Abstract
A radical-scavenging, guided phytochemical study of the latex of Calotropis Procera afforded five lignans (1–5), including a new one (4). The structural determination was accomplished using 1D- and 2D-NMR, high-resolution electrospray ionization mass spectrometry (HRESIMS), and correlation with known compounds. Among the isolated compounds, acylated lignans (3–5) showed stronger antioxidant activity than non-acylated derivatives (1,2). Anti-inflammatory activity was evaluated by determining the inhibitory potential against 5- and 15-lipoxygenase enzymes. The highest anti-inflammatory activity was observed in compound 4, with IC50s values of 7.6 µM and 2.7 µM against 5-LOX and 15-LOX, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytochemicals are endless sources of natural antioxidants that play an important role in health promotion and protection against harmful free radicals, which cause serious diseases. In general, natural antioxidants are safer to consume than their synthetic counterparts, with fewer environmental hazards (Khasawneh et al. 2011; Abdel-Mageed et al. 2012, 2014). The antioxidant activity of plants is mainly attributed to the presence of polyphenolics that counteract reactive oxygen species (ROS). ROS can induce oxidative damage in biomolecules, leading to many diseases, such as cancer, rheumatoid arthritis, diabetes, cirrhosis, and arteriosclerosis (Gupta and Sharma 2006; Gill and Tuteja 2010).
Lipoxygenases (LOXs) are non-heme, iron-containing enzymes that catalyze the oxygenation of polyunsaturated fatty acids such as arachidonic acid and linoleic acid to their corresponding hydroperoxy derivatives. LOXs are sub-classified, according to their positional specificity of arachidonic acid oxygenation, as 5-, 8-, 11-, 12-, and 15-LOXs. 5-LOX catalyzes the oxidation of arachidonic acid at the 5 position to yield 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE) which converts later to leukotrienes. Leukotrienes play an important role in many inflammatory conditions, such as inflammatory bowel disease, arthritis, asthma, cancer, and allergic diseases. 15-LOX, which catalyzes the oxidation of arachidonic acid at the 15 position to yield 15-hydroxyeicosatetraenoic acid (15-HETE), is subdivided into 15-LOX-1 and 15-LOX-2. 15-LOX-1 is highly expressed in airway endothelial cells and leukocytes, while 15-LOX-2 is expressed in different tissues, such as the liver, kidney, lung, prostate, colon, cornea, and brain. 15-LOXs have been implicated in atherogenesis, asthma, chronic bronchitis, and cell differentiation (Rioux and Castonguay 1998; Vernon et al. 1999; Walther et al. 1999; Mabalirajan et al. 2013; Wisastra and Dekker 2014). Thus, selective lipoxygenase inhibitors, as a class of therapeutic agent, may exhibit medicinal benefits in the prevention and treatment of these inflammatory conditions (Yves et al. 1994; Rackova et al. 2007).
Calotropis procera (Asclepiadaceae) is an erect, succulent shrub known by various names, such as Usher, Dead Sea apple, Sodom apple, swallow wort, and milkweed. The shrub is widely distributed in tropical and subtropical areas of America, Africa, and Asia (Ansari and Ali 2001; Mijatovic et al. 2007; Begum et al. 2010; Doshi et al. 2011; Sabrin et al. 2015). The plant is able to produce large quantities of latex that is known for its pharmacological and medicinal activities (Ramos et al. 2007, 2009; Silva et al. 2010). Latex from different Calotropis species is a rich source of biologically active compounds and previous phytochemical investigations have revealed the presence of cardenolides, steroids, lipids, terpenes, resins, and alkaloids (Mahajan and Badgujar 2008; Kawo et al. 2009; Nadia et al. 2015). It has also exhibited various different medicinal activities, such as anticancer (Choedon et al. 2006; Soares de Oliveira et al. 2007; Kamel et al. 2010; Meena et al. 2010; Harne et al. 2012), anti-inflammatory (Sangraula et al. 2002; Arya and Kumar 2005; Kumar and Roy 2009), anthelmintic (Al-Qarawi et al. 2001; Shivkar and Kumar 2003), antimicrobial (Sehgal et al. 2005; Farzin et al. 2008; Kareem et al. 2008; Yesmin et al. 2008), antioxidant (Roy et al. 2005; Joshi et al. 2009), antidiarrheal (Kumar et al. 2001), analgesic (Dewan et al. 2000), antipyretic (Dewan et al. 2000; Larhsini et al. 2002), schizonticidal (Sharma and Sharma 2000), insecticidal (Moursy 1997; Ramos et al. 2006), antidiabetic (Roy et al. 2005), and hepatoprotective (Setty et al. 2007) activities.
During the course of our ongoing research activities regarding the isolation and identification of drug leads from plants growing in Egypt, we had the opportunity to investigate the latex of C. procera to identify its phenolic constituents and investigate their potential biological activities. To the best of our knowledge, this study is the first report to highlight in detail the phenolic content of C. procera latex and its biological activities.
In the present study, bioassay-guided fractionation of the active ethyl acetate and aqueous fractions of C. procera latex led to the isolation of five lignan glycosides (1–5), including a new one (4) (Fig. 1). In this study, we report the structural characterization of the new compound and assess the antioxidant and lipoxygenase inhibition activities of the isolated phenolic compounds.
Materials and methods
General experimental procedures
One-dimensional (1D-) and two-dimensional (2D-) NMR spectra were obtained on a Bruker Avance DRX400 spectrometer. High-resolution electrospray ionization mass spectrometry (HRESIMS) measurements were obtained on a Bruker micrOTOF mass spectrometer. Optical rotations were measured on a Perkin-Elmer Model 343 polarimeter. High-performance liquid chromatography (HPLC) separations and purifications were carried out using an Agilent 1200 series gradient pump, monitored using a DAD G1315B variable-wavelength ultraviolet (UV) detector and a Phenomenex RP column (C18, 250 × 10 mm, 5 µm) and an Agilent Chromatorex Zorbax SB C3 (5 μm) semi-preparative column (9.4 × 250 mm). UV absorption was performed with a UV–vis spectrometer (Lambda 25; Perkin-Elmer Instruments). Thin-layer chromatography (TLC) pre-coated RP-18 F254 plates (0.25 mm; Merck, Germany) and silica gel 60 F254 (0.25 mm, ALUGRAM® SIL G/UV254; Macherey–Nagel, Germany) were used in the isolation process. Column chromatography (CC) was performed using silica gel (Kieselgel 60 Å, 40–63 μM mesh size; Fluorochem, UK) and Diaion HP-20 (Nippon, Rensui Co., Japan). All medium pressure or flash chromatography was performed using a Biotage Flash system (Charlottesville, VA).
Plant material
The latex of the C. procera plant was collected during June–July from uncultivated land in Assiut, Egypt (longitude: 31°11′9.3336″E, latitude: 27°10′41.9232″N). The herbarium specimen was authenticated by Prof. Dr. Ahmed Shoreit, Botany Dept., Faculty of Science, Assiut University. A voucher specimen was deposited in the Botany Department Herbarium (No. 2011CP), Faculty of Science, Assiut University, Assiut, Egypt.
Extraction and isolation
By cutting thin strips of leaves and stems from the shrub, 500 mL of the latex of C. procera was collected and allowed to exude into a collecting glass vessel over a period of hours. The collected latex was mixed with 95 % ethanol and subjected to sonication at room temperature, centrifugation, and then evaporation to obtain the supernatant and produce a light yellowish residue (39.0 g, 7.8 %). The residue was mixed with 500 mL of distilled H2O and subjected to successive solvent fractionation with n-hexane, chloroform, and ethyl acetate until complete exhaustion to produce an n-hexane fraction (14 g), chloroform fraction (10.2 g), ethyl acetate fraction (7 g), and aqueous fraction (7 g).
The ethyl acetate and aqueous fractions, combined due to their similarity on TLC (14 g), were loaded into the top of a Diaion HP-20 column (25 × 1.5 cm) and eluted with distilled water and methanol (5 × 500 mL) in a gradient mode of analysis. Fractions eluted by 100 % (MeOH) were combined and concentrated under reduced pressure to produce 11.5 g, and then further purified by subjecting them to flash or medium pressure liquid chromatography (MPLC) on a silica gel column using CHCl3–MeOH mixtures in a manner of increasing polarities. Several fractions of 10 mL each were collected and monitored by TLC silica gel, and similar fractions were pooled together to obtain five main fractions (A–E), using CHCl3–MeOH (90:10) and CHCl3–MeOH–H2O (80:20:2) and (70:30:3) as solvent systems and 15 % v/v sulfuric acid in ethanol and/or 0.2 % 2,2-diphenyl-1-picrylhydrazyl (DPPH) in MeOH as spraying agents. Fractions B and C were selected for further purification and subjected to preparative reversed-phase HPLC (Agilent Chromatorex Zorbax SB C3 9.4 × 250 mm, 5 μm; Phenomenex RP C18, 250 × 10 mm, 5 µm) columns using a gradient of 5–100 % acetonitrile–H2O over 40 min to give compounds 3 (8.1 mg), 4 (2.7 mg), and 5 (2.4 mg) from fraction B and compounds 1 (15.7 mg) and 2 (11.3 mg) from fraction C.
Compound (4)
Yellow amorphous powder (2.7 mg); [α]22D +14.55 (c 0.1, MeOH); UV: λmax MeOH (log ε): 220 (4.33), 264 (4.18), 290 (3.65) nm; IR (KBr) v max 3397, 1708 cm−1; HRESIMS m/z: 679.2011 [M + Na]+ (calcd. for C33H36O14, 679.2003). 1H (400 MHz, DMSO_d 6 ) and 13C (100 MHz, DMSO_d 6 ) NMR data in Table 1.
Acid hydrolysis of isolated lignan glycosides
A methanolic solution (5 mL) of compounds 1 and 2 (3 mg each) was mixed with 1 N HCl (4 mL) and refluxed for 4 h. Then, the solution was concentrated under reduced pressure, diluted with H2O (8 mL), and extracted with ethyl acetate to obtain the aglycone. The glycone (sugar part) was obtained from the aqueous part after concentration, and it was identified as d-glucose by paper chromatography using BuOH:AcOH:H2O (4:1:5) as a mobile phase and aniline phthalate as a spraying agent with heating at 110 °C (Rehman et al. 2005).
Alkaline hydrolysis of isolated lignan glycosides
Mild alkaline hydrolysis was achieved by treating a methanolic solution (1 mL) of compounds 3–5 (0.2 mg) with sodium methoxide (1.6 mg, 30 mmol) for 48 h at room temperature (Bai et al. 2013; Parhira et al. 2014). The reaction was terminated by adding formic acid. Two products of (+)-pinoresinol 4-O-β-d-glucopyranoside and a methyl ester of 4-hydroxy-3-methoxy benzoic acid were clearly detected by TLC analysis for compound 3, (+)-pinoresinol 4-O-β-d-glucopyranoside and 3,4-dihydroxy benzoic acid were detected for compound 4, and (+)-pinoresinol 4-O-β-d-glucopyranoside and ferulic acid (FER) were detected for compound 5 using CHCl3–MeOH (90:10) and CHCl3–MeOH–H2O (80:20:2), (70:30:3), and (50:50:5) as solvent systems.
DPPH radical scavenging assay
First, a rapid TLC screening method using 0.2 % DPPH in MeOH was used to examine the radical scavenging activity of the isolated compounds (1–5). Thirty minutes after spraying, the active compounds appeared as yellow spots against a purple background.
Second, a spectrophotometric assay was carried out according to the method of Abdel-Mageed et al. (2010). Briefly, a methanolic solution (2.0 mL) of a wide range of concentrations (2.5–120 μM) of the isolated compounds (1–5) was mixed with a methanolic solution (2.0 mL) of 100 μM of DPPH. The mixture was vortexed for one minute and then left to stand in the dark for 30 min at room temperature. The change in color of the antioxidant compound from deep violet to light yellow was measured spectrophotometrically at 517 nm. The experiment was carried out in triplicate, using ascorbic acid as a positive control. The percentage of reduction in DPPH, Q, referring to “inhibition” or “quenching,” was calculated by the following formula (Abdel-Mageed et al. 2012; Ibraheim et al. 2012; Abdel-Mageed et al. 2014; Wagner et al. 2014):
where A B = absorption of the blank sample (t = 0 min) and A A = absorption of the tested extract solution (t = 30 min).
Lipoxygenase inhibition assay
Lipoxygenase-inhibiting activity was carried out spectrophotometrically according to the method of Tappel (1962), with a slight modification using lipoxygenase (EC 1.13.11.33), (EC 1.13.11.34) and linoleic acid. The reaction mixture contained 10 µl of test compound solution, 160 µl of 100 mM sodium phosphate buffer (pH 8.0), and 20 µl of lipoxygenase solution, which were mixed and incubated for 10 min at 25 °C. The reaction was then initiated by the addition of 10 µl of linoleic acid (substrate) solution, and the change in absorbance at 234 nm was followed for 6 min. The test compounds and the control were dissolved in methanol. All of the reactions were performed in triplicate in a 96-well micro-plate in Spectra-Max 384 plus (Molecular Devices, U.S.A.). The IC50 values were then calculated using the EZ-Fit enzyme kinetics program (Perrella Scientific Inc., Amherst, NH). The percentage (%) of inhibition was calculated as follows: (E-S)/E-100, where E is the activity of the enzyme without the test compound and S is the activity of the enzyme with the test compound (Tappel 1962; Abdel-Mageed et al. 2014).
Results and discussion
A combination of different chromatographic techniques as medium-pressure liquid chromatography (MPLC) and HPLC of the ethyl acetate and aqueous fractions afforded five lignans (1–5) (Fig. 1). Their structures were elucidated by extensive 1D- and 2D-NMR analysis, accurate mass measurements, and by comparing them with the reported data of the known compounds. The four known lignans were identified as (+)-pinoresinol 4-O-β-d-glucopyranoside (1) (Dong-Mei et al. 2012); (+)-medioresinol 4-O-β-d-glucopyranoside (Eucommin A) (2) (Takeshi et al. 1985); (+)-pinoresinol 4-O-[6″-O-vanillyl]-β-d-glucopyranoside (3) (Parhira et al. 2014); and pinoresinol-4′-O-[6″-O-(E)-feruloyl]-β-d-glucopyranoside (5) (Ouyang et al. 2007). All physical and spectral data of these compounds were in full agreement with the reported data (Supplementary data S1).
Compound 4 was obtained as a yellow amorphous powder; \(\left[ \alpha \right]^{ 2 2}_{\text{D}} + 1 4. 5 5 { }\left( {c0. 1,{\text{ MeOH}}} \right)\). HRESIMS showed pseudomolecular ion peaks at m/z 679.2011 [M + Na]+ consistent with a molecular weight of 656 amu. The molecular formula was established as C33H36O14, thus implying 16 degrees of unsaturation. The UV λmax MeOH (log ε): 220 (4.33), 264 (4.18), 290 (3.65) nm, while the IR spectrum showed strong absorption bands attributed to the hydroxyl and ester groups at 3397 and 1708 cm−1, respectively. The 1D- and 2D-NMR spectra in DMSO-d 6 of 4 revealed the lignan glycoside pattern and were very similar to those of 3 (Table 1). The 1H and 13C NMR spectral data showed the existence of three sets of aromatic ABX system; an anomeric proton [δ H 4.93 (d, J = 7.2 Hz, H-1″), δ C 99.9, C-1″] of β-glucopyranosyl moiety; two methoxyls [δ H 3.75, δ C 55.7 and δ H 3.78, δ C 55.7]; two aliphatic SP 3 methines [δ H 2.95 (m, H-8), δ C 53.8, C-8 and δ H 3.01 (m, H-8`), δ C 53.5, C-8`]; two oxygen-bearing sp3 methines [δ H 4.62 (d, J = 5.2 Hz, H-7), δ C 84.8, C-7 and δ H 4.59 (m, H-7′), δ C 85.2, C-7′]; two oxygen-bearing sp 3 methylenes [δ H 4.08 (m, H-9a), 3.74 (m, H-9b), δ C 70.9, C-9 and δ H 4.13 (m, H-9′a), 3.74 (m, H-9′b), δ C 71.0, C-9′], and an ester carbonyl (δ C 165.5, C-7′′′).
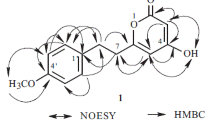
Comparing the above mentioned NMR spectroscopic data of 4 with those of 1 indicated the presence of (+)-pinoresinol 4-O-β-d-glucopyranoside esterified with phenolic acid, which later was identified as protocatechuic (PCA) acid. Esterification occurred at C-6″, as evidenced from 1H–13C HMBC correlations from C-7′′′ (δ C 165.5) to H2-6″ (δ H 4.52 and 4.25) (Fig. 2). Esterification at C-6″ leads to downfield of its chemical shift to (δ C 63.7) instead of (δ C 60.9) in 1 (Supplementary data S2-9S). In addition, the NMR data of 4 was closely similar to those of 3, with the exception of replacement of vanillyl moiety in 3 by protocatechuoyl in 4. The replacement of vanillyl by protocatechuoyl is accompanied by exchanging the methoxyl group (δ H 3.77, δ C 55.7) at C-3′′′ with a hydroxyl group (δ H 9.82, OH-3′′′), leading to a slight upfield shift in its resonance, from δ C 147.5 (3) to δ C 145.5 (4), with a downfield shift of C-2′′′ to δ C 116.3 (4) rather than δ C 112.9 (3) (Table 1) (Kirley et al., 2010). Moreover, key HMBC correlations from C-3′′′ to OH-3′′′ and H-5′′′ (δ H 6.76), as well as from C-4′′′ (δ C 150.5) to OH-4′′′ (δ H 10.13), H-2′′′ (δ H 7.37), and H-6′′′ (δ H 7.43), confirmed the protocatechuoyl moiety. Therefore, the structure of compound 4 was assigned as (+)-pinoresinol 4-O-[6″-O-protocatechuoyl]-β-d-glucopyranoside.
Alkaline hydrolysis of compound 4 using strong alkali as sodium methoxide leads to the production of two compounds of (+)-pinoresinol 4-O-β-d-glucopyranoside and a methyl ester of 3,4-dihydroxy benzoic acid, which were clearly detected by TLC analysis. (+)-pinoresinol 4-O-β-d-glucopyranoside was identified by comparing its HPLC retention time with that of 1. Methyl ester of 3,4-dihydroxy benzoic acid was identified by its accurate mass [M + Na]+ at m/z 191.0318 (calculated for C8H8O4Na, [M + Na]+ at m/z 191.0318).
Esterification of lignan glycosides with oxygenated aromatic acids is uncommon, and few members were identified from natural sources. Esterification was reported with gallic acid, FER, and vanillic acid (VAN), which have recently been reported in the latex of Calotropis gigantea (Ouyang et al. 2007; Matsunami et al. 2009; Parhira et al. 2014).
The antioxidant and anti-inflammatory activities of the isolates were also investigated in this study. For antioxidant activity, the radical scavenging activity of 1–5 was determined spectrophotometrically against stable DPPH• using the method of Abdel-Mageed (Abdel-Mageed et al. 2010; Ibraheim et al. 2012; Abdel-Mageed et al. 2012, 2014; Wagner et al. 2014) with luteolin as a positive control. As shown in Table 2, compound 4 exhibited the strongest radical scavenging activity (IC50 10.6 µM), followed by compound 5 (IC50 13.1 µM), while compounds 1 and 2 had the weakest antioxidant activity (IC50 62.3 and 57.2 µM, respectively). Only compounds 4 and 5 were slightly more potent than luteolin (IC50 15.2 µM).
An improvement in antioxidant activity was unambiguously noticeable by esterification of the lignoid structure with aromatic acids, such as VAN in 3, PCA in 4, and FER in 5. The DPPH• scavenging efficiency of the isolated lignans was in the following order: 4 > 5 > 3. This order can be explained by understanding that the acyl phenol carboxylic acid (PhA) moieties (e.g., PCA, VAN, and FER) have strong radical scavenging activity (RSA) and can potentiate the RSA of the lignoid carrier to various degrees. The degree of potentiation depends on the RSA strength of the acyl PhA group, which mainly depends on its structure characteristics, and on their oxygenation patterns. For further clarification, comparisons of the RSA of the PhA of isolated lignans (3–5) have been reported in the following order in previous studies: PCA > FER > VAN. This order can be explained by understanding that (1) RSA is improved by hydroxylation, so PCA exhibits stronger antioxidant activity than FER and VAN; (2) the methylation of free hydroxyl groups reduces activity; and (3) hydroxylated cinnamate derivatives in general are more effective than their benzoic acid counterparts (FER > VAN) (Mathew et al., 2015). The higher hydroxylated cinnamate activity is attributed to the presence of the bulky −CH=CH−COO− group, which can stabilize the resultant phenoxy radicals by resonance. Moreover, the presence of the –CH=CH−COO− group in cinnamic acid derivatives ensures greater hydrogen donating ability and subsequent radical stabilization than those of the COO– group in benzoic acid derivatives. In benzoic acid, the carboxylate group has electron withdrawing properties, which produce a negative influence on its hydrogen-donating ability, and therefore, on its scavenging ability. Thus, VAN exhibited lower RSA activity than FER due to the adjacency of the carboxylate group to the phenyl ring (Cuvelier et al. 1992; Natella et al. 1999; Yamagami et al. 2005; Mathew et al. 2015).
A preliminary structure–activity relationship among these lignans can be summarized as follows: (1) increased oxygenation of the lignoid unit, in the form of either hydroxyl or methoxyl groups—preferably, hydroxyl groups—leads to improved antioxidant activity, as observed with compounds 1 and 2; (2) in general, esterification of the lignoid unit by oxygenated aromatic acids significantly improves the activity to various degrees, depending on the antioxidant ability of the acyl entity; (3) an increase in the oxygenation patterns of phenyl moieties increases antioxidant activity; and (4) hydroxylated cinnamates exhibit greater antioxidant power than their benzoic acid counterparts do.
In the present study, the anti-inflammatory activity of pure compounds was also evaluated, using a group of key enzymes related to inflammation, including arachidonate 5-lipoxygenase (5-LOX) and 15-LOX, and using luteolin as a positive control (Table 2). It is obvious that isolated lignans exhibited selective inhibitory action against the 15-LOX enzyme rather than the 5-LOX enzyme. The acylated lignans (3–5) showed significantly higher inhibitory activity against 5- and 15-LOX than the non-acylated members (1 and 2) did. Among the acylated lignans, compound 4 exhibited the highest inhibitory action, with IC50s 7.6 and 2.7 µM against 5-LOX and 15-LOX, respectively. The weakest activity was displayed by compound 1, with IC50s 34.1 µM (5-LOX) and 12.4 µM (15-LOX). None of the isolated lignans was stronger than luteolin (IC50s 4.1 and 2.3 µM).
The bioassay results of the LOX enzymes and the structure characteristics implied the following structure–activity relationships: (1) acylation of the lignoid unit by PhA moieties (e.g., PCA, VAN, and FER) improved their activity; (2) compounds with two adjacent hydroxyl groups, as in compound 4, inhibited LOX enzymes effectively; and (3) methylation of one hydroxyl group of the ortho di-hydroxylated system may reduce the inhibitory action on LOX enzymes. These results agree with those of previous studies on the inhibition of LOX enzymes that suggested that the two adjacent hydroxyl groups on an aromatic ring are essential to elicit inhibitory effects on lipoxygenase activities. As such, compounds with one hydroxyl group on an aromatic ring had little inhibitory effect on lipoxygenase activities compared to those with ortho di-hydroxyl groups (Kohyama et al. 1997).
It is worth noting that 15-LOX plays an important role in the pathogenesis of asthma, and its overexpression in non-epithelial cells leads to bronchial epithelial injury (Mabalirajan et al. 2013). Acylated lignans exhibited good selectivity against 15-LOX, with IC50 values ranging from 2.7 to 5.2 µM, making them promising candidates for further research on developing anti-inflammatory agents, specifically targeting asthma pathogenesis.
In conclusion, five lignans are isolated, one of which (4) is a new compound, from the latex of Calotropis procera grown in Egypt. This paper is considered the first report highlighting the phenolic content of C. procera latex and its biological activities. Acylated lignans, especially members with ortho phenolic di-hydroxyl groups, showed significant antioxidant and anti-inflammatory activity, while the non-acylated counterparts exhibited moderate activity. Acylated lignans are an uncommon group, and few members have been identified in various natural sources. Therefore, our new compound (4) is considered a valuable addition to the growing number of previously isolated members. Further investigation of these promising compounds should be pursued for the development of novel antioxidants and anti-inflammatory drugs using natural sources.
References
Abdel-Mageed WM, Milne BF, Wagner M, Schumacher M, Sandor P, Pathom-aree W, Goodfellow M, Bull AT, Horikoshi K, Ebel R, Diederich M, Fiedler HP, Jaspars M (2010) Dermacozines, a new phenazine family from deep-sea dermacocci isolated from a Mariana Trench sediment. Org Biomol Chem 8:2352–2362
Abdel-Mageed WM, Backheet EY, Khalifa AA, Ibraheim ZZ, Ross SA (2012) Antiparasitic antioxidant phenylpropanoids and iridoid glycosides from Tecoma mollis. Fitoterapia 83:500–507
Abdel-Mageed WM, Bayoumi SAH, Radwan AA, Salem-Bekhit MM, Abd-Alrahman SH, Basudan OA, Sayed HM (2014) Simmondsia chinensis: a rich source of bioactive flavonoids and lignans. Ind Crops Prod 60:99–103
Al-Qarawi AA, Mahmoud OM, Sobaih MA, Haroun EM, Adam SE (2001) A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet Res Commun 25:61–70
Ansari SH, Ali M (2001) Norditerpenic ester and pentacyclic triterpenoids from root bark of Calotropis procera (Ait) R. Br Pharmazie 56:175–177
Arya S, Kumar VL (2005) Antiinflammatory efficacy of extracts of latex of Calotropis procera against different mediators of inflammation. Mediators Inflamm 2005:228–232
Bai LP, Ho HM, Ma DL, Yang H, Fu WC, Jiang ZH (2013) Aminoglycosylation can enhance the G-quadruplex binding activity of epigallocatechin. PLoS ONE 8:e53962
Begum N, Sharma B, Pandey RS (2010) Evaluation of Insecticidal Efficacy of Calotropis Procera and Annona Squamosa Ethanol Extracts Against Musca Domestica. J Biofert Biopest 1:1–6
Choedon T, Mathan G, Arya S, Kumar VL, Kumar V (2006) Anticancer and cytotoxic properties of the latex of Calotropis procera in a transgenic mouse model of hepatocellular carcinoma. World J Gastroenterol 12:2517–2522
Cuvelier ME, Richard H, Berset C (1992) Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship. Biosci Biotechnol Biochem 56:324–325
Dewan S, Kumar S, Kumar VL (2000) Antipyretic effect of latex of Calotropis procera. Indian J Pharmacol 32:252
Dong-Mei W, Wen-Jun P, Yong-Hong W, Yu-Juan Z, Shan-Shan W (2012) A New Isorhamnetin Glycoside and Other Phenolic Compounds from Callianthemum taipaicum. Molecules 17:4595–4603
Doshi H, Satodiya H, Thakur MC, Parabia F, Khan A (2011) Phytochemical screening and biological activity of Calotropis Procera (Ait). R. Br. (Asclepiadaceae) against selected bacteria and Anopheles stephansi Larvae. Int J Plant Res 1:29–33
Farzin MP, Kothari IL, Parabia MH (2008) Antibacterial activity of solvent fractions of crude water decoction of apical twigs and latex of Calotropis procera (Ait.) R. Br. Nat Prod Radiance 7:30–34
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gupta VK, Sharma SK (2006) Plants as natural antioxidants. Nat Prod Radiance 5:326–334
Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M (2012) Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B Biointerfaces 95:284–288
Ibraheim ZZ, Abdel-Mageed WM, Dai H, Guo H, Zhang L, Jaspars M (2012) Antimicrobial antioxidant daucane sesquiterpenes from Ferula hermonis Boiss. Phytother Res 26:579–586
Joshi R, Sharma A, Jat BL (2009) Analysis of antioxidant activity in extracts of Calotropis procera (Ait.) R. Br. J Appl Biosci 17:899–903
Kamel HS, Nagy M, Heidi Z, Johannes FI, Bernd S (2010) Secondary metabolites from Calotropis procera (Aiton). Phytochem Lett 3:212–216
Kareem SO, Akpan I, Ojo OP (2008) Antimicrobial activities of Calotropis procera on selected pathogenic microorganisms. African J Biomed Res 11:105–110
Kawo AH, Mustapha A, Abdullahi BA, Rogo LD, Gaiya ZA, Kumurya AS (2009) Phytochemical properties and antibacterial activities of the leaf and latex extracts of Calotropis procera (Ait. F.). Bayero J Pure Appl Sci 2:34–40
Khasawneh MA, Elwy HM, Fawzi NM, Hamza AA, Chevidenkandy A, Hassan AH (2011) Antioxidant activity, lipoxygenase inhibitory effect and polyphenolic compounds from Calotropis procera (Ait.) R. Br. Res J Phytochem 5:80–88
Kirley MC, Mary ASL, Edilberto RS (2010) Amburosides C-H and 6-O-Protocatechuoyl Coumarin from Amburana cearensis. J Braz Chem Soc 21:1746–1753
Kohyama N, Nagata T, Fujimoto SI, Sekiya K (1997) Inhibition of arachidonate lipoxygenase activities by 2-(3,4-dihydroxyphenyl)ethanol, a phenolic compound from olives. Biosci Biotech Biochem 61:347–350
Kumar VL, Roy S (2009) Protective effect of latex of Calotropis procera in Freund’s complete adjuvant induced monoarthritis. Phytother Res 23:1–5
Kumar S, Dewan S, Sangraula H, Kumar VL (2001) Anti-diarrhoeal activity of the latex of Calotropis procera. J Ethnopharmacol 76:115–118
Larhsini M, Markouk M, Jaouhari JT, Bekkouche K, Lazrek HB, Jana M (2002) The antipyretic activity of some Moroccan medicinal plants. Phytother Res 16:97–98
Mabalirajan U, Rehman R, Ahmad T, Kumar S, Leishangthem GD, Singh S, Dinda AK, Biswal S, Agrawal A (2013) 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci Rep 3:1540–1550
Mahajan RT, Badgujar SB (2008) Phytochemical investigation of some lactiferous plants belonging to Khandesh region of Maharashtra. Ethnobot Leaflets 12:1145–1152
Mathew S, Abraham TE, Zakaria ZA (2015) Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. doi:10.1007/s13197-014-1704-0
Matsunami K, Otsuka H, Kondo K, Shinzato T, Kawahata M, Yamaguchi K, Takeda Y (2009) Absolute configuration of (+)-pinoresinol 4-O-[6′’-O-galloyl]-beta-d-glucopyranoside, macarangiosides E, and F isolated from the leaves of Macaranga tanarius. Phytochemistry 70:1277–1285
Meena AK, Yadav MM, Niranjan US, Singh B, Nagariya AK, Sharma K, Gaurav A, Sharma S, Rao MM (2010) A review of Calotropis procera Linn and its ethnobotany, phytochemical and pharmacological profile. Drug Invent Today 2:185–190
Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R (2007) Cardiotonic steroids on the road to anti-cancer therapy. BBA Rev Cancer 1776:32–57
Moursy LE (1997) Insecticidal activity of Calotropis procera extracts of the flesh fly, Sarcophaga haemorrhoidalis fallen. J Egypt Soc Parasitol 2:505–514
Nadia HM, Miaomiao L, Wael MA, Lamya HA, Huanqin D, Mady AI, Gamal B, Ronald JQ, Xueting L, Lixin Z, Ahmed AMS (2015) Cytotoxic cardenolides from the latex of Calotropis procera. Bioorg Med Chem Lett 25:4615–4620
Natella F, Nardini M, di Felice M, Scaccini C (1999) Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J Agric Food Chem 47:1453–1459
Ouyang MA, Wein YS, Zhang ZK, Kuo YH (2007) Inhibitory Activity against Tobacco Mosaic Virus (TMV) Replication of Pinoresinol and Syringaresinol Lignans and Their Glycosides from the Root of Rhus javanica var. roxburghiana. J Agric Food Chem 55:6460–6465
Parhira S, Yang ZF, Zhu GY, Chen QL, Zhou BX, Yu-Tao WYT, Liu L, Bai LP, Jiang ZH (2014) In Vitro anti-influenza virus activities of a New Lignan Glycoside from the Latex of Calotropis gigantea. PLoS ONE 9:e104544
Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L (2007) Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflammation 4:15–21
Ramos MV, Bandeira GP, De Freitas CD, Nogueira NA, Alencar NM, De Sousa PA, Carvalho AF (2006) Latex constituents from Calotropis procera (R. Br.) display toxicity upon egg hatching and larvae of Aedes aegypti (Linn.). Mem Inst Oswaldo Cruz 101:503–510
Ramos MV, Aguiar VC, Melo VMM, Mesquita RO, Silvestre PP, Oliveira JS, Macedo NM, Alencar NM (2007) Immunological and allergenic responses induced by latex fractions of Calotropis procera (Ait.) R. Br. J Ethnopharmacol 111:115–122
Ramos MV, Oliveira JS, Figueiredo JG, Figueireido IS, Kumar VL, Bitencourt FS, Cunha FQ, Oliveira RS, Bomfim LR, Vitor LFJ, Alencar NM (2009) Involvement of NO in the inhibitory effect of Calotropis procera latex protein fractions on leukocyte rolling, adhesion and infiltration in rat peritonitis model. J Ethnopharmacol 125:387–392
Rehman AU, Malik A, Riaz N, Ahmed H, Nawaz SA, Choudhary MI (2005) Lipoxygenase Inhibiting Constituents from Indigofera hetrantha. Chem Pharm Bull 53:263–266
Rioux N, Castonguay A (1998) Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis 19:1393–1400
Roy S, Sehgal R, Padhy BM, Kumar VL (2005) Antioxidant and protective effect of latex of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol 102:470–473
Sabrin RMI, Gamal AM, Lamiaa AS, Laetitia MYB, Robert K, Diaa TAY (2015) Calotroposides H-N, new cytotoxic oxypregnane oligoglycosides from the root bark of Calotropis procera. Steroids 96:63–72
Sangraula H, Dewan S, Kumar VL (2002) Evaluation of anti-inflammatory activity of latex of Calotropis procera in different models of inflammation. Inflammopharmacology 9:257–264
Sehgal R, Arya S, Kumar VL (2005) Inhibitory effect of extracts of latex of Calotropis procera against Candida albicans: A preliminary study. Indian J Pharmacol 37:334–335
Setty SR, Quereshi AA, Swamy AHM, Patil T, Prakash T, Prabhu K, Veeran G (2007) Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 78:451–454
Sharma P, Sharma JD (2000) In-vitro schizonticidal screening of Calotropis procera. Fitoterapia 71:77–79
Shivkar YM, Kumar VL (2003) Anthelmintic activity of latex of Calotropis procera. Pharm Biol 41:263–265
Silva MCC, Silva AB, Teixeira FM, Sousa PCP, Rondon RMM, Junoir JERH, Sampiao LRL, Oliveira SL, Holonda ANM, Vasconcelos SMM (2010) Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac J Trop Med 3:332–336
Soares de Oliveira J, Pereira Bezerra D, Teixeira de Freitas CD, Delano BMFJ, Odorico de Moraes M, Pessoa C, Costa-Lotufo LV, Ramos MV (2007) In vitro cytotoxicity against different human cancer cell lines of laticifer proteins of Calotropis procera (Ait.) R. Br. Toxicol in Vitro 21:1563–1573
Takeshi D, Takako I, Sansei N (1985) The constituents of Eucommia ulmoides Oliv II. Isolation and structures of three new lignan glycosides. Chem Pharm Bull 33:3651–3657
Tappel AL (1962) Methods in Enzymology, vol 5. Academic Press, New York, p 539
Vernon ES, Cathy AH, Ernest TH, Levy K, Ronald AL, James AC, Caroline CS, Gary JK (1999) Lipoxygenase Inhibitors as Potential Cancer Chemopreventives. Cancer Epidemiol Biomarkers Prev 8:467–483
Wagner M, Abdel-Mageed WM, Ebel R, Bull AT, Goodfellow M, Fiedler HP, Jaspars M (2014) Dermacozines H-J isolated from a deep-sea strain of Dermacoccus abyssi from Mariana Trench sediments. J Nat Prod 77:416–420
Walther M, H-g Holzhütter, Kuban RJ, Wiesner R, Rathmann J, Kühn H (1999) The Inhibition of Mammalian 15-Lipoxygenases by the anti-inflammatory drug ebselen: dual-type mechanism involving covalent linkage and alteration of the iron ligand sphere. Mol Pharmacol 56:196–203
Wisastra R, Dekker FJ (2014) Inflammation, cancer and oxidative lipoxygenase activity are intimately Linked. Cancers 6:1500–1521
Yamagami C, Akamatsu M, Motohashi N, Hamada S, Tanahashi T (2005) Quantitative structure-activity relationship studies for antioxidant hydroxybenzalacetones by quantum chemical- and 3-D-QSAR(CoMFA) analyses. Bioorg Med Chem Lett 15:2845–2850
Yesmin MN, Uddin SN, Mubassara S, Akond MA (2008) Antioxidant and antibacterial activities of Calotropis procera Linn. Am Eurasian J Agric Environ Sci 4:550–553
Yves D, Christine B, Daniel D, Chi CC, Jean PF, John WG, Jocelyne G, John HH, Cyryl SM (1994) Naphthalenic lignan lactones as selective, nonredox 5-lipoxygenase inhibitors. Synthesis and biological activity of (methoxyalkyl)thiazole and methoxytetrahydropyran hybrids. J Med Chem 37:512–518
Acknowledgments
The authors would like to extend their sincere appreciation to the deanship of Scientific Research at King Saud University for its funding of this research through Research Group Project no. RGP-VPP-326.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdel-Mageed, W.M., Mohamed, N.H., Liu, M. et al. Lipoxygenase inhibitors from the latex of Calotropis Procera . Arch. Pharm. Res. (2016). https://doi.org/10.1007/s12272-016-0725-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12272-016-0725-9