Abstract
One new lignan, trichobenzolignan (1), and seven known compounds, ligballinol (2), (−)-pinoresinol (3), ehletianol C (4), luteolin 7-O-β-D-glucopyranoside (5), chrysoeriol-7- O-β-D-glucopyranoside (6), 10α-cucurbita-5,24-dien-3β-ol (7), and arvenin I (8). Their structures were established on the basis of spectral and chemical evidence, which were in agreement with those reported in literature. The cytotoxic activities of these compounds were evaluated on four cancer cell lines such as A-549 (human lung cancer), HT-29 (human colon adenocarcinoma), OVCAR (human ovarian carcinoma), and MCF-7 (human breast cancer). As the results, compound 7 showed significant activity on HT-29 and OVCAR cancer cell lines with IC50 of 4.1 and 6.5 µM, respectively. Compounds 1, 5, 6, and 8 exhibited moderate activities in all cancer cell lines with IC50 ranging from 11.3 to 42.8 µM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichosanthes kirilowii MAXIM belongs to the cucumber family (Cucurbitaceae) and is one of the fundamental herbals in Vietnam. Its roots have been used in Vietnam traditional medicine as an anti-inflammatory agent, a cough and sore throat medicines (Chi 2012). Previous phytochemical investigations of T. kirilowii have yielded lignans (Moon et al. 2008), flavones (Rahman and Moon 2007), and triterpenes (Akihisa et al. 1992; 1994; Kimura et al. 1995). Compounds showed cytotoxic (Kondo et al. 1995; Moon et al. 2008; Takahashi et al. 2009), antitumor (Ryu et al. 1994), anti-inflammatory (Akihisa et al. 1994), and antibacterial activities (Jang et al. 2007). As part of our ongoing investigation on cytotoxic compounds from the roots of T. kirilowii, the phytochemical investigation led to the isolation of one new lignan and seven known compounds. We report herein the isolation, structural elucidation, and evaluation of the in vitro cytotoxic activities of compounds against four human cancer cell lines, including A-549, HT-29, OVCAR, and MCF-7.
Materials and methods
General experimental procedures
All NMR spectra were recorded on a Agilent 400-MR-NMR Spectrometer operated at 400 and 100 MHz for hydrogen and carbon, respectively. NMR measurements, including 1H, 13C, HSQC, and HMBC experiments, were carried out using 5-mm probe tubes at temperature of 22.2 °C in CD3OD solutions, with TMS as the internal standard. Chemical shifts are reported in parts per million from TMS. The HR-EI-MS was obtained on a JEOL JMS-AX505WA. Circular dichroism spectrums were determined on a Chirascan™ CD spectrometer. Optical rotations were determined on a Jasco DIP-370 automatic polarimeter. Column chromatography was performed using a silica-gel (Kieselgel 60, 70–230 mesh and 230–400 mesh, Merck) or YMC RP-18 resins (30–50 μm, Fujisilisa Chemical Ltd.), thin layer chromatography (TLC) using a pre-coated silica-gel 60 F254 (0.25 mm, Merck) and RP-18 F254S plates (0.25 mm, Merck).
Plant material
The roots of T. kirilowii were collected in Hoabinh, Vietnam in September, 2012, and identified by Dr. Ninh Khac Ban, Institute of Marine Biochemistry, VAST, Vietnam. A voucher specimen (TK1209) was deposited at the Herbarium of Institute of Marine Biochemistry, VAST, Vietnam.
Extraction and isolation
The roots of T. kirilowii (2.5 kg) were extracted with MeOH three times under reflux for 15 h to yield 120 g of a dark solid MeOH extract, which was then suspended in water and successively partitioned with CHCl3 and ethyl acetate (EtOAc) to obtain CHCl3 (TK1, 80.0 g), EtOAc (TK2, 8.0 g), and water (TK3, 32.0 g) layers after removing solvent in vacuo. The TK1 layer was chromatographed on a silica gel column and eluted with n-hexane–acetone gradient (40:1 → 1:1, v/v) to obtain five fractions, TK1A (15.0 g), TK1B (30.0 g), TK1C (8.0 g), TK1D (8.5 g), and TK1E (10.0 g). The TK1C fraction was chromatographed on a silica gel column eluting with CHCl3–acetone (8:1, v/v) to give three fractions TK1C1-TK1C3. The TK1C2 fraction was chromatographed on an YMC RP-18 column eluting with acetone–water (3:1, v/v) to yield 7 (8.0 mg). The TK1C3 fraction was chromatographed on an YMC RP-18 column eluting with MeOH–water (3:1, v/v) to yield 2 (6.0 mg) and 3 (9.0 mg). The TK1E fraction was chromatographed on a silica gel column eluting with CHCl3–MeOH (10:1, v/v) to give four factions, TK1E1-TK1E4. The TK1E1 fraction was chromatographed on an YMC RP-18 column eluting with acetone–water (1:1, v/v) to yield 1 (7.0 mg). The TK1E2 fraction was chromatographed on a silica gel column eluting with CHCl3–acetone–water–formic acid (1:3:0.2:001, v/v/v/v) to yiled 4 (20.0 mg). The TK1E3 fraction was chromatographed on an YMC RP-18 column eluting with MeOH–water (2:1, v/v) to yield 8 (30.0 mg). The water soluble fraction TK3 was chromatographed on a Diaion HP-20P column eluting with water containing increasing concentrations of MeOH (0, 25, 50, 75, and 100 % MeOH) to obtain four fractions, TK3A–TK3D. The TK3C fraction was chromatographed on Sephadex LH-20 column eluting with MeOH–water (1:1, v/v) to yield 5 (10.0 mg) and 6 (15.0 mg).
Trichobenzolignan (1)
A white amorphous powder; ([α] 25 D = −38.0, c = 0.15, MeOH); IR (KBr) νmax: 3,360, 1,601, 1,480, 1,260 cm−1; CD (c 0.001 mg/mL, MeOH, Δε (nm): −4.3 (244), +2.6 (222); 1H-NMR (CD3OD) and 13C-NMR (CD3OD): see Table 1.
Ligballinol (2)
A white amorphous powder; ([α] 25 D = −24.0, c = 0.1, MeOH); 1H-NMR (CD3OD) δH 7.17 (d, J = 8.4 Hz, H-2/6/2′/6′), 6.74 (d, J = 8.4 Hz, H-3/5/3′/5′), 6.74 (d, J = 8.4 Hz, H-5),7.17 (d, J = 8.4 Hz, H-6/6′) 4.67 (d, J = 4.4 Hz, H-7/7′), 3.09 (m, H-8/8′), 3.77 (d, J = 3.6, 9.2 Hz, Ha-9/9′),4.17 (m, Hb-9/9′); 13C-NMR (CD3OD) δC 133.0 (C-1/1′), 128.7 (C-2/2′, 6/6′), 116.2 (C-3/3′, 5/5′), 158.2 (C-4/4′), 116.2 (C-5/5′), 128.7 (C-6/6′), 87.4 (C-7/7′), 55.3 (C-8/8′), 72.5 (C-9/9′).
(−)-Pinoresinol (3)
A white amorphous powder; ([α] 25 D = −45.0, c = 0.2, MeOH); 1H-NMR (CD3OD) δH 6.91 (d, J = 2.0 Hz, H-2, 2′), 6.74 (d, J = 8.0 Hz, H-5, 5′), 6.75(dd, J = 2.0, 8.0 Hz, H-6, 6′), 4.66 (d, J = 4.0 Hz, H-7, 7′), 3.08 (m, H-8, 8′), 3.79 (dd, J = 3.2, 9.2 Hz, Ha-9, 9′), 4.18 (dd, J = 6.8, 9.2 Hz, Hb-9, 9′), 3.81 (s, 3, 3′-OMe); 13C-NMR (CD3OD) δC 133.8 (C-1, 1′), 110.9 (C-2, 2′), 149.1 (C-3, 3′), 147.3 (C-4, 4′), 116.1 (C-5, 5′), 120.0 (C-6, 6′), 87.4 (C-7, 7′), 55.3 (C-8, 8′), 72.6 (C-9, 9′), 56.4 (3, 3′-OMe).
Ehletianol C (4)
A white amorphous powder; ([α] 25 D = −13.0, c = 0.3, MeOH); 1H-NMR (CD3OD) δH 7.01 (d, 1.8 Hz, H-2), 6.73, H-5), 6.84 (dd, 1.8, 8.4 Hz, H-6), 4.84, H-7), 4.22z (m, H-8), 3.46 (dd, 4.8, 12.6 Hz, Ha-9), 3.70 (dd, 5.4, 12.0 Hz, Hb-9), 6.86 (d, 1.8) Hz, H-2′), 6.97 (d, 8.4 Hz, H-5′), 6.71 (dd, 1.8, 8.4 Hz, H-6′), 2.49 (dd, 12.0, 13.8 Hz, Ha-7′), 2.92 (dd, 4.8, 13.8 Hz, Hb-7′), 2.70 (m, H-8′), 3.96 (dd, 6.0, 7.8 Hz, Ha-9′), 3.70 (dd, 4.8, 7.8 Hz, Hb-9′), 6.90 (d, 1.8 Hz, H-2″), 6.76, H-5″), 6.75, H-6″), 4.74 (d, 7.2 Hz, H-7″), 2.35 (m, H-8″), 3.63 (dd, 6.6, 11.4 Hz, Ha-9″), 3.82, Hb-9″), 3.80 (s, 3-OMe), 3.83 (s, 3′-OMe), 3.84 (s, 3″-OMe); 13C-NMR (CD3OD) δC 133.8 (C-1), 111.7 (C-2), 148.8 (C-3), 147.2 (C-4), 115.8 (C-5), 120.8 (C-6), 74.1 (C-7), 87.5 (C-8), 61.9 (C-9), 136.7 (C-1′), 114.2 (C-2′), 151.8 (C-3′), 147.9 (C-4′), 119.5 (C-5′), 122.4 (C-6′), 33.7 (C-7′), 43.7 (C-8′), 73.5 (C-9′), 135.7 (C-1″), 110.7 (C-2″), 149.0 (C-3″), 147.1 (C-4″), 116.0 (C-5″), 119.8 (C-6″), 84.0 (C-7″), 54.0 (C-8″), 60.5 (C-9″), 56.3 (3-OMe), 56.4 (3′-OMe), 56.6 (3″-OMe).
Luteolin 7-O-β-D-glucopyranoside (5)
A yellow amorphous powder; ([α] 25 D = −47.0, c = 0.15, MeOH); 1H-NMR (DMSO-d6) δH 6.75 (s, H-3), 6.44 (d, J = 2.4 Hz, H-6), 6.79 (d, J = 2.4 Hz, H-8), 7.42 (d, J = 2.4 Hz, H-2′), 6.94 (d, J = 8.4 Hz, H-5′), 7.60 (d, J = 8.4 Hz, H-6′), 5.08 (d, J = 7.2 Hz, H-1″), 3.26 (dd, J = 7.2, 8.4 Hz, H-′2′), 3.30 (t, J = 8.4 Hz, H-3″), 3.18 (t, J = 8.4 Hz, H-4″), 3.45 (m, H-5″), 3.48 (dd, J = 5.4, 10.2 Hz, Ha-6″), 3.71(d, J = 10.2 Hz, Hb-6″); 13C-NMR (DMSO-d6) δC 164.5 (C-2), 103.2 (C-3), 181.9 (C-4), 161.1 (C-5),99.6 (C-6), 163.0 (C-7), 94.7 (C-8), 156.9 (C-9), 105.4 (C-10), 121.4 (C-1′), 113.6 (C-2′), 145.8 (C-3′), 149.9 (C-4′), 116.0 (C-5′), 119.2 (C-6′), 99.9 (C-1″), 73.1 (C-2″), 76.4 (C-3″), 69.6 (C-4″), 77.2 (C-5″), 60.6 (C-6″).
Chrysoeriol 7-O-β-D-glucopyranoside (6)
A yellow amorphous powder; ([α] 25 D = −40.0, c = 0.1, MeOH); 1H-NMR (DMSO-d6) δH 6.99 (s, H-3), 6.45 (d, J = 2.4 Hz, H-6), 6.86 (d, J = 2.4 Hz, H-8), 7.59 (s, H-2′), 7.20 (d, J = 8.0 Hz, H-5′), 7.38 (d, J = 8.0 Hz, H-6′), 3.89 (s, 3-OMe), 5.06 (d, J = 7.2 Hz, H-1″), 3.27 (dd, J = 7.2, 8.4 Hz, H-2″), 3.30 (t, J = 8.4 Hz, H-3″), 3.17 (t, J = 8.4 Hz, H-4″), 3.45 (m Hz, H-5″), 3.47 (dd, J = 5.4, 10.2 Hz, Ha-6″), 3.72 (d, J = 10.2 Hz, Hb-6″); 13C-NMR (DMSO-d6) δC 164.2 (C-2), 103.5 (C-3), 182.1 (C-4), 161.1 (C-5), 99.5 (C-6), 163.0 (C-7), 95.0 (C-8), 156.9 (C-9), 105.4 (C-10), 121.4 (C-1′), 110.3 (C-2′), 148.1 (C-3′), 150.9 (C-4′), 115.8 (C-5′), 120.5 (C-6′), 56.0 (3-OMe), 100.0 (C-1″), 73.1 (C-2″), 76.5 (C-3″), 69.6 (C-4″), 77.3 (C-5″), 60.6 (C-6″).
10α-Cucurbita-5,24-dien-3β-ol (7)
A yellow amorphous powder ([α] 25 D = +35.0, c = 0.1, MeOH); 1H-NMR (CDCl3) δH 3.40 (br s, H-3), 5.51 (d, 6.5 Hz, H-6), 1.73 (dd, 6.5, 21.0 Hz, Ha-7), 2.30 (dd, 7.5, 21.0 Hz, Hb-7), 1.53 (s, H-26), 1.61 (s, H-27), 0.73 (s, H-28), 1.07 (s, H-29), 0.95 (s, H-30); 13C-NMR (CDCl3) δC 21.2 (C- 1), 29.0 (C-2), 76.7 (C-3), 41.5 (C-4), 141.3 (C-5), 121.6 (C-6), 24.5 (C-7), 43.7 (C-8), 34.5 (C-9), 37.9 (C-10), 32.4 (C-11), 30.5 (C-12), 46.3 (C-13), 49.2 (C-14), 34.8 (C-15), 28.0 (C-16), 50.5 (C-17), 15.5 (C-18), 28.2 (C-19), 35.9 (C-20), 18.7 (C-21), 36.5 (C-22), 24.9 (C-23), 125.3 (C-24), 131.0 (C-25), 17.7 (C-26), 25.8 (C-27), 17.9 (C-28), 27.3 (C-29), 25.6 (C-30).
Arvenin I (8)
A yellow amorphous powder; ([α] 25 D = −65.0, c = 0.2, MeOH); 1H-NMR (CD3OD) δH 4.84 (H-2), 5.77 (d, J = 1.2 Hz, H-6), 2.54 d, J = 15.6 Hz, H-12), 4.51 (t, J = 8.0 Hz, H-16), 2.51 (d, J = 6.8 Hz, H-17), 0.83 (s, H-18), 0.99 (s, H-19), 1.36 (s, H-21), 6.78 (d, J = 15.6 Hz, H-23), 6.92 (d, J = 15.6 Hz, H-24), 1.52 (s, H-26), 1.50 (s, H-27), 1.33 (s, H-28), 1.28 (s, H-29), 1.23 (s, H-30), 4.28 (d, J = 8.0 Hz, H-1′), 1.96 (CH 3CO); 13C-NMR (CD3OD) δC 35.9 (C-1), 79.6 (C-2), 213.5 (C-3), 52.4 (C-4), 141.6 (C-5), 121.4 (C-6), 24.8 (C-7), 44.1 (C-8), 49.9 (C-9), 35.0 (C-10), 215.8 (C-11), 49.7 (C-12), 51.7 (C-13), 49.1 (C-14), 46.5 (C-15), 71.8 (C-16), 60.2 (C-17), 20.7 (C-18), 20.1 (C-19), 80.3 (C-20), 25.6 (C-21), 205.4 (C-22), 122.6 (C-23), 151.5 (C-24), 81.1 (C-25), 26.5 (C-26), 26.8 (C-27), 19.4 (C-28), 29.3 (C-29), 21.8 (C-30), 104.2 (C-1′), 75.4 (C-2′), 77.9 (C-3′), 71.5 (C-4′), 78.2 (C-5′), 62.9 (C-6′), 21.9 (CH3CO), 171.9 (CH3 CO).
Cytotoxic assay
Effects of 1–8 on the growth of human cancer cells were determined by measuring the cytotoxic activity using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Nhiem et al. 2009). Four human cancer cell lines, including A-549 (human lung cancer), OVCAR (human ovarian carcinoma), HT-29 (human colon adenocarcinoma), and MCF-7 (human breast cancer) were grown in RPMI 1640 medium supplemented with 10 % fetal bovine serum and penicillin/streptomycin (100 U/mL and 100 g/mL, respectively) at 37 °C in a humidified 5 % CO2 atmosphere. The exponentially growing cells were used throughout the experiments. The MTT assays were performed as follows: human cancer cells (1.5–2.5 × 105 cells/mL) were treated for 3 days with 1, 10, 30 and 100 μM of compounds. Mitoxantrone (Sigma-Aldrich, USA; purity of mitoxantrone was ≥97 %) was used to final concentrations of 1, 3, 10, and 20 μM as a reference compound. After incubation, 0.1 mg (50 μL of a 2 mg/mL solution) MTT (Sigma, Saint Louis, MO, USA) was added to each well and the cells were then incubated at 37 °C for 4 h. The plates were centrifuged at 1,000 rpm for 5 min at room temperature and the media was then carefully aspirated. Dimethylsulfoxide (150 μL) was then added to each well to dissolve the formazan crystals. The plates were read immediately at 540 nm on a microplate reader (Amersham Pharmacia Biotech., USA). All the experiments were performed three times and the mean absorbance values were calculated. The results are expressed as the percentage of inhibition that produced a reduction in the absorbance by the treatment of crude extract or solvent fractions compared to the untreated controls. A dose–response curve was generated and the inhibitory concentration of 50 % (IC50) was determined for each compound as well as each cell line.
Results and discussion
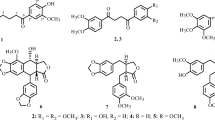
Using various chromatographic methods, one new lignan, three known lignans, two flavonone glycosides, and two cucurbitane-type triterpenes were isolated from the methanol extract of the roots of T. kirilowii.
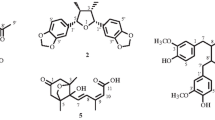
Compound 1 was obtained as a white amorphous powder and its molecular formula was determined to be C18H18O4 by HR-EI-MS at m/z 298.1203 (Calcd C18H18O4 for 298.1205). The 1H-NMR spectrum of 1 showed signals for four protons of 1,4-disubstituted benzene at δH 6.73 (2H, d, J = 8.0 Hz) and 7.16 (2H, d, J = 8.0 Hz); three protons of 1,2,4-trisubstituted aromatic ring with ABX coupling patterns at δH 6.72 (1H, d, J = 8.0 Hz), 7.20 (1H, dd, J = 2.0, 8.0 Hz), and 7.33 (1H, d, J = 2.0 Hz), and two olefin protons at δH 6.19 (1H, dt, J = 6.0, 16.0 Hz) and 6.54 (1H, d, J = 16.0 Hz). The 13C-NMR and DEPT spectra of 1 revealed signals for 18 carbons, including five quaternary at δC 129.5, 131.5, 134.2, 158.5, and 161.0, eleven methine at δC 54.7, 88.7, 110.0, 116.3 (2 × C), 123.8, 127.1, 128.2 (2 × C), 128.7, 132.0, and two oxymethylene carbons at δC 63.9 and 65.1. The 1H- and 13C-NMR data of 1 suggested the presence of dihydrobenzofuran skeleton and similar to those of cupressoside B (2) except for the different from propyl moiety at C-1′ (Xu et al. 2006). The HMBC correlations between H-7 (δH 5.45) and C-1 (δC 134.2), C-2/C-6 (δC 128.2), and C-3′ (δC 131.5), C-4′ (δC 161.0), between H-2/H-6 (δH 7.16) and C-1 (δC 134.2), C-4 (δC 158.5), and C-7 (δC 88.7) suggested the position of p-hydroxylphenyl at C-7. The HMBC correlations from H-7′ (δH 6.54) to C-1′ (δC 129.5), C-2′ (δC 123.8), C-6′ (δC 128.7), C-8′ (δC 127.1), and C-9′ (δC 63.9) confirmed the presence of double bond at C-7′/C-8′ and hydroxyl group at C-9′ (Fig. 1). The E configuration of the double bond was based on the coupling constant between H-7′ and H-8′, J H-7′-H-8′ = 16.0 Hz. The large coupling constant of H-7 and H-8, J H-7-H-8 = 6.0 Hz confirmed the configurations of two protons at C-7 and C-8 to be trans. The CD spectrum of 1 ([Δε (nm): −4.3 (244) and +2.6 (222)]) proved the configurations at C-7 and C-8 to be 7R and 8S by comparing with those of cupressoside B (a negative peat at 238 nm and a positive peak at 221 nm) (Xu et al. 2006). Based on the above evidence, compound 1 was elucidated to be 2-(4-hydroxyphenyl)-5-(3-hydroxyprop-1E-en-1-yl)-2R,3S-dihydrobenzofuran and named trichobenzolignan.
The known compounds were identified as ligballinol (2) (Wang et al. 2009), (−)-pinoresinol (3) (Moon et al. 2008), ehletianol C (4) (Yoshikawa et al. 1995), luteolin 7-O-β-D-glucopyranoside (5) (Chiruvella et al. 2007), chrysoeriol 7-O-β-D-glucopyranoside (6) (Schwaiger et al. 2006), and 10α-cucurbita-5,24-dien-3β-ol (7) (Nes et al. 1991), and arvenin I (8) (Kawahara et al. 2004). Their structures were established on the basis of spectral and chemical evidence, which were in agreement with those reported in literature (Fig. 2).
All compounds were evaluated for their cytotoxic activities against four human cancer cell lines, A-549, HT-29, OVCAR, and MCF-7 (Table 2). As the results, compound 7 showed significant activity on HT-29 and OVCAR cancer cell lines with IC50 values of 4.1 and 6.5 µM, respectively, comparing with those of mitoxantrone, an anticancer agent with IC50 of 3.1 and 8.4 µM. Compounds 1, 5, 6, and 8 exhibited moderate activities in all cancer cell lines with IC50 ranging from 11.3 to 42.8 µM.
References
Akihisa, T., W.C.M.C. Kokke, J.A. Krause, D.S. Eggleston, S.-I. Katayama, Y. Kimura, and T. Tamura. 1992. 5-Dehydrokarounidiol [D:C-Friedo-oleana-5, 7, 9(11)-triene-3α, 29-diol], a novel triterpene from Trichosanthes kirilowii MAXIM. Chemical & Pharmaceutical Bulletin 40: 3280–3283.
Akihisa, T., K. Yasukawa, Y. Kimura, M. Takido, W.C.M.C. Kokke, and T. Tamura. 1994. Five D: C-Friedo-oleanane triterpenes from the seeds of Trichosanthes kirilowii MAXIM. and their anti-inflammatory effects. Chemical & Pharmaceutical Bulletin 42: 1101–1105.
Chi, V. V. 2012. The Dictionary of Medicinal Plants in Vietnam. Hanoi: Medical Publishing House, Vol 1.
Chiruvella, K.K., A. Mohammed, G. Dampuri, R.G. Ghanta, and S.C. Raghavan. 2007. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O-glucoside isolated from callus cultures of Soymida febrifuga. International Journal of Biomedical Science 3: 269–278.
Jang, K.C., J.H. Lee, S.C. Kim, E.Y. Song, N.Y. Ro, D.Y. Moon, Y.C. Um, and K.H. Park. 2007. Antibacterial and radical scavenging activities of 1-C-(p-hydroxyphenyl)-glycerol from Trichosanthes kirilowii. Journal of Applied Biological Chemistry 50: 17–21.
Kawahara, N., A. Kurata, T. Hakamatsuka, S. Sekita, and M. Satake. 2004. Two new cucurbitacin glucosides, opercurins A and B, from the Brazilian folk medicine “Buchinha” (Luffa operculata). Chemical & Pharmaceutical Bulletin 52: 1018–1020.
Kimura, Y., T. Akihisa, K. Yasukawa, M. Takido, and T. Tamura. 1995. Structures of five hydroxylated sterol from the seeds of Trichosanthes kirilowii MAXIM. Chemical & Pharmaceutical Bulletin 43: 1813–1817.
Kondo, T., M. Inoue, H. Mizukami, and Y. Ogihara. 1995. Cytotoxic activity of bryonolic acid isolated from transformed hairy roots of Trichosanthes kirilowii var. japonica. Biological and Pharmaceutical Bulletin 18: 726–729.
Moon, S.S., A.A. Rahman, J.Y. Kim, and S.H. Kee. 2008. Hanultarin, a cytotoxic lignan as an inhibitor of actin cytoskeleton polymerization from the seeds of Trichosanthes kirilowii. Bioorganic & Medicinal Chemistry 16: 7264–7269.
Nes, W.D., R.Y. Wong, M. Benson, and T. Akihisa. 1991. Conformational analysis of 10α-cucurbitadienol. Chemical Communications 0: 1272–1274.
Nhiem, N.X., N.H. Tung, P.V. Kiem, C.V. Minh, Y. Ding, J.H. Hyun, H.K. Kang, and Y.H. Kim. 2009. Lupane triterpene glycosides from leave of Acanthopanax koreanum and their cytotoxic activity. Chemical & Pharmaceutical Bulletin 57: 986–989.
Rahman, M.A.A., and S.S. Moon. 2007. Isoetin 5’-methyl ether, a cytotoxic flavone from Trichosanthes kirilowii. Bulletin of Korean Chemical Society 28: 1261–1264.
Ryu, S.Y., S.H. Lee, S.U. Choi, C.O. Lee, Z. No, and J.W. Ahn. 1994. Antitumor activity of Trichosanthes kirilowii. Archives of Pharmacal Research 17: 348–353.
Schwaiger, S., C. Seger, B. Wiesbauer, P. Schneider, E.P. Ellmerer, S. Sturm, and H. Stuppner. 2006. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium Cass.) constituents. Phytochemical Analysis 17: 291–298.
Takahashi, N., Y. Yoshida, T. Sugiura, K. Matsuno, A. Fujino, and U. Yamashita. 2009. Cucurbitacin D isolated from Trichosanthes kirilowii induces apoptosis in human hepatocellular carcinoma cells in vitro. International Immunopharmacology 9: 508–513.
Wang, X.W., H.P. Zhang, F. Chen, X. Wang, and W.Y. Wen. 2009. A new lignan from Gynostemma pentaphyllum. Chinese Chemical Letters 20: 589–591.
Xu, J.F., D.H. Cao, N.H. Tan, Z.L. Liu, Y.M. Zhang, and Y.B. Yang. 2006. New lignan glycosides from Cupressus duclouxian (Cupessaceae). Journal of Asian Natural Products Research 8: 181–185.
Yoshikawa, K., H. Kageyama, and S. Arihara. 1995. Phenolic glucosides and lignans from Ehretia ovalifolia. Phytochemistry 39: 659–664.
Acknowledgments
This research was supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2011.23 and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0025129).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minh, C.V., Nhiem, N.X., Yen, H.T. et al. Chemical constituents of Trichosanthes kirilowii and their cytotoxic activities. Arch. Pharm. Res. 38, 1443–1448 (2015). https://doi.org/10.1007/s12272-014-0490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0490-6