Abstract
Ten compounds, 1′,3′-propanediol,2′-amino-1′-(1,3-benzodioxol-5-yl) (1), artanomaloide (2), canin (3), eupatilin (4), quercetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (5), 1,3-di-O-caffeoylquinic acid (6), isoquercitrin (7), pinoresinol-4-O-β-d-glucoside (8), scopolin (9), and isofraxidin-7-O-β-d-glucopyranoside (10) were isolated from the aerial parts of A. selengensis. The structures of compounds (1–10) were identified based on 1D and 2D NMR, including 1H–1H COSY, HSQC, HMBC and NOESY spectroscopic analyses. Among them, compound 1 was isolated from this plant for the first time as a naturally occurring compound. The inhibitory activity of these isolated compounds against interleukin-6 (IL-6) production in TNF-α stimulated MG-63 cells was also examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisia selengensis Turcz. is a perennial herb belonging to the Compositae that grows mainly in wetlands, and waterside in Korea (Lee 1993). The aerial parts of this plant have been used traditionally as an anti-inflammation, hemostasis, invigorating the blood circulation, and relieving dysmenorrhea (Ahn 1998; Hu and Feng 1999). Only a few phytochemical investigation on this plant resulted in the isolation of a sesquiterpene endoperoxide, and sesquiterpene (Hu and Feng 1999; Jang and Lee 1993). Concerning the biological studies of A. selengensis, anti-tumor, antioxidant and immune-modulating activities of its various extracts has been reported so far (Koo et al. 1994; Shi et al. 2010).
In an ongoing investigation into anti-inflammatory compounds from natural products, the methanol extract of A. selengensis was found to inhibit IL-6 production in TNF-α stimulated MG-63 cells. By means of repeated column chromatography using silica gel, Sephadex LH-20, and LiChroprep RP-18, ten compounds were isolated from the aerial parts of A. selengensis. The structures of these compounds were identified as 1′,3′-propanediol,2′-amino-1′-(1,3-benzodioxol-5-yl) (1), artanomaloide (2), canin (3), eupatilin (4), quercetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (5), 1,3-di-O-caffeoylquinic acid (6), isoquercitrin (7), pinoresinol-4-O-β-d-glucoside (8), scopolin (9), and isofraxidin-7-O-β-d-glucopyranoside (10), by comparing their spectroscopic data with those reported in the literature. These compounds were isolated from this plant for the first time. Furthermore, compound 1 was isolated for the first time as a new natural product even though it was synthesized previously. For theses isolated compounds, the inhibitory activity of IL-6 production in TNF-α stimulated MG-63 cells was examined. Among these compounds, artanomaloide (2), and canin (3) showed potent inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells.
This paper reports the isolation, structure elucidation, and the inhibitory activity of IL-6 production of isolated compounds from the aerial parts of A. selengensis. In addition, the complete NMR assignments of compound 1 are presented here for the first time.
Materials and methods
General procedure
Optical rotations were measured using an Autopol-IV polarimeter (Rudolph Research Flangers). The UV spectra were obtained on a Shimadzu UV/Visible Spectrophotometer. The IR spectra were recorded on an IMS 85 (Bruker). The HR-TOF -MS spectra were recorded on a Q-TOF (Synapt HDMS system, Waters, USA) mass spectrometer. The NMR spectra were recorded on a Varian Unity Inova 500 and Unity Inova 600 spectrometer (KBSI-Gwangju center). Semi-preparative HPLC was performed using a Waters HPLC system equipped with Waters 600 Q-pumps, a 996 photodiode array detector, and a YMC-Pack ODS-A column (250 × 10 mm i.d., 5 μm), flow rate 4.0 mL/min. TLC and column chromatography were performed on precoated Si Gel F254 plates (Merck, art. 5715), RP-18 F254 plates (Merck, art. 15389) and silica gel 60 (40–63 and 63–200 μm, Merck), MCI gel CHP20P (75–150 μ, Mitsubishi Chemical Co.), Sephadex LH-20 (25–100 μm, Sigma), as well as LiChroprep RP-18 (40–63 μm, Merck).
Plant material
The aerial parts of A. selengensis Turcz. (Compositae) were collected from the Herbarium of College of Pharmacy, Chosun University, Korea, in Aug 2007. A voucher specimen was deposited in the Herbarium of College of Pharmacy, Chosun University (CSU-1041-17).
Extraction and isolation
The air-dried aerial parts of A. selengensis (3.5 kg) were extracted three times with MeOH under reflux and 218.9 g of residues were produced. The MeOH extract was suspended in water, which was then partitioned sequentially with equal volumes of dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and n-butanol (BuOH). Each fraction was evaporated in vaccuo to yield residues of CH2Cl2 (61.8 g), EtOAc (12.1 g), n-BuOH (27.6 g), and water extract (117.4 g). The CH2Cl2 fraction (26.0 g) was chromatographed over a silica gel column chromatography (CC), using a gradient solvent system of Hexane:EtOAc (10:1 → 1:5), to give six subfractions (D1–D6). Subfraction D5 (1.70 g) was subjected to a silica gel CC eluting with a gradient solvent system of Hexane:EtOAc (3:1 → 1:2) to yield nine subfractions (D5-1–D5-9). Subfraction D5-5 (210.81 mg) was eluted with Hexane:Acetone (4:1) to yield thirteen subfractions (D5-5-1–D5-5-13). Subfraction D5-5-9 (41.84 mg) was subjected to RP-18 CC eluting with a gradient solvent system of MeOH: H2O (1:1.5 → 2:1) to give 2 (8.56 mg), 3 (9.12 mg), and 4 (3.53 mg), respectively. Subfraction D3 (3.1 g) was purified by repeated silica gel CC (Hexane:EtOAc, 10:1 → 2:1), followed by MCI gel CC (MeOH:H2O, 50:1), to give 1 (12.37 mg). The n-BuOH fraction (2.5 g) was chromatographed over a HP-20 CC, using a gradient solvent system of MeOH:H2O (0:100 → 100:0) to give six subfractions (B1–B6). Subfraction B4 (0.55 g) was subjected to a silica gel CC eluting with a gradient solvent system of CHCl3:MeOH:H2O (7.5:1:0.1 → 1:1:0.1) to yield twenty-four subfractions (B4-1–B4-24). Subfraction B4-18, -19, and -20 (61.09 mg) was eluted with CHCl3:MeOH:H2O (3:1:0.1) to yield 5 (4.18 mg), and 6 (3.77 mg). Subfraction B4-9-21, and -22 (39.24 mg) was purified by silica gel CC eluting with a gradient solvent system of CHCl3:MeOH:H2O (4:1:0.1 → 2:1:0.1), followed by Lichroprep RP-18 CC (MeOH:H2O, 1:3 → 1:2.5) to give 7 (3.07 mg), and 8 (3.10 mg). Subfraction B3 (2.54 g) was purified by MCI gel CC eluting with a gradient solvent system of MeOH:H2O (1:4 → 1:1), followed by silica gel CC (CHCl3:MeOH:H2O,10:1:0.1 → 1:1:0.1) to give 9 (6.78 mg), and 10 (1.49 mg).
1′,3′-Propanediol,2′-amino-1′-(1,3-benzodioxol-5-yl) (1)—White amorphous powder; \([\alpha ]_{\text{D}}^{25}\) 12.7° (CHCl3; c 0.1); HR-ESI-MS (positive mode) m/z: 212.0962 [M + H]+ (calcd for C10H14NO4, 212.0923); 1H NMR (500 MHz, CDCl3) δ: 3.05 (1H, dd, J = 4.5, 6.5 Hz, H-2′), 3.87(1H, dd, J = 3.5, 9.5 Hz, H-3′a), 4.23 (1H, dd, J = 6.5, 9.5 Hz, H-3′b), 4.71 (1H, d, J = 4.5 Hz, H-1′), 5.95 (2H, s, O-CH2-O), 6.78 (1H, d, J = 8.0 Hz, H-7), 6.80 (1H, dd, J = 1.5, 8.0 Hz, H-6), 6.85 (1H, br s, H-4); 13C NMR (125 MHz, CDCl3) δ: 101.0 (O-CH2-O), 106.5 (C-4), 134.9 (C-5), 119.3 (C-6), 108.1 (C-7), 147.9 (C-8), 147.0 (C-9), 85.7 (C-1′), 54.3 (C-2′), 71.7 (C-3′).
Artanomaloide (2)—Colorless gum; \([\alpha ]_{\text{D}}^{25}\) −17° (CHCl3; c 0.2); HR-EI-MS m/z: 548.2440 [M]+ (calcd for C32H36O8 548.2447); 1H NMR (500 MHz, CDCl3) δ: 1.23 (3H, s, H-14′), 1.51 (1H, d, J = 12.0 Hz, H-13a), 1.54 (3H, s, H-15′), 1.83 (2H, m, H-9′), 1.84 (1H, m, H-8′a), 1.98 (1H, d, J = 9.8 Hz, H-5′), 2.04 (3H, s, -OAc), 2.10 (1H, m, H-8′b), 2.31 (3H, br s, H-14), 2.36 (1H, d, J = 2.5 Hz, H-9a), 2.37 (3H, s, H-15), 2.43 (1H, d, J = 12.0 Hz, H-13b), 2.90 (1H, dd, J = 10.5, 13.0 Hz, H-9b), 3.06 (1H, m, H-7), 3.15 (1H, m, H-7′), 3.78 (1H,br d, H-6), 3.79 (1H, br d, H-5), 4.22 (1H, t, J = 9.5 Hz, H-6′), 5.15 (1H, dd. J = 2.5, 10.5 Hz, H-8), 5.47 (1H, d, J = 3.0 Hz, H-13′a), 5.88 (1H, d, J = 5.5 Hz, H-3′), 6.04 (1H, d, J = 3.0 Hz, H-13′b), 6.17 (1H, s, H-3), 6.28 (1H, d, J = 5.5 Hz, H-2′); 13C NMR (125 MHz, CDCl3) δ: 136.0 (C-1), 197.3 (C-2), 136.6 (C-3), 174.6 (C-4), 51.2 (C-5), 81.0 (C-6), 57.6 (C-7), 68.1 (C-8), 44.1 (C-9), 145.9 (C-10), 61.5 (C-11), 178.5 (C-12), 38.0 (C-13), 20.5 (C-14), 20.2 (C-15), 65.1 (C-1′), 143.3 (C-2′), 134.2 (C-3′), 58.2 (C-4′), 67.9 (C-5′), 81.9 (C-6′), 44.9 (C-7′), 22.1 (C-8′), 35.7 (C-9′), 73.1 (C-10′), 142.8 (C-11′), 172.7 (C-12′), 119.9 (C-13′), 29.6 (C-14′), 15.2 (C-15′), 25.0, 172.7 (OAc).
Canin (3)—Colorless powder; \([\alpha ]_{\text{D}}^{25}\) −14.7° (CHCl3; c 0.4); HR-ESI-MS (positive mode) m/z: 279.1236 [M + H]+ (calcd for C15H19O5 279.1230); 1H NMR (500 MHz, CDCl3) δ: 1.27 (3H, s, H-14), 1.57 (3H, s, H-15), 1.84 (1H, m, H-9a), 2.05 (1H, m, H-9b), 2.10 (1H, m, H-8a), 2.33 (1H, m, H-8b), 2.63 (1H, d, J = 11.5 Hz, H-5), 3.45 (1H, m, H-7), 3.70 (1H, br s, H-2), 4.07 (1H, br s, H-3), 4.34 (1H, dd, J = 9.5, 11.5 Hz, H-6), 5.49 (1H, d, J = 3.0 Hz, H-13a), 6.21 (1H, d, J = 3.0 Hz, H-13b); 13C NMR (125 MHz, CDCl3) δ: 73.3 (C-1), 64.6 (C-2), 64.3 (C-3), 83.3 (C-4), 57.8 (C-5), 79.8 (C-6), 45.0 (C-7), 23.5 (C-8), 35.0 (C-9), 72.2 (C-10), 139.3 (C-11), 169.4 (C-12), 120.0 (C-13), 26.5 (C-14), 22.1 (C-15).
Eupatilin (4)—Yellow amorphous powder; EI-MS m/z: 344 [M]+; 1H NMR (500 MHz, CDCl3) δ: 3.94 (3H, s, OCH3), 3.98 (3H, s, OCH3), 3.99 (3H, s, OCH3), 6.61 (1H, s, H-3), 6.56 (1H, s, H-8), 6.98 (1H, d, J = 9.0 Hz, H-5′), 7.34 (1H, d, J = 2.0 Hz, H-2′), 7.53 (1H, dd, J = 2.0, 8.5 Hz, H-6′); 13C NMR (125 MHz, CDCl3) δ: 163.9 (C-2), 104.5 (C-3), 182.6 (C-4), 153.2 (C-5), 132.6 (C-6), 158.7 (C-7), 90.6 (C-8), 153.1 (C-9), 106.1 (C-10), 123.8 (C-1′), 108.7 (C-2′), 149.3 (C-3′), 152.2 (C-4′), 111.1 (C-5′), 120.1 (C-6′), 60.86 (OCH3), 56.33 (OCH3), 56.11 (OCH3).
Quercetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (5)—Yellow amorphous powder; 1H NMR (500 MHz, CD3OD) δ: 1.12 (3H, d, J = 6.0 Hz, H-6′″), 3.08 (1H, m, H-4″), 3.24 (1H, d, J = 7.5 Hz, H-3″), 3.24 (1H, d, J = 7.5 Hz, H-2″), 3.29 (1H, d, J = 9.0 Hz, H-4″′), 3.31 (2H, m, H-6″), 3.40 (1H, m, H-5″′), 3.60 (1H, m, H-5″), 3.63 (1H, dd, J = 2.9, 9.5 Hz, H-3″″), 3.83 (1H, dd, J = 2.0, 2.9 Hz, H-2″′), 4.52 (d, J = 1.2 Hz, H-1″′), 5.11 (1H, d, J = 7.5 Hz, H-1″), 6.21 (1H, d, J = 2.0 Hz, H-6), 6.40 (1H, d, J = 2.0 Hz, H-8), 6.87 (1H, d, J = 8.5 Hz, H-5′), 7.63 (1H, dd, J = 2.0, 8.5 Hz, H-6′), 7.67 (1H, d, J = 2.0 Hz, H-2′); 13C NMR (125 MHz, CD3OD) δ: 159.4 (C-2), 135.7 (C-3), 179.6 (C-4), 163.1 (C-5), 100.2 (C-6), 166.5 (C-7), 95.1 (C-8), 158.7 (C-9), 104.9 (C-10), 123.7 (C-1′), 117.8 (C-2′), 146.0 (C-3′), 150.0 (C-4′), 116.2 (C-5′), 123.2 (C-6′), 105.7 (C-1″), 75.9 (C-2″), 76.4 (C-3″), 71.5 (C-4″), 78.3 (C-5″), 68.7 (C-6″), 102.6 (C-1″′), 74.1 (C-4″′), 71.4 (C-5″′), 72.3 (C-3″′), 69.9 (C-2″′), 18.0 (C-6″′).
Isoquercitrin (6)—Yellow amorphous powder; \([\alpha ]_{\text{D}}^{25}\) −85° (MeOH; c 0.06); EI-MS m/z: 464 [M]+; 1H NMR (500 MHz, CD3OD) δ: 3.71–3.35 (6H, m, H-2″-6″), 5.25 (1H, d, J = 8.0 Hz, H-1″), 6.20 (1H, d, J = 2.0 Hz, H-6), 6.39 (1H, d, J = 2.0 Hz, H-8), 6.87 (1H, d, J = 8.5 Hz, H-5′), 7.59 (1H, dd, J = 2.0, 8.5 Hz, H-6′), 7.78 (1H, d, J = 2.0 Hz, H-2′); 13C NMR (125 MHz, CD3OD) δ: 159.1 (C-2), 135.7 (C-3), 179.6 (C-4), 163.2 (C-5), 100.1 (C-6), 166.5 (C-7), 94.9 (C-8), 158.6 (C-9), 104.4 (C-10), 123.2 (C-1′), 117.7 (C-2′), 146.1 (C-3′), 150.0 (C-4′), 116.1 (C-5′), 123.3 (C-6′), 105.7 (C-1″), 75.9 (C-2″), 78.5 (C-3″), 71.3 (C-4″), 78.3 (C-5″), 62.7 (C-6″).
1,3-Di-O-caffeoylquinic acid (7) - Yellowish gum; \([\alpha ]_{\text{D}}^{25}\) −24.7° (MeOH; c 0.21); 1H NMR (500 MHz, CD3OD) δ: 1.98 (1H, m, H-6a), 2.25 (1H, dd, J = 3.5, 13.0 Hz, H-2b), 2.69 (2H, m, H-2a,-6b), 3.72 (1H, dd, J = 3.5, 9.5 Hz, H-4), 4.22 (1H, dd, J = 3.5, 6.5 Hz, H-5), 5.45 (1H, m, H-3) (quinic acid moiety); 6.32 (2H, d, J = 16.0 Hz, H-2), 6.77 (2H, dd, J = 2.5, 8.0 Hz, H-8), 6.94 (2H, dd, J = 2.0, 8.5 Hz, H-9), 7.05 (2H, t, J = 2.0 Hz, H-5), 7.57 (2H, dd, J = 4.5, 16.0 Hz, H-3) (caffeoyl groups); 13C NMR (125 MHz, CD3OD) δ: 82.7 (C-1), 35.5 (C-2), 70.5 (C-3), 73.2 (C-4), 69.6 (C-5), 37.1 (C-6), 176.8 (C-7) (quinic acid moiety); 167.8, 166.9 (C-1), 115.5, 114.2 (C-2), 145.5, 145.4 (C-3), 126.8, 126.5 (C-4), 113.7, 113.6 (C-5), 145.3, 144.8 (C-6), 148.2, 147.8 (C-7), 115.1, 115.0 (C-8), 121.6, 121.4 (C-9) (caffeoyl groups).
Pinoresinol-4-O-β-d-glucoside (8)—Yellow amorphous powder; \([\alpha ]_{\text{D}}^{25}\) −84.0° (MeOH; c 0.16); 1H NMR (500 MHz, CD3OD) δ: 3.13 (2H, m, H-8, 8′), 3.39–3.86 (sugar H), 3.84 (3H, s, OCH3), 3.85 (3H, s, OCH3), 3.86 (2H, m, H-9a, 9a′),4.24 (2H, m, H-9b, 9b′), 4.71 (1H, d, J = 4.0 Hz, H-7′), 4.76 (1H, d, J = 4.5 Hz, H-7), 4.87 (1H, H-1″), 6.77 (1H, d, J = 8.5 Hz, H-5′), 6.81 (1H, dd, J = 1.5, 8.5 Hz, H-6′), 6.92 (1H, dd, J = 1.5, 8.5 Hz, H-6), 6.94 (1H, d, J = 1.5 Hz, H-2′), 7.03 (1H, d, J = 2.0 Hz, H-2), 7.15 (1H, d, J = 8.0 Hz, H-5); 13C NMR (125 MHz, CD3OD) δ: 137.6 (C-1), 133.9 (C-1′), 111.7 (C-2), 111.1 (C-2′), 147.6 (C-3), 147.4 (C-3′), 151.1 (C-4), 149.3 (C-4′), 118.1 (C-5), 116.2 (C-5′), 119.9 (C-6), 120.2 (C-6′), 87.2 (C-7), 87.6 (C-7′), 55.5 (C-8), 55.7 (C-8′), 72.8 (C-9), 72.8 (C-9′), 56.9 (C-OCH3), 56.5 (C-OCH3), 102.9 (C-1″), 75.0 (C-2″), 78.0 (C-3″), 71.5 (C-4″), 78.3 (C-5″), 62.6 (C-6″).
Scopolin (9)—White powder; 1H NMR (500 MHz, DMSO-d 6): δ 3.15 (1H, m), 3.28 (2H, m), 3.45 (2H, m), 3.69 (1H, m), 3.82 (3H, s, 6-OCH3), 5.08 (1H, d, J = 7.5 Hz, H-1′), 6.33 (1H, d, J = 9.0 Hz, H-3), 7.15 (1H, s, H-8), 7.30 (1H, s, H-5), 7.96 (1H, d, J = 9.5 Hz, H-4); 13C NMR (125 MHz, DMSO-d 6) δ: 160.5 (C-2), 113.3 (C-3), 144.2 (C-4), 109.6 (C-5), 146.0 (C-6), 149.9 (C-7), 103.0 (C-8), 148.9 (C-9), 112.2 (C-10), 56.0 (6-OCH3), 99.6 (C-1′), 73.0 (C-2′), 76.7 (C-3′), 69.6 (C-4′), 77.1 (C-5′), 60.6 (C-6′).
Isofraxidin-7-O-β-d-glucopyranoside (10)—Colorless amorphous powder; \([\alpha ]_{\text{D}}^{25}\) +44.1° (MeOH; c 0.25); 1H NMR (500 MHz, DMSO-d 6) δ: 3.09 (2H, m), 3.24 (2H, m), 3.38 (1H, m), 3.59 (1H, m), 3.82 (3H, s, 6-OCH3), 3.91 (3H, s, 8-OCH3), 5.15 (1H, d, J = 5.0 Hz, H-1′), 6.40 (1H, d, J = 9.5 Hz, H-3), 7.13 (1H, s, H-5), 7.96 (1H, d, J = 9.5 Hz, H-4); 13C NMR (125 MHz, DMSO-d 6) δ: 159.8 (C-2), 114.7 (C-3), 144.4 (C-4), 105.4 (C-5), 149.4 (C-6), 141.6 (C-7), 140.2 (C-8), 142.4 (C-9), 114.5 (C-10), 56.5 (6-OCH3), 61.3 (8-OCH3), 102.2 (C-1′), 74.1 (C-2′), 77.5 (C-3′), 70.0 (C-4′), 76.5 (C-5′), 60.7 (C-6′).
Bioassay of IL-6
IL-6 bioassay was carried out using a slight modification of an established method (Kim et al. 2003; Liu et al. 2006). Briefly, 500 μL of the MG-63 cells (3 × 104 cells/mL) in DMEM containing 10 % FBS were dispensed into a 24-well plate; the culture was incubated for 24 h at 37 °C. Then, 5 μL of TNF-α (10 ng/mL), 5 μL of BAY 11-7085 (10 ng/mL), and 5 μL of the DMSO with or without the compounds (100 μg/mL) were added. After incubation at 37 °C with 5 % CO2 for 24 h, the medium was stored at −20 °C until measurement. The IL-6 content of the medium was measured in an ELISA procedure. 96-well plates were coated with 100 μL of purified rat anti-human IL-6 monoclonal antibody in 0.1 M NaHCO3 (pH 9.6) by overnight incubation at 4 °C. The wells were blocked with 200 μL of 3 % BSA in PBS for 2 h at room temperature (RT) and then incubated with 100 μL of specific antibody for 2 h at RT. 100 μL of HRP conjugated rabbit anti-goat IgG (1:1,000 dilution) was added to each well and incubated for 2 h at RT. 100 μL of TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution was added and incubated for 10 min at RT. The color reaction was stopped with 50 μL of 0.4 N HCl and the optical density was read at 450 nm using a Microplate Reader (Molecular Devices Co., Ltd., U.S.A.).
Results and discussion
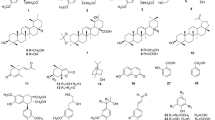
The MeOH extract of the aerial parts of A. selengensis was partitioned into CH2Cl2, EtOAc, n-BuOH-soluble fractions. Separation of the CH2Cl2 and n-BuOH soluble fraction with silica gel, MCI gel filtration, and repeated RP-18 CC led to the isolations of compounds 1–10 (Fig. 1).
Compound 1 was obtained as white amorphous powder, \([\alpha ]_{\text{D}}^{25}\) 12.7° (MeOH). Its molecular formula was determined to be C10H14NO4 by HR-ESI-MS data at m/z 212.0962 [M+ H]+ (calcd for C10H14NO4 212.0923). In the IR spectrum, absorption bands for hydroxyl (3,400 cm−1) and aromatic ring (1600, 1518 cm−1) groups were observed. The 1H NMR spectrum of 1 showed ABX-trisubstituted aromatic protons at δH 6.78 (1H, d, J = 8.0 Hz, H-7), 6.80 (1H, dd, J = 1.0, 8.0 Hz, H-6), 6.85 (1H, br s, H-4), one hydroxyl propyl proton at δH 3.87(1H, dd, J = 3.5, 9.5 Hz, H-3′a), 4.23 (1H, dd, J = 6.5, 9.5 Hz, H-3′b), one oxymethine proton at δH 4.71 (1H, d, J = 4.5 Hz, H-1′), one aminomethine proton at δH 3.05 (1H, dd, J = 4.5, 6.5 Hz, H-2′) and one methylene dioxy proton at δH 5.95 (2H, s, H-2). In the 13C NMR spectrum, 10 carbon signals appeared, which included two oxygenated quaternary carbons at δC 147.9 (C-8), and 147.0 (C-9), three aromatic carbons at δC 106.5 (C-4), 119.3 (C-6), and 108.1 (C-7), one quaternary carbon at δC 134.9 (C-5), one oxygenated carbon at δC 85.7 (C-1′), one aminomethine carbon at δC 54.3 (C-2′), one methylene dioxycarbon at δC 101.0 (C-2) and one hydroxyl propyl carbon δC at 71.7 (C-3′). From these results, compound 1 was indicated to be a phenylpropanoid derivative with one amino and two hydroxyl groups. In the HMBC spectrum, correlations between H-2 and C-9/C-8, H-1′ and C-5/C-3′ were observed. Furthermore, in the 1H-1H COSY spectrum, the hydroxyl proton at δH 3.87 (H-3′a) and 4.23 (H-3′b) showed couplings with H-2′, and H-1′ (Fig. 2). Accordingly, compound 1 was determined as 1′,3′-propanediol,2′-amino-1′-(1,3-benzodioxol-5-yl), as shown in Fig. 1. Compound 1 was isolated for the first time as a new natural product even though it was synthesized previously (Cellitti et al. 2008). Furthermore, its spectral data are presented here for the first time.
Artanomaloide (2) was obtained as colorless gum, \([\alpha ]_{\text{D}}^{25}\) −17°. The 1H, 13C NMR, and HSQC spectroscopic data of 2 showed the presence of 32 carbons, which were assignable to four tertiary methyl groups [δH 1.23 (3H, s, H-14′), 1.54 (3H, s, H-15′), 2.31 (3H, br s, H-14), 2.37 (3H, s, H-15); δC 29.6 (C-14′), 15.2 (C-15′), 20.5 (C-14), 20.2 (C-15), respectively], three carbonyl groups [δC 197.3 (C-2), 178.5 (C-12), 172.7 (C-12′)], three acetal methine groups [δH 3.78 (1H, br s, H-6); δC 81.0 (C-6), δH 4.22 (1H, t, J = 9.5 Hz, H-6′); δC 81.9 (C-6′), δH 5.15 (1H, dd, J = 2.5, 10.5 Hz, H-8); δC 68.1 (C-8)], an exo methylene group [δH 5.47 (1H, d, J = 3.0 Hz, H-13′a) and δH 6.04 (1H, d, J = 3.0 Hz, H-13′b); δC 119.9 (C-13′)], one oxygenated quaternary carbon δC 73.1(C-10′), one acetyl group [δH 2.04 (3H, s); δC 172.7], two olefinic groups [δH 6.17 (1H, s, H-3); δC 136.6 (C-3), δH 5.88 (1H, d, J = 5.5 Hz, H-3′); δC 134.2 (C-3′), δH 6.28 (1H, d, J = 5.5 Hz, H-2′); δC 143.3 (C-2′), respectively], four methylene groups δC 44.1(C-9), 38.0 (C-13), 35.7 (C-9′), and 22.1 (C-8′), four methine groups [δH 1.98 (d, J = 9.8 Hz, H-5′); δC 67.9 (C-5′), δH 3.06 (1H, m, H-7); δC 57.6 (C-7), δH 3.15 (1H, m, H-7′); δC 44.9 (C-7′), δH 3.79 (1H, br s, H-5); δC 51.2 (C-5), respectively], and two quaternary carbons δC 65.1(C-1′) and 61.5 (C-11). From these results, compound 2 was indicated to be a dimeric sesquiterpene lactone (Jakupovic et al. 1987). Furthermore, in the HMBC spectrum, long range correlations between H-3/H-6 and C-1, H-7 and C-11, H-3 and C-2 were observed. In addition, correlations between H-5′ and C-11, H-7′ and C-12′, H-6′ and C-11′ were supported the proposed structure of 2. Accordingly, compound 2 was determined as artanomaloide on the basis of the above evidences, together with a comparison with the literature (Jakupovic et al. 1987).
Compound 3 was obtained colorless powder, \([\alpha ]_{\text{D}}^{25}\) −14.7°. The 1H-, 13C NMR, and HSQC spectroscopic data of 3 showed the presence of 15 carbons, which were assignable to two tertiary methyl groups [δH 1.27 (3H, s, H-14); δC 26.5 (C-14) and δH 1.57(3H, s, H-15); δC 22.1 (C-15), respectively], an exo methylene group [δH 6.21 (1H, d, J = 3.0 Hz, H-13b) and δH 5.49 (1H, d, J = 3.0 Hz, H-13a); δC 120.0], three acetal methine groups [δH 4.34 (1H, dd, J = 9.5, 11.5 Hz, H-6); δC 79.8 (C-6), δH 4.07 (1H, br s, H-3); δC 64.3 (C-3) and δH 3.70 (1H, br s, H-2); δC 64.6 (C-2), respectively], three oxygenated quaternary carbons δC 72.2 (C-10), 73.3 (C-1), and 83.3 (C-4), one carbonyl carbon δC 169.4 (C-12), two methine groups [δH 2.63 (1H, d, J = 11.5 Hz, H-5); δC 57.8 (C-5) and δH 3.45 (1H, m, H-7); δC 45.0 (C-7), respectively], two methylene groups [δH 1.84 (1H, m, H-9a), δH 2.05 (1H, m, H-9b); δC 35.0 (C-9) and δH 2.10 (1H, m, H-8a), δH 2.33 (1H, m, H-8b); δC 23.5 (C-8), respectively] and one quaternary carbon δC 139.3 (C-11). These observations suggested that compound 3 was a 1,2;3,4-diepoxyguaianolide sesquiterpene lactone with two tertiary methyls and one hydroxyl group. Furthermore, HMBC and NOESY spectral data were good agreement with the reported data (Li et al. 2010). Accordingly, compound 3 was determined as canin on the basis of the above evidences, together with a comparison with the literature (Li et al. 2010).
The known compounds 4–10 were also identified as eupatilin (4) (Li et al. 2010), quercetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (5) (Kim et al. 2013), isoquercitrin (6) (Duan et al. 2009), 1,3-di-O-caffeoylquinic acid (7) (An et al. 2008; Jiang et al. 2010; Lee et al. 2013), pinoresinol-4-O-β-d-glucoside (8) (Wang et al. 2012; Kim et al. 2005), scopolin (9) (Chung et al. 1999; Lee et al. 2005), and isofraxidin-7-O-β-d-glucopyranoside (10) (Heo et al. 2005; Hu et al. 2011), respectively, by comparing the NMR spectral data with those reported in the literature. All compounds have not been previously isolated from this plant.
IL-6 is a cytokine, originally identified as a T cell derived factor that regulates B-cell growth and differentiation (Hirano et al. 1986). Human IL-6 is an important component of the inflammatory cascade. Dysregulation of IL-6 production has been implicated in a variety of inflammatory/autoimmune disease states, including rheumatoid arthritis, cardiac myxoma, Castleman’s disease, and mesangial proliferative glomerulonephritis (Hirano et al. 1990). The proinflammatory cytokines IL-1 and TNF-α markedly stimulate the production IL-6 (Van Damme et al. 1987).
The inhibitory activity of the isolated compounds (1–10) against IL-6 production in TNF-α stimulated MG-63 cells was examined. None of these isolates exhibited cellular cytoxicity in MG 63 cells at the tested concentration (data not shown). Among these compounds, compounds 2, 3 and 7 showed potent inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells, while compounds 5, 6, and 9 showed moderate inhibitory activity (Fig. 3; Table 1).
Inhibitory effect of compounds 1–10 against IL-6 production in TNF-α stimulated MG-63cells. MG-63 cells (3 × 104) were incubated for 24 h. Cultures were incubated with or without compounds (100 μg/mL) for 30 min and then stimulated with TNF-α (10 ng/mL) for 24 h. IL-6 in the supernatant was measured by ELISA as described in “Materials and methods” section. Results are expressed as the mean ± S.E. from three different experiments. BAY 11-7085 was used as a positive control. *P < 0.05 or **P < 0.01 compared with TNF-α treated value
In conclusion, this paper reports the isolation, characterization, and inhibitory activity of 10 isolates, including one new compound and nine known compounds, from the aerial parts of A. selengensis.
References
Ahn, D.K. 1998. Illustrated book of Korean medicinal herbs, 550. Seoul: Kyo-Hak Publishing.
An, R.-B., D.-H. Sohn, G.-S. Jeong, and Y.-C. Kim. 2008. In vitro hepatoprotective compounds from Suaeda glauca. Archives of Pharmacal Research 31: 594–597.
Cellitti, S.E., D.H. Jones, L. Lagpacan, X. Hao, Q. Zhang, H. Hu, S.M. Brittain, A. Brinker, J. Caldwell, B. Bursulaya, G. Spraggon, A. Brock, Y. Ryu, T. Uno, P.G. Schultz, and B.H. Geierstanger. 2008. In vivo incorporation of unnatural amino acids to probe structure, dynamics, and ligand binding in a large protein by nuclear magnetic resonance spectroscopy. Journal of American Chemical Society 130: 9268–9281.
Chung, S.-C., B.Y. Hwang, G.-J. Oh, S.-J. Kang, M.-J. Kim, W.-H. Choi, K.S. Lee, and J.S. Ro. 1999. Chemical components from the stem bark of Rhus javanica L. Korean Journal of Pharmacognosy 30: 295–300.
Duan, C.H., J.-N. Lee, C.-M. Lee, G.-T. Lee, and K.-K. Lee. 2009. Phytochemical constituents from Metasequoia glyptostroboids leaves. Natural Product Sciences 15: 12–16.
Hirano, T., K. Yasukawa, H. Harada, T. Taga, Y. Watanabe, T. Matsuda, S. Kashiwamura, K. Nakajima, K. Koyama, A. Iwamatsu, S. Tsunsawa, F. Sakiyama, H. Matsui, Y. Takahara, T. Taniguchi, and T. Kishimoto. 1986. Complementary DNA for a novel human interleukin (BSF-2) that induced lymphocytes to produce immunoglobulin. Nature 324: 73–76.
Heo, J.E., J.L. Jin, Y.Y. Lee, and H.S. Yun-Choi. 2005. Chemical constituents of the aerial parts of Chloranthus japonicas Sieb. Natural Product Sciences 11: 41–44.
Hirano, T., S. Akira, T. Taga, and T. Kishimoto. 1990. Biological and clinical aspects of interleukin 6. Immunology Today 11: 443–449.
Hu, J.-F., and X.-Z. Feng. 1999. New guaianolides from Artemisia selengensis. Journal of Asian Natural Product Report 1: 169–176.
Hu, H.-B., X.-D. Zheng, Y.-F. Jian, J.-X. Liu, and J.-H. Zhu. 2011. Constituents of the root of Anemone tomentosa. Archives of Pharmacal Research 34: 1097–1105.
Jang, W.Y., and K.R. Lee. 1993. A new endoperoxide from Artemisia selengensis. Korean Journal of Pharmacognosy 24: 107–110.
Jakupovic, J., Z.-L. Chen, and F. Bohlmann. 1987. Artanomaloide, a dimeric guaianolide and phenylalanine derivatives from Artemisia anola. Phytochemistry 26: 2777–2779.
Jiang, J., B.B. Zhang, and Z.X. Liao. 2010. A new dicaffeoyl quinic acid from Nannoglottis ravida. Chinese Chemical Letters 21: 203–205.
Kim, B.H., E.Y. Chung, J.-C. Ryu, S.-H. Jung, K.R. Min, and Y. Kim. 2003. Anti-inflammatory mode of isoflavone glycosides sophoricoside by inhibition of interleukin-6 and cyclooxygenase-2 in inflammatory response. Archives of Pharmacal Research 26: 306–311.
Kim, D.K., J.P. Lim, J.W. Kim, H.W. Park, and J.S. Eun. 2005. Antitumor and anti-inflammatory constituents from Celtis sinensis. Archives of Pharmacal Research 28: 39–43.
Kim, H.J., B.-G. Kim, and J.-H. Ahn. 2013. Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Applied Microbiology Biotechnology 97: 5275–5282.
Koo, K.A., J.H. Kwak, K.R. Lee, O.P. Zee, E.-R. Woo, H.K. Park, and H.J. Youn. 1994. Antitumor and immunomodulating activities of the polysaccharide fraction from Artemisia selengensis and Artemisia iwayomogi. Archives of Pharmacal Research 17: 371–374.
Lee, T.B. 1993. Illustrated flora of Korea, 759. Seoul: Hyang-Moon Publishing.
Lee, J.H., W.J. Jeon, E.S. Yoo, C.M. Kim, and Y.S. Kwon. 2005. The chemical constituents and their antioxidant activity of the stem of Rhododendron mucronulatum. Natural Product Sciences 11: 97–102.
Lee, Y.G., J.-Y. Cho, C.-M. Kim, S.-H. Lee, W.-S. Kim, T.-I. Jeon, K.-H. Park, and J.-H. Moon. 2013. Coumaroyl quinic acid derivatives and flavonoids from Immature Pear (Pyrus pyrifolia Nakai) fruit. Food Science Biotechnology 22: 803–810.
Li, D., X.H. Han, S.S. Hong, C. Lee, M.S. Lee, D. Lee, M.K. Lee, and B.Y. Hwang. 2010. Inhibitors of nitric oxide production from Artemisia princeps. Natural Product Sciences 16: 143–147.
Liu, Q.H., J.-E. Jeong, E.J. Choi, Y.H. Moon, and E.-R. Woo. 2006. A new furofuran lignan from Geranium thunbergii. Archives of Pharmacal Research 29: 1109–1113.
Shi, F., X. Jia, C. Zhao, and Y. Chen. 2010. Antioxidant activities of various extracts from Artemisia selengensis Turcz (LuHao). Molecules 15: 4934–4946.
Van Damme, J., G. Opdenakker, R.J. Simpson, M.R. Rubira, S. Cayphas, A. Vink, A. Billiau, and J. Van Snick. 1987. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced in interleukin 1 and tumor necrosis factor. Journal of Experimental Medicine 165: 914–919.
Wang, D.-M., W.-J. Pu, Y.-H. Wang, Y.-J. Zhang, and S.-S. Wang. 2012. A New isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules 17: 4595–4603.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A3005531).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, A.R., Ko, H.J., Chowdhury, M.A. et al. Chemical constituents on the aerial parts of Artemisia selengensis and their IL-6 inhibitory activity. Arch. Pharm. Res. 38, 1059–1065 (2015). https://doi.org/10.1007/s12272-014-0543-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0543-x