. Characterization of temporal expressions of FOXO and pFOXO proteins in the hippocampus by kainic acid in mice: involvement of NMDA and non-NMDA receptors. Arch. Pharm. Res. 39, 660–667 (2016). https://doi.org/10.1007/s12272-016-0733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0733-9