Abstract
Matrix metalloproteinase-13 (MMP-13) plays a critical role in degrading major collagens in human cartilage under some pathological conditions such as osteoarthritis. To establish the therapeutic potential against cartilage degradation, the effects of 12 naturally-occurring triterpenoids and steroids on MMP-13 induction were examined in the human chondrocyte cell line, SW1353. They included coreanoside F1, suavissimoside R1, spicatoside A, 25(S)-ruscogenin, methyl protogracillin, hederagenin, loniceroside A, loniceroside B, loniceroside C, smilaxin A, smilaxin C, and ursolic acid. Among these, only spicatoside A and 25(S)-ruscogenin were found to inhibit MMP-13 expression in IL-1β-treated SW1353 cells at a pharmacologically-relevant concentration of 10 μM. These effects were also supported by the finding that spicatoside A (20 μM) reduced glycosaminoglycan release from IL-1α-treated rabbit joint cartilage culture to some degree. When the cellular mechanisms of action of spicatoside A in MMP-13 inhibition were investigated, the blocking point was not found among the MMP-13 signaling molecules examined such as mitogen-activated protein kinases, activator protein-1, and nuclear transcription factor-κB. Instead, spicatoside A was found to reduce MMP-13 mRNA stability. All of these findings suggest that spicatoside A and 25(S)-ruscogenin have a therapeutic potential for protecting against cartilage breakdown in arthritic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the cartilage, residing chondrocytes synthesize and release matrix metalloproteinases (MMP) to the extracellular matrix (ECM) (Hadler-Olsen et al. 2011). Of these, MMP-1 and -13 are collagenases that degrade collagen matrix of the cartilage. They participate in normal turn-over of the cartilage materials, but in some situations such as arthritic conditions or aging process, they are highly induced and degrade the ECM rapidly, resulting in pathological conditions like osteoarthritis (OA) (Mitchell et al. 1996; Takaishi et al. 2008). The important role of MMP-13 in osteoarthritic conditions was also proved in MMP-13 deficient mice (Little et al. 2009). Therefore, the inhibitors and/or down-regulators of MMP-1 and -13 may have a therapeutic potential as a cartilage-protective agent in cartilage degradation disorders. Actually, one MMP inhibitor (Periostat®) is clinically available for treating periodontitis (Caton and Ryan 2011). In this regard, many chemicals or natural products are worthy to be evaluated for their inhibitory action against MMPs.
Triterpenoids and steroids as plant constituents have been reported to show various pharmacological activities in vitro and in vivo. They include anti-cancer activity, anti-inflammatory activity, anti-allergic activity, etc. (Kim et al. 1999; Yadav et al. 2010). However, there have been a few investigations of these constituents with respect to their effects on MMPs and cartilage protection. Recently, several ginsenosides isolated from Panax ginseng (dammarane-type triterpenoids) were found to inhibit MMP-13 expression and the ginsenosides, F4 and Rg3, protected against cartilage breakdown in rabbit cartilage culture (Lee et al. 2014). In the present study, 12 chemically-different triterpenoids and steroids were examined for their down-regulatory action against MMP-13 expression in the human chondrocyte cell line, SW1353, for the purpose of establishing the therapeutic potential for chondroprotection. Some cellular mechanisms of action were also studied.
Materials and methods
Chemicals
Human IL-1α, IL-1β, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dexamethasone, actinomycin D and anti-MMP-13 antibody were purchased from Sigma-Aldrich (St. Louis, MO). DMEM and other cell culture reagents including FBS, were products of Thermo Scientific (Logan, UT). All antibodies relating to mitogen-activated protein kinase (MAPK) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling were purchased from Cell Signaling Technologies (Danvers, MA). Lamin B1 antibody was purchased from Bioworld technology (Minneapolis, MN).
Triterpenoids and steroids from plant origin (Fig. 1)
Coreanoside F1, suavissimoside R1, spicatoside A, methyl protogracillin, hederagenin, loniceroside A, loniceroside B, smilaxin A, smilaxin C, and ursolic acid were isolated from several plant extracts according to the previously described (Kim et al. 1999). Loniceroside C was isolated from Lonicera japonica (Kwak et al. 2003). Ruscogenin was obtained by the hydrolysis of spicatoside A. Their purities were examined by TLC and they gave one spot.
Animals
Male New Zealand White rabbits (6 weeks old) were purchased from Central Experimental Animal Co. (Seoul, Korea). The animals were maintained in an animal facility (KNU) at 20–22 °C under 40–60 % relative humidity and a 12 h/12 h (light/dark) cycle. The experimental design using the animals was approved by the local committee for animal experimentation of Kangwon National University (KIACUC-13-0003). The animals were handled according to the guideline described in the Food and Drug Administration (Korea) Guide for the Care and Use of Laboratory Animals throughout the experiments.
SW1353 cell culture and MMP-13 induction
SW1353 cells (human chondrosarcoma cell line) purchased from American Type Culture Collection (Manassas, VA) were cultured and treated with IL-1β according to the previously described procedures (Lim and Kim 2011). Briefly, the cells were normally maintained in DMEM with 10 % FBS, glutamine and penicillin/streptomycin. For an induction of MMP-13, IL-1β (10 ng/ml) with/without test compounds was added to the cells in serum-free DMEM. After 24 h incubation, MMP-13 expressed and released in the media was examined by Western blotting analysis. After separating on SDS-PAGE, the blots were treated with anti-MMP-13 antibody in 5 % skim milk in TBST. An ECL system (GE Healthcare, UK) was used to visualize the band. The densities of the bands were analyzed with ImageQuant LAS4000 mini (GE Healthcare, UK). All test compounds were initially dissolved in DMSO and diluted with serum-free DMEM to adjust the final DMSO concentration of 0.1 % (v/v). Other compounds except several compounds showing cytotoxicity at 10 μM were treated to cells at a concentration of 10 μM. Spicatoside A, ursolic acid, smilaxin A and smilaxin C were tested at 5, 5, 0.1 and 5 μM, respectively, concentration without cytotoxicity. Cell viability was checked using MTT bioassay (Mossman 1983). The treatment of 0.1 % DMSO had no effect on cell viability and the levels of MMP-13 expression. For expression of MMP-13 and GAPDH mRNA, total RNA was extracted using NucleoSpin RNA (MACHEREY–NAGEL GmbH & Co. KG, Düren, Germany). cDNA was synthesized with ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Quantitative PCR was carried out with THUNDERBIRD SYBR qPCR mix (Toyobo, Osaka, Japan) using C1000 Touch Thermal Cycler (Biorad Lab., CA). Primers for human MMP-13 and GAPDH were purchased from Qiagen (Hilden, Germany). MMP-13 mRNA expression was normalized to GAPDH.
Cellular mechanisms of inhibition of MMP-13 induction
Using total cellular lysates, expression and phosphorylation of MAPKs and JAK/STAT were examined. For the detection of MAPKs, cells were treated with IL-1β for 30 min after pretreatment with spicatoside A for 2 h. For STAT1/2, spicatoside A (1, 2 and 5 μM) was pretreated for 2 h and then cells were incubated for further 4 h in the presence of IL-1β. Total cellular proteins were extracted with Pro-Prep solution (iNtRON Biotechnology) containing 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM sodium orthovanadate and 1 mM sodium fluoride. Expression of NF-κB p65, c-Jun, and c-Fos were identified in nuclear fractions. Cells were treated with IL-1β for 1 h after pretreatment with spicatoside A (1, 2 and 5 μM) for 2 h. For an extraction of nuclear proteins, cells were resuspended in 400 μl of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.5 mM PMSF, 1 μg/ml aprotinin and 1 μg/ml leupeptin) and incubated on ice for 10 min. After 25 μl of 10 % NP-40 was added, cells were vortexed for 10 s and centrifuged at 5000 rpm for 2 min. The nuclear pellet was vigorously vortexed in buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF, 1 μg/ml aprotinin and 1 μg/ml leupeptin) and centrifuged at 13,000 rpm for 10 min. BCA protein assay (Pierce, IL, USA) was used to determine protein concentration in the nuclear fraction. Proteins were separated, blotted and visualized as described above. Lamin B1 was used as an internal control of nuclear proteins. To investigate the effect of spicatoside A on MMP-13 mRNA stability, cells were treated with IL-1β for 1 h and then the media was washed out, following treatment with spicatoside A (5 μM) and/or actinomycin D (2 μM) for 2 h. The level of MMP-13 mRNA was measured in total RNA extracted from the cells as described above.
Effects of spicatoside A on glycosaminoglycan (GAG) release from rabbit cartilage culture
According to the previously described procedures (Lim et al. 2011), articular cartilages were excised from the femoral condyles of rabbit knee and incubated in DMEM containing 5 % FBS for 1–2 days. Approximately 30 mg cartilage fragments per well were seeded on 48 well plates and media were changed to DMEM containing 1 % FBS in 400 μl/well. Cartilages were treated with 10 ng/ml of IL-1α and test compounds for 3 days. The amounts of released GAG in the supernatant were measured with Blyscan sulfated glycosaminoglycan assay kit (Biocolor, Northern Ireland, UK) according to the manufacturer’s protocol.
Statistical analysis
Experimental values are represented as arithmetic mean ± SD. Statistical analysis was evaluated using one-way ANOVA followed by Dunnett’s analysis.
Results
Effects of various triterpenoids and steroids on MMP-13 expression
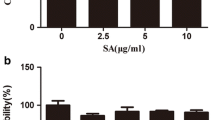
First, the cytotoxicity in IL-1β-treated SW1353 cells was examined using MTT bioassay. At a pharmacologically-relevant concentration (10 μM), several compounds including ursolic acid, smilaxin A and smilaxin C showed strong cytotoxicity in IL-1β-treated SW1353 cells when incubated for 24 h (Fig. 2). Spicatoside A slightly reduced the viability of SW1353 cells (9.8 % reduction). Smilaxin A showed cytotoxicity at 0.5–10 μM (data not shown).
Viability of SW1353 cells treated with on IL-1β and triterpenoids or steroids (MTT bioassay). All compounds were treated at 10 μM in SW1353 cells for 24 h and MTT bioassay was carried out. DEX is an abbreviation of dexamethasone. *P < 0.05, **P < 0.01, Significantly different from the IL-1β-treated control group (n = 3). Note spicatoside A, ursolic acid, smilaxin A and smilaxin C reduced viability of SW1353 cells
IL-1β treatment of SW1353 cells for 24 h strongly induced MMP-13 expression as shown in Fig. 3. Initially, when the activity of 8 triterpenoids and steroids was screened, only spicatoside A and 25(S)-ruscogenin were found to strongly suppress MMP-13 expression at 10 μM (Fig. 3a), although spicatoside A caused slight reduction in cell viability (Fig. 2). As expected, dexamethasone (10 μM), a reference compound, strongly inhibited MMP-13 expression. To identify the MMP-13 down-regulatory effect of spicatoside A at non-cytotoxic concentration, its activity was re-examined including effects of several derivatives. As shown in Fig. 3b, only spicatoside A (5 μM) among the tested derivatives inhibited MMP-13 induction at non-cytotoxic concentration. These results indicate that only spicatoside A (steroidal saponin) and its aglycone, 25(S)-ruscogenin (steroidal sapogenin), reduced MMP-13 expression in IL-1β-treated SW 1353 cells without any cytotoxic effect.
Effects of triterpenoids and steroids on induced MMP-13 expression in SW1353 cells by IL-1β. The compounds and IL-1β were simultaneously treated. The released MMP-13 levels in the media were examined by Western blotting analysis. a Effects of the compounds on MMP-13 expression at 10 μM, 1 coreanoside F1 (10 μM), 2 suavissimoside R1 (10 μM), 3 spicatoside A (10 μM), 4 methyl protogracillin (10 μM), 5 dexamethasone (10 μM), 6 hederagenin (10 μM), 7 loniceroside A (10 μM), 8 25(S)-ruscogenin (10 μM), 9 loniceroside B (10 μM), 10 loniceroside C (10 μM) b Effects of the compounds on MMP-13 expression at non-cytotoxic concentrations, 11 ursolic acid (5 μM), 12 smilaxin C (5 μM), 13 spicatoside A (5 μM), 14 dexamethasone (10 μM), 15 smilaxin A (0.1 μM)
Concentration-dependent inhibition of MMP-13 expression by spicatoside A and 25(S)-ruscogenin
For studying the concentration-dependent effect, SW1353 cells were incubated with different concentrations of spicatoside A and 25(S)-ruscogenin. As shown in Fig. 4, spicatoside A concentration-dependently inhibited MMP-13 expression at 0.5–5 μM. On the other hand, 25(S)-ruscogenin only inhibited MMP-13 expression at 10–30 μM, but not in a concentration-dependent manner.
Concentration-dependent inhibition of MMP-13 expression in IL-1β-treated SW1353 cells by spicatoside A and 25(S)-ruscogenin. The compounds and IL-1β were simultaneously treated. The released MMP-13 levels in the media were examined by Western blotting analysis. DEX is an abbreviation of dexamethasone. *P < 0.05, **P < 0.01, significantly different from the IL-1β-treated control group (n = 3)
Cellular mechanism of MMP-13 down-regulation by spicatoside A
Next, the cellular mechanism of MMP-13 down-regulation by spicatoside A was investigated using Western blotting technique. Since it is known that NF-κB, p38 MAPK/c-Fos/AP-1, and JAK/STAT pathways are important for the expression of MMP-13 gene in IL-1β-treated SW1353 cells (Mengshol et al. 2000; Lim and Kim 2011; Lim et al. 2011), the effects of spicatoside A on these signaling pathways were examined. To identify the effect on the phosphorylation of three MAPKs and STAT1/2, cell lysates were prepared and examined as described above. Additionally, activation of c-Jun, c-Fos and NF-κB were examined in nuclear proteins. However, spicatoside A did not affect phosphorylation of all three MAPKs and activation of several transcription factors (Fig. 5). These results indicate that MMP-13 reduction by spicatoside A may not be mediated through NF-κB, p38 MAPK/c-Fos/AP-1, and JAK/STAT pathways crucially involved in the process of MMP-13 induction.
Insignificant role of MAPKs, STAT1/2, c-Jun, c-Fos and NF-κB in MMP-13 down-regulation by spicatoside A. The cellular level of the signaling molecule was examined by Western blotting analysis. a Effect on p38 MAPK, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation, cells were treated with IL-1β for 30 min after pretreatment with spicatoside A for 2 h. b Effect on the activation of transcription factors, For STAT1/2 activation, cells were treated with IL-1β for 4 h after spicatoside A pretreatment. For c-Jun, c-Fos and NF-κB activation, cells were treated with IL-1β for 1 h after spicatoside A pretreatment and the nuclear proteins were used for Western blotting analysis. Each blot is a representative of three separate experiments
Effect of spicatoside A on MMP-13 mRNA stabilization
The effect of spicatoside A on MMP-13 mRNA stabilization was examined by RT-PCR analysis. When cells were simultaneously treated with spicatoside A and IL-1β for 6 h, spicatoside A reduced the MMP-13 mRNA level (Fig. 6a). This reduction may be the sum of the reduced level of MMP-13 mRNA transcription and the reduced stability of MMP-13 mRNA. Importantly, when cells were treated with spicatoside A (5 μM) and actinomycin D (2 μM) after IL-1β was washed out, MMP-13 mRNA level was further decreased (41.5 % reduction) compared to that in the actinomycin D-treated group (Fig. 6b), suggesting that spicatoside A down-regulates MMP-13 expression, at least in part, by decreasing MMP-13 mRNA stability.
Effects of spicatoside A on the stability of MMP-13 mRNA. The levels of MMP-13 mRNA were examined using real-time PCR method. a Spicatoside A and IL-1β were simultaneously treated for 6 h. DEX is an abbreviation of dexamethasone. b After IL-1β treatment for 1 h, the media were washed out and cells were treated with spicatoside A (5 μM) and/or actinomycin D (2 μM) for 2 h. *P < 0.05, **P < 0.01, significantly different from the IL-1β-treated control group (n = 3) or IL-1β-treated group with actinomycin D (n = 3)
Inhibition of glycosaminoglycan (GAG) release from rabbit cartilage by spicatoside A
For examining the effect of spicatoside A on cartilage degradation, rabbit cartilage tissue culture was used. When treatment with IL-1α was performed for 3 days, rabbit cartilage was degraded and GAG was released into the media (approximately 2.3 fold increase) as shown in Fig. 7. Under this condition, spicatoside A blocked the release of GAG (34.2 %) at 20 μM, although not statistically significantly mainly due to the large SD value. Dexamethasone (10 μM) used as a reference drug strongly inhibited GAG release (65.8 % inhibition).
Discussion
The present study has clearly demonstrated that spicatoside A and its aglycone, 25(S)-ruscogenin, down-regulate MMP-13 induction in human chondrocytes, and spicatoside A also inhibits cartilage degradation in rabbit cartilage culture to some degree. The cellular mechanism of MMP-13 down-regulation by spicatoside A was found to be a reduction of MMP-13 mRNA stability, at least in part. Other triterpenoids and steroids examined did not exert inhibition of MMP-13 expression up to the concentration of 10 μM.
The concentration of the compounds tested initially was limited to 10 μM, because in the human body, the maximum concentration of each compound attainable by oral treatment with plant extracts or the compounds themselves is thought to be approximately 10 μM or lower. Concentrations higher than 10 μM may not be attained even by the pharmacological treatment. In this pharmacologically-relevant concentration range, only 25(S)-ruscogenin was found to be active without any cytotoxic effect and spicatoside A caused inhibition at a concentration of less than 10 μM.
In this study, the cellular mechanisms of action of MMP-13 down-regulation by spicatoside A were elucidated. In SW1353 cells or primary human chondrocyte cell culture, several signaling pathways are known to be critically involved in MMP-13 induction (Mengshol et al. 2000; Pei et al. 2006; Lim and Kim 2011). But, contrary to our expectation, spicatoside A neither affects the MAP kinase pathway nor inhibits the activation of major transcription factors which are known to be critically involved in MMP-13 induction. Some other signaling molecules that were unexamined might have been affected. On the other hand, it was found that spicatoside A inhibits MMP-13 expression possibly by decreasing MMP-13 mRNA stabilization, at least in part. The possibility of inhibition of the translation process and/or the release process of MMP-13 to the outside may not be completely excluded. The detailed cellular mechanisms of action need to be elucidated further.
Several classes of natural products were previously found to inhibit collagenase expression in certain condition of chondrocytes or synovial fibroblasts. For example, a flavonoid derivative, epigallocatechin-3-gallate, was found to inhibit MMP-1, -3 and -13 expression in several cells (Ahmed et al. 2004; Corps et al. 2004). We have found that certain flavones including apigenin and several synthetic flavones such as 2′,3′,5,7-tertrahydroxyflavone down-regulated MMP-13 expression in IL-1β-treated human chondrosarcoma cell line SW1353. Especially, they inhibited c-Fos/AP-1 activation and JAK/STAT activation (Lim et al. 2011). One diterpenoid, atractylenolide I, also suppressed MMP-13 expression in SW1353 cells (Park et al. 2011). Recently, we have demonstrated that some ginsenosides (dammarane-type triterpenoid saponins) inhibited MMP-13 induction in IL-1β-treated SW1353 cells and blocked cartilage breakdown. The most prominent inhibitor, ginsenoside F4, interferes with the p38 MAPK pathway (Lee et al. 2014). The present investigation has for the first time demonstrated that steroidal saponin (spicatoside A) and steroidal sapogenin (25(S)-ruscogenin) exert MMP-13 down-regulatory effect.
Spicatoside A is a major ingredient of the tubers of Liriope platyphylla, used as a remedy for improving learning and memory and for treating pulmonary inflammatory disease in traditional herbal medicine (Hur et al. 2009; Lee et al. 2005). Spicatoside A was reported to enhance memory consolidation and increase mucin production from airway epithelial cell (Kwon et al. 2014; Park et al. 2014). However, there has been no report showing the effect of Liriope platyphylla and its constituents on MMPs. In this regard, the present investigation may provide a new possibility of traditional plants containing spicatoside A as a therapeutic agent in disease treatment related with MMP expression such as OA.
In conclusion, the present study clearly demonstrates that spicatoside A and 25(S)-ruscogenin down-regulated MMP-13 expression in human chondrocyte cell line, SW1353, at pharmacologically relevant concentrations. It can be suggested that spicatoside A and 25(S)-ruscogenin may be beneficial for protecting against cartilage degradation in certain conditions such as arthritis and aging process. The results also provide a potential therapeutic possibility for protection against ECM degradation in other area of the body such as the buccal space.
References
Ahmed, S., N. Wang, M. Lalonde, V.M. Goldberg, and T.M. Haqqi. 2004. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. Journal of Pharmacology and Experimental Therapeutics 308: 767–773.
Caton, J., and M.E. Ryan. 2011. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacological Research 63: 114–120.
Corps, A.N., V.A. Curry, D.J. Buttle, B.L. Hazleman, and G.P. Riley. 2004. Inhibition of interleukin-1-stimulated collagenase and stromelysin expression in human tendon fibroblasts by epigallocatechin gallate ester. Matrix Biology 23: 163–169.
Hadler-Olsen, E., B. Fadnes, I. Sylte, L. Uhlin-Hansen, and J.O. Winberg. 2011. Regulation of matrix metalloproteinase activity in health and disease. FEBS Journal 278: 28–45.
Hur, J., P. Lee, E. Moon, I. Kang, S.H. Kim, M.S. Oh, and S.Y. Kim. 2009. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. European Journal of Pharmacology 620: 9–15.
Kim, S.Y., K.H. Son, H.W. Chang, S.S. Kang, and H.P. Kim. 1999. Inhibition of mouse ear edema by steroidal and triterpenoid saponins. Archives of Pharmacal Research 22: 313–316.
Kwak, W.J., C.K. Han, H.W. Chang, H.P. Kim, S.S. Kang, and K.H. Son. 2003. Loniceroside C, an anti-inflammatory saponin from Lonicera japonica. Chemical & Pharmaceutical Bulletin 51: 333–335.
Kwon, G., H.E. Lee, D.H. Lee, H. Woo, S.J. Park, Q. Gao, Y.J. Ahn, K.H. Son, and J.H. Ryu. 2014. Spicatoside A enhances memory consolidation through the brain-derived neurotrophic factor in mice. Neuroscience Letters 572: 58–62.
Lee, J.H., H. Lim, O. Shehzad, Y.S. Kim, and H.P. Kim. 2014. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. European Journal of Pharmacology 724: 145–151.
Lee, Y.C., J.C. Lee, Y.B. Seo, and Y.B. Kook. 2005. Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. Journal of Ethnopharmacology 101: 144–152.
Lim, H., and H.P. Kim. 2011. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Archives of Pharmacal Research 34: 109–117.
Lim, H., H. Park, and H.P. Kim. 2011. Effects of flavonoids on matrix metalloproteinase-13 expression of interleukin-1-treated articular chondrocytes and their cellular mechanisms: Inhibition of c-Fos/AP-1 and JAK/STAT signaling pathways. Journal of Pharmacological Sciences 116: 221–231.
Little, C.B., A. Barai, D. Burkhardt, S.M. Smith, A.J. Fosang, Z. Werb, M. Shah, and E.W. Thopson. 2009. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis and Rheumatism 60: 3723–3733.
Mengshol, J.A., M.P. Vincent, C.I. Coon, A. Barchowsky, and C.E. Brinckehoff. 2000. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase-13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: Differential regulation of collagenase a and collagenase 3. Arthritis and Rheumatism 43: 801–811.
Mitchell, P.G., H.A. Magna, L.M. Reeves, L.L. Lopresti-Morrow, S.A. Yocum, P.J. Rosner, K.F. Geoghegan, and J.E. Hambor. 1996. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. Journal of Clinical Investigation 97: 761–768.
Mossman, T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. Journal of Immunological Methods 65: 55–56.
Park, H.Y., H. Lim, H.P. Kim, and Y.S. Kwon. 2011. Downregulation of matrix metalloproteinase-13 by the root extract of Cyathula officinalis Kuan and its constituents in IL-1β-treated chondrocytes. Planta Medica 77: 1528–1530.
Park, S.H., H.J. Lee, J. Ryu, K.H. Son, S.Y. Kwon, S.K. Lee, Y.S. Kim, J.H. Hong, J.H. Seok, and C.J. Lee. 2014. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine 21: 172–176.
Pei, Y., A. Harvey, X.P. Yu, S. Chandrasekhar, and K. Thirunavukkarasu. 2006. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells; potential role of Runx2 in mediating p38 effects. Osteoarthritis and Cartilage 14: 749–758.
Takaishi, H., T. Kimura, S. Dalah, Y. Okada, and J. D’Armiento. 2008. Joint disease and matrix metalloproteinases: A role for MMP-13. Current Pharmaceutical Biotechnology 9: 47–54.
Yadav, V.R., S. Prasad, B. Sung, R. Kannappan, and B.B. Aggarwal. 2010. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2: 2428–2466.
Acknowledgments
This study was supported by BK21-plus Project from the Korean Ministry of Education. The bioassay facility of New Drug Res. Institute (KNU) was used and greatly acknowledged.
Conflict of interest
The authors indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, H., Min, D.S., Kang, Y. et al. Inhibition of matrix metalloproteinase-13 expression in IL-1β-treated articular chondrocytes by a steroidal saponin, spicatoside A, and its cellular mechanisms of action. Arch. Pharm. Res. 38, 1108–1116 (2015). https://doi.org/10.1007/s12272-015-0581-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0581-z