Abstract
Myocardial ischemia/reperfusion injury (I/RI) and ventricular remodeling are the critical pathological basis of heart failure. Danlou tablet (Dan) is a kind of Chinese patent medicine used in angina pectoris treatment in China. However, it remains unclear whether and how Dan could protect against cardiac remodeling after myocardial I/RI. In this study, both preventive and therapeutic administration of Dan attenuated ventricular remodeling and cardiac dysfunction at 3 weeks after myocardial I/RI. Dan inhibited Bax/Bcl2 ratio and Caspase3 cleavage in heart tissues and also inhibited apoptosis of human AC16 cells and neonatal rat cardiomyocytes stressed by oxygen and glucose deprivation/reperfusion. Mechanistically, Dan inhibited myocardial apoptosis through phosphorylating AKT and FoxO3a, thereby inhibiting downstream BIM and PUMA expressions. Collectively, these results demonstrate that Dan treatment is effective to protect against cardiac remodeling and dysfunction after myocardial I/RI and provide theoretical basis for its cardioprotection and clinical application in treating ischemic cardiac diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial ischemia/reperfusion injury (I/RI) refers to the pathological process of progressive aggravation of the ischemic myocardium, although the normal perfusion of the ischemic myocardium can be restored after the partial or complete acute occlusion of the coronary artery [1]. Myocardial I/RI is a complex process involving several associated factors, including decreased cellular adenosine triphosphate (ATP) levels, increased inflammation, calcium overload, and oxidative stress which can cause cardiomyocyte injury and death [2]. Late in the myocardium injury, the accumulation of inflammatory cytokines and the activation of neurohumoral factors will lead to structural and functional changes in the heart which is known as ventricular remodeling [3]. The adverse remodeling will further exacerbate cardiomyocyte apoptosis and cardiac fibrosis and eventually lead to heart failure.
At present, there is still a lack of effective means to prevent and treat I/RI. Some studies have shown that the antioxidants could eliminate free radical accumulation induced by I/RI and reduce oxidative stress [4]. Drug preconditioning with sodium-hydrogen exchange blockers and sodium-calcium exchange blockers affected the outcome of myocardial I/RI [5]. Pharmacological preconditioning is a method to attenuate I/RI by stimulating or mimicking endogenous substances in the body, such as opioid-induced preconditioning was beneficial to protect the heart from I/RI [6]. Multidrug combination strategies, such as co-administration of doxycycline, ML-7, and eNOS inhibitor could have a synergistic effect which reduced I/RI and improved cardiac function [7]. However, effective strategies to inhibit cardiac remodeling and heart failure after I/RI are still lacking in clinical application.
Danlou Tablet (Dan) is a kind of traditional Chinese medicine, which has been used in the treatment of angina pectoris and acute coronary syndrome in China [8, 9]. The main ingredients of Dan are Trichosanthes kirilowii maxim, Paeoniae radix rubra, Ligusticum Chuanxiong hort, Salvia miltiorrhiza bunge, Radix Puerariae, Astragalus propinquus schischkin, etc. Dan has been reported to relieve unstable angina and hyperlipidemia, and improve vascular endothelial function [10, 11]. Our previous work and other reports have demonstrated that Dan was effective to inhibit acute myocardial I/RI in vivo [12, 13]. However, whether and how Dan could have protective effect against cardiac remodeling and improve cardiac function at long-term after myocardial I/RI remained largely unclear.
In the present study, we established in vivo model and in vitro mimic model of myocardial I/RI using mice, AC16 cells, and neonatal rat cardiomyocytes (NRCM). We explored the potential function of Dan in preventing cardiac remodeling and dysfunction after I/RI, and further explored the molecular mechanism underlying its protection in oxygen glucose deprivation/reperfusion (OGD/R)-treated cardiomyocytes in vitro and in myocardial I/RI model in vivo. The protective role and mechanism of Dan in attenuating myocardial I/RI and ventricular remodeling will provide new method and strategy for the prevention and treatment of myocardial I/RI.
Methods
Myocardial I/RI Model and Dan Treatment
Eight-week-old male C57BL6/J mice were purchased from Weitong Lihua Experimental Animal Technology Co., Ltd. (Zhejiang, China). All the animals were housed in the specific pathogen free (SPF) laboratory animal facility of Shanghai University (Shanghai, China). Mice were randomized into Sham group, I/RI group, and I/RI treated with Dan group. For the preventive experiment, myocardial I/RI mouse model was established after oral administration of 700 mg/kg/day (dissolved with saline) of Dan for one week. Mice were anesthetized with intraperitoneal injection of 4% chloral hydrate (10 mL/g body weight). Then myocardial I/RI surgery was performed by ligating the left anterior descending (LAD) coronary artery for 30 min followed by reperfusion for 3 weeks according to the previous study [14]. Sham mice received the same operation procedures without ligation of LAD coronary artery. After three days of rest post I/RI surgery, mice were continued to be treated with Dan (700 mg/kg/day), and the control group was given the same dose of saline. For the therapeutic experiment, mice were subjected to myocardial I/RI surgery and from the next day of surgery received oral administration of 700 mg/kg/day Dan for 3 weeks. All the I/RI surgery operations were conducted under blinded condition. A low mortality rate (10% ~ 20%) of the I/RI model can be achieved in our laboratory as our previously reported [14]. There was no difference in the mortality rate between the I/RI group and I/RI + Dan group. After three weeks of reperfusion, mice were sacrificed and hearts were collected for further analyses. All animal experiments were approved by the Committee for the Ethics of Animal Experiments of Shanghai University and performed in accordance with the Guidelines on the Care and Use of Laboratory Animals for biomedical research published by the National Institutes of Health (No. 85–23, revised 1996).
Echocardiography for Cardiac Function
A Visual Sonics Vevo 2100 (FUJIFILM Visual Sonics Inc) equipped with an MS-550D ultrasound probe was used to detect transthoracic echocardiography in mice to measure the index of small animal cardiac function. Animals were anesthetized with isoflurane. Hearts were imaged with M-mode in long-axis view of the left ventricle at papillary muscle level. Left ventricular ejection fraction (EF, %) and fractional shortening (FS, %) were determined for cardiac function.
Hematoxylin & Eosin Staining
Hematoxylin & Eosin (H&E) staining was used to observe the morphology of liver and heart tissues. After three weeks of I/RI, liver or heart tissues were harvested and cut into 5 μm-thick tissue sections. Hematoxylin and eosin were sequentially added to tissue sections, and images were acquired with microscope (Leica DM3000, Germany).
Masson's Trichrome Staining
Masson's trichrome staining was used to investigate cardiac pathological fibrosis. After three weeks of I/RI, the hearts of experimental mice were harvested and cut into 5 μm-thick tissue sections. Masson's trichrome staining was performed according to manufacturer’s instructions (Solarbio, G1340) and images were acquired with microscope (Leica DM3000, Germany). The percentage of fibrotic area to total cardiac area was analysed using ImageJ software 1.50i (NIH, USA).
Cardiomyocyte Culture
Human AC16 cardiomyocytes and neonatal rat cardiomyocytes (NRCM) were used to study the functional role of Dan in cardiomyocytes. The NRCM were isolated and cultured according to the previous study [15]. In brief, left ventricles were freshly obtained from 1- to 3-day old neonatal Sprague–Dawley rats and minced into 1 mm2 small pieces on ice. The Collagenase II (Gibco, 17,101,015) and Pancreatin from porcine pancreas (Sigma, P3292) were used to digest NRCM. Then, Percoll (GE healthcare, 17–0891-01) centrifugation was used to isolate NRCM. NRCM were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum and 10% horse serum for further experiments. Human AC16 cardiomyocytes were cultured and passaged in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum for further experiments.
Oxygen Glucose Deprivation/Reperfusion (OGD/R) Model and Cell Treatment
To investigate the role of Dan in cardiomyocytes, AC16 cardiomyocytes and NRCM were cultured in serum-free and glucose-deprived DMEM medium under an oxygen deprivation atmosphere at 37℃ for 12 h and 8 h, respectively. Then, the two kinds of cells were cultured in DMEM with normal serum, glucose, and oxygen conditions for another 12 h to mimic I/RI in vitro. The 50 μg/ml and 100 μg/ml of Dan were used in OGD/R-induced AC16 apoptosis model, followed by flow cytometry analysis. And the 50 μg/ml of Dan was finally selected for further study in OGD/R-stressed AC16 and NRCM. To further study the contribution of AKT to the protective role of Dan, NRCM were treated with AKT inhibitor MK2206 (10 nM, Selleck S1078) or AKT activator SC79 (10 μM, Selleck S7863) for 24 h, simultaneously treated with Dan for 48 h under OGD/R condition.
Flow Cytometry for Apoptosis
After AC16 cells were stressed with OGD/R, cell apoptosis was analyzed using Annexin V-FITC/PI Apoptosis Kit (Bioworld, Nanjing, China) according to the manufacturer’s instructions. Briefly, the cells were trypsinized and washed twice with PBS. After being added with binding buffer and incubating with 5 μL of Annexin V-FITC for 15 min and 10 μL of PI for 5 min in the dark, the cells were analyzed for apoptosis by flow cytometry (Beckman Coulter, USA).
TUNEL Staining
After treatment, NRCM were fixed by 4% paraformaldehyde (PFA) and stained with TUNEL FITC Apoptosis Detection Kit according to the manufacturer’s instructions (Vazyme, China). Immunofluorescent staining for α-actinin (Sigma, A7811) was performed to label NRCM. Hoechst was used to label the nuclei of NRCM. NRCM stained with TUNEL and α-actinin were imaged under a fluorescence microscope (Leica DMi8, Germany). The percentage of TUNEL-positive NRCM to total NRCM was analyzed using ImageJ software 1.50i (NIH, USA).
Western Blot
For AC16 cells and NRCM cultured in vitro or heart tissues from I/RI mouse model in vivo, cells or tissues were lysed with RIPA lysis buffer (Beyotime, China) complemented with 1% phenylmethylsulfonyl fluoride (PMSF) and protease and phosphatase inhibitor (Beyotime, China) at 4℃ and subsequently centrifuged at 12,000 g for 20 min. A total of 10 to 30 μg proteins were used to perform Western blot analysis. The primary antibodies used are as follows: Bax (Abclonal, A0207), Bcl2 (Abclonal, A2845), Caspase 3 (Cell Signaling, 9662), p-AKT(S473) (Cell Signaling, 4060), total-AKT (Proteintech, 10,176–2-AP), p-FoxO3a(T32) (Cell signaling, 9464), p-FoxO3a(S253) (Cell signaling, 9466), FoxO3a, (Beyotime, AF609), BIM (Abclonal, A19702), and PUMA (Proteintech, 55,120–1-AP). GAPDH or β-actin was used as an internal control. Protein bands were visualized using enhanced chemiluminescence (ECL) kit and analysed using ImageJ software.
Real-Time PCR
Total RNA was isolated from mouse heart tissues using TRIzol RNAiso Plus Kit (TaKaRa). RNA was then reverse-transcribed to cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo K1622). Real-time PCR for pathological remodeling genes including atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-myosin heavy chain (β-MHC) and fibrosis-related genes including collagen I and collagen III was performed using TaKaRa SYBR Premix Ex Taq™ (Tli RNaseH Plus, Japan) on Roche LightCycler480 PCR System. 18 s was used as an internal control. The primers used were listed in Table 1.
Statistical Analysis
All data were presented as mean ± standard deviation (SD) and analyzed by SPSS 20.0 or GraphPad Prism 8. The normal distribution of data was analyzed by Shapiro–Wilk normality test. For data with normal distribution, Student's t-test, one-way ANOVA followed by Bonferroni or Dunnett’s T3 test, or Two-way ANOVA followed by Tukey’s post hoc test was used as appropriate. For those which did not pass normality test, Kruskal–Wallis test was performed as appropriate. A p value < 0.05 was considered statistically significant.
Results
Role of Dan in Cardiac Remodeling and Dysfunction after I/R Injury
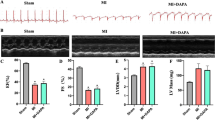
To explore the role of Dan in cardiac remodeling and dysfunction after long-term exposure to myocardial I/RI, adult C57BL6/J mice were administrated with Dan (700 mg/kg/d) for one week, then the myocardial I/RI mice model was established. After I/RI, mice were further administrated with Dan (700 mg/kg/d) for another three weeks. At the end of the experiment, cardiac function of the experimental mice was examined using echocardiography. Our results showed that Dan could improve cardiac function after three weeks of myocardial I/RI in mice; both left ventricular EF (%) and FS (%) of the I/RI group were decreased compared with the sham group, while left ventricle systolic function was significantly improved in mice treated with Dan compared with the I/RI group (Fig. 1A). H&E staining results showed that administration of Dan for three weeks did not induce pathological structure changes in liver tissues in I/RI mice (Fig. 1B). Meanwhile, no difference was found in myocardial hypertrophy among different groups as determined by heart weight (HW), heart weight/body weight (HW/BW) ratio, heart weight/tibia length (HW/TL) ratio, and H&E staining for heart tissues (Figure S1A and S1B). Masson staining showed that the fibrosis area was significantly increased in the heart of mice with I/RI compared with the sham group, while the fibrosis area of the Dan group was significantly reduced compared with the I/RI group (Fig. 1C). The pathological remodeling genes and fibrosis-related genes in heart tissues were further detected by fluorescence quantitative PCR. It was found that treatment with Dan significantly decreased the expression of ANP, β-MHC, and collagen III, and tended to downregulate BNP and collagen I compared with the I/RI group (Fig. 1D and 1E). Collectively, these results demonstrated that Dan could prevent ventricular remodeling and attenuate cardiac dysfunction induced by three-week of myocardial I/RI.
Effect of preventive administration of Dan in cardiac remodeling and dysfunction after 3 weeks of I/RI. (A) Adult C57BL6/J mice were orally administrated with Dan (700 mg/kg/d) or normal saline for one week. Then myocardial I/RI model was established. During three weeks of I/RI, Dan (700 mg/kg/d) or saline was orally administrated. At three weeks post I/RI, left ventricular ejection fraction (EF, %) and fractional shortening (FS, %) were measured by echocardiography (n = 6:9:9). (B) Representative images of H&E staining to evaluate the effect of chronic administration of Dan on the structure of liver tissues. Scale bar = 100 μm. (C) Masson’s trichrome staining was used to detect the effect of Dan on cardiac fibrosis after I/RI (n = 6:7:7). Scale bar = 100 μm. (D and E) Real-time PCR was performed to determine the expressions of ANP, BNP, and β-MHC (D) and Collagen I and Collagen III (E) after 3 weeks of I/RI in the presence or absence of Dan treatment (n = 6:9:9). Statistics were performed using non-parametric Kruskal–Wallis test for A, D (ANP) and E, and one-way ANOVA followed by Bonferroni or Dunnett’s T3 test for C and D (BNP and β-MHC). *, p < 0.05; **, p < 0.01; ***, p < 0.001
Dan Treatment Inhibits Cardiomyocyte Apoptosis In Vivo and In Vitro
Western blot was used to investigate whether Dan affected the expression of apoptosis-related proteins in cardiac tissues after three weeks of myocardial I/RI in mice. Our results showed a marked increase of the Bax/Bcl2 ratio and cleaved-Caspase3/Caspase3 ratio after three weeks of myocardial I/RI, however, Dan treatment significantly decreased the Bax/Bcl2 ratio and cleaved-Caspase3/Caspase3 ratio compared with the I/RI group (Fig. 2A). These results provided evidence that long-term administration of Dan in vivo could inhibit myocardial apoptosis after three weeks of myocardial I/RI. We previously reported that Dan treatment was effective to inhibit apoptosis of primary NRCM in vitro [12]. Here, we further determined whether Dan could also protect cardiomyocyte apoptosis using human AC16 cells. The OGD/R-treated AC16 cell model was firstly used to explore the appropriate concentration of Dan in inhibiting cell apoptosis. Flow cytometry analysis showed that OGD/R stress significantly increased the apoptotic ratio of AC16 cells compared with the control group. Meanwhile, we noticed that both 50 μg/mL and 100 μg/mL of Dan had similar effects in reducing the apoptotic ratio of AC16 cells compared with the OGD/R group (Fig. 2B). As there was no significant difference between 50 μg/mL and 100 μg/mL of Dan in inhibiting AC16 cell apoptosis, 50 μg/ml of Dan was selected for further study in vitro. Western blot was then used to detect apoptosis-related proteins and showed that OGD/R significantly upregulated the Bax/Bcl2 ratio in AC16 cells, while Dan could significantly reduce the Bax/Bcl2 ratio compared with the OGD/R group (Fig. 2C). Collectively, these results indicated that Dan was efficient to inhibit cardiomyocyte apoptosis after long term exposure to I/RI both in vivo and in vitro.
Dan treatment inhibits myocardial apoptosis in vivo and in vitro. (A) Western blot for apoptosis-associated proteins in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). (B) Flow cytometry analysis for oxygen glucose deprivation/reperfusion (OGD/R)-induced apoptosis of human AC16 cells in the presence or absence of Dan treatment (n = 3). (C) Western blot for apoptosis-associated proteins in OGD/R-induced apoptosis of human AC16 cells in the presence or absence of Dan treatment (n = 3). Statistics were performed using one-way ANOVA followed by Bonferroni or Dunnett’s T3 test and two-way ANOVA followed by Tukey’s post hoc test as appropriate. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Dan Prevents Cardiac I/RI with Activation of AKT/FoxO3a Pathway
Forkhead box O3a (FoxO3a) is a critical transcription factor that can be phosphorylated by AKT and has been proved to be protective against myocardial I/RI [16]. To determine whether Dan activated AKT/FoxO3a signaling pathway in myocardial I/RI, Western blot was used to detect the phosphorylation of AKT and its downstream FoxO3a in heart tissues of three-week of I/RI mice. The results showed that the phosphorylation levels of AKT and FoxO3a were significantly decreased after three-week myocardial I/RI in mice, while treatment with Dan was efficient to increase the phosphorylation levels of AKT and FoxO3a compared with the IRI group (Fig. 3A and Fig. 3B). It has been known that phosphorylated FoxO3a can be transported out of the nucleus, thus inhibiting the transcription of pro-apoptotic proteins such as BIM and PUMA [17]. We then further determined BIM and PUMA expressions in the heart after I/RI. Our results demonstrated that three weeks of myocardial I/RI caused significant upregulation of BIM and PUMA in mice heart tissues compared with the sham group, whereas treatment with Dan inhibited the expression of BIM and PUMA compared with the I/RI group (Fig. 3C). These results indicated that Dan could enhance the phosphorylation of AKT and FoxO3a and downregulate the pro-apoptotic proteins BIM and PUMA, which might contribute to its protective effect against myocardial I/RI in vivo.
Dan prevents cardiac I/RI with activation of AKT/FoxO3a pathway. (A) Western blot for phosphorylation of AKT in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). (B) Western blot for phosphorylation of FoxO3a (T32) and FoxO3a (S253) in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). (C) Western blot for BIM and PUMA expressions in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). Statistics were performed using non-parametric Kruskal–Wallis test for A and B (p-FoxO3a (T32)), and one-way ANOVA followed by Bonferroni or Dunnett’s T3 test for B (p-FoxO3a (S253)) and C. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Dan Prevents Cardiomyocyte Apoptosis with Activation of AKT/FoxO3a Pathway In Vitro
We next investigated whether Dan could regulate AKT/FoxO3a signaling pathway in OGDR-stressed cardiomyocytes in vitro. Our results showed that Dan increased the phosphorylation level of AKT in the OGD/R-stressed NRCM model (Fig. 4A). The phosphorylation of FoxO3a (T32 and S253) was also increased by Dan treatment in the OGD/R-stressed NRCM model (Fig. 4B). Furthermore, we demonstrated that Dan could significantly reduce BIM and PUMA expressions in OGDR-stressed NRCM (Fig. 4C). These findings were consistent to the in vivo results, showing that Dan could activate AKT/FoxO3a signaling pathway and inhibit the expression of pro-apoptotic proteins PUMA and BIM in cardiomyocytes under apoptotic stress.
Dan prevents cardiomyocyte apoptosis with activation of AKT/FoxO3a pathway in vitro. (A) Western blot for phosphorylation of AKT in oxygen glucose deprivation/reperfusion (OGD/R)-induced apoptosis of neonatal rat cardiomyocytes (NRCM) in the presence or absence of Dan treatment (n = 3). (B) Western blot for phosphorylation of FoxO3a (T32) and FoxO3a (S253) in OGD/R-induced apoptosis of NRCM in the presence or absence of Dan treatment (n = 3). (C) Western blot for BIM and PUMA expressions in OGD/R-induced apoptosis of NRCM in the presence or absence of Dan treatment (n = 3). Statistics were performed using Student's t-test. *, p < 0.05; **, p < 0.01
Dan Prevents Cardiomyocyte Apoptosis through Activating AKT/FoxO3a Pathway
To further elucidate whether Dan protects cardiomyocytes against apoptosis by activating AKT and subsequently influencing FoxO3a activity, Dan and AKT inhibitor MK-2206 or AKT activator SC79 were co-used in NRCM under OGD/R condition. TUNEL staining showed that the protective effect of Dan against cardiomyocyte apoptosis was attenuated after treatment of AKT inhibitor MK-2206 (Fig. 5A). But the combination of Dan and AKT activator SC79 did not have additive effect in reducing cardiomyocyte apoptosis (Fig. 5B). These results suggested that AKT activation was necessary for Dan to inhibit OGD/R-induced cardiomyocyte apoptosis. Next, we further determined AKT and FoxO3a phosphorylation and PUMA and BIM expressions in NRCM treated with Dan and/or MK2206, and found that Dan treatment significantly enhanced AKT and FoxO3a phosphorylations and downregulated PUMA and BIM expressions in the OGDR-induced NRCM model, however, these changes associated with Dan were partially attenuated by co-treatment of MK2206 (Fig. 5C and 5D). Collectively, these results provided direct evidence that AKT/FoxO3a activation was necessary to mediate the protective effect of Dan against cardiomyocyte apoptosis.
Dan prevents cardiomyocyte apoptosis through activating AKT/FoxO3a pathway. (A and B) TUNEL/α-actinin stainings for oxygen glucose deprivation/reperfusion (OGD/R)-induced apoptosis of neonatal rat cardiomyocytes (NRCM) treated with AKT inhibitor MK-2206 (A) or AKT activator SC79 (B) in the presence or absence of Dan treatment (n = 4–6). Scale bar = 100 μm. (C) Western blot for phosphorylation of AKT and FoxO3a in NRCM treated with Dan and/or MK2206 under OGD/R condition (n = 6). (D) Western blot for BIM and PUMA expressions in NRCM treated with Dan and/or MK2206 under OGD/R condition (n = 6). Statistics were performed using two-way ANOVA followed by Tukey’s post hoc test. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Therapeutic Administration of Dan is Protective against Cardiac Remodeling and Dysfunction after I/R Injury
Clinical myocardial infarction usually occurs unexpectedly, and in most of patients, acute myocardial infarction could not be prevented. Thus, we further investigated whether therapeutic administration of Dan could still be protective against cardiac I/RI. For the therapeutic experiment, mice were subjected to myocardial I/RI surgery and from the next day of surgery received oral administration of Dan for 3 weeks. Our data showed that therapeutic treatment of Dan was still effective to preserve cardiac function and inhibit cardiac fibrosis and remodeling after 3 weeks of I/RI (Fig. 6A-6D). No difference was found in heart weight (Figure S2A) or myocardial hypertrophy (Figure S2B) among different groups. Moreover, therapeutic treatment of Dan increased Akt and FoxO3a phosphorylation (Fig. 6E and 6F) and downregulated pro-apoptotic BIM and PUMA expressions (Fig. 6G) in the I/RI heart. Collectively, these data indicate that therapeutic administration of Dan was still effective to attenuate cardiac remodeling and dysfunction after myocardial I/RI.
Effect of therapeutic administration of Dan in cardiac remodeling and dysfunction after 3 weeks of I/RI. (A) For therapeutic experiment, mice were subjected to myocardial I/RI surgery and from the next day of surgery received oral administration of 700 mg/kg/day Dan for 3 weeks. At 3 weeks post I/RI, left ventricular ejection fraction (EF, %) and fractional shortening (FS, %) were measured by echocardiography (n = 6:7:6). (B) Masson’s trichrome staining was used to detect the effect of Dan on cardiac fibrosis after I/RI (n = 6:7:6). Scale bar = 100 μm. (C and D) Real-time PCR was performed to determine the expressions of ANP, BNP, and β-MHC (C) and Collagen I and Collagen III (D) after 3 weeks of I/RI in the presence or absence of Dan treatment (n = 6:7:6). (E and F) Western blot for phosphorylation of AKT (E) and FoxO3a (F) in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). (G) Western blot for BIM and PUMA expressions in I/RI mice hearts in the presence or absence of Dan treatment (n = 6). Statistics were performed using non-parametric Kruskal–Wallis test for D (Collagen III), and one-way ANOVA followed by Bonferroni or Dunnett’s T3 test if not specifically indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001
Discussion
Dan has been shown to have protective effects against cardiovascular injuries such as myocardial ischemia, arrhythmia, atherosclerosis, and dyslipidemia [10, 11, 18, 19]. We previously reported that Dan had a protective effect in acute myocardial I/RI in mice, which was manifested by reduced infarct size and myocardial apoptosis in vivo and in vitro [12]. The cardioprotection of Dan was associated with the activation of proliferator-activated receptor γ (PPARγ) in acute myocardial I/RI hearts. In this study, we further investigated the role of Dan in chronic myocardial I/RI and demonstrated that both preventive and therapeutic administration of Dan improved cardiac function and prevented ventricular remodeling after long term exposure to I/RI in mice. We further elucidated that Dan activated AKT/FoxO3a pathway at both animal and cellular levels. Mechanistically, AKT activation is necessary to mediate the protective effect of Dan against myocardial apoptosis, closely associated with FoxO3a phosphorylation and reduced downstream PUMA and BIM expressions.
In this study, we first demonstrated that preventive administration of Dan could inhibit cardiac fibrosis, prevent ventricular remodeling, and improve cardiac function at 3 weeks after myocardial I/RI. Long-term administration of Dan in vivo could inhibit Bax/Bcl2 ratio and Caspase3 cleavage in heart tissues after myocardial I/RI. Thus, in addition to our previous study showing the protective effect of Dan in acute I/RI [12], the present study indicates that Dan is also protective against cardiac remodeling and dysfunction long after I/RI. Moreover, our data showed that therapeutic administration of Dan is still effective to attenuate cardiac remodeling and dysfunction after 3 weeks of I/RI, indicating a potential therapeutic approach of Dan in clinical use for patients with acute myocardial infarction.
The phosphoinositol-3 kinase (PI3K)/AKT pathway is involved in a variety of cellular processes, such as cell survival, cell proliferation, and cell metabolism [20]. AKT is considered to be a major mediator of the PI3K/AKT pathway, ultimately leading to phosphorylation of some important downstream targets [21]. In addition, some negative regulators such as PTEN, a lipid phosphatase, lead to the loss of downstream p-AKT by hydrolysis of phosphatidylinositol 3,4,5-trisphosphate (PIP-3) to phosphatidylinositol 4,5-bisphosphate (PIP-2) [22]. It has been reported that the PI3K/AKT pathway was involved in the regulation of neuronal apoptosis during rat brain development and the activation of AKT protein kinase can further phosphorylate FoxO3a, leading to the translocation of phosphorylated FoxO3a to the cytoplasm thus inhibiting cell apoptosis [23]. In the heart, AKT has been well known for its protective effects against myocardial apoptosis as well as other cardiovascular injuries [24, 25]. In addition, during exercise-induced physiological cardiac growth, insulin-like growth factor-1 (IGF-1)-induced activation of PI3K/AKT pathway can improve cardiac function and promote cardiomyocyte survival [26, 27]. In the present study, we demonstrated that Dan could inhibit human AC16 cardiomyocyte and NRCM apoptosis in vitro with an obvious activation of AKT. Function-rescue experiments further showed that MK-2206, an AKT inhibitor, could eliminate the protective effect of Dan against cardiomyocyte apoptosis, while SC79, an AKT activator, could not further enhance the protective effect of Dan. Thus, these results suggest that Dan treatment is protective against cardiac remodeling and dysfunction after long term exposure to myocardial I/RI, which is closely related to its anti-apoptotic effect in the myocardium through AKT activation.
FoxO3a belongs to the forkhead Box O (FoxO) family members, which are transcription factors involved in the regulation of various cellular behaviors, such as cell apoptosis, DNA damage, and cell proliferation [28,29,30,31,32]. Due to its multiple biological functions, the FoxO family dysfunction is involved in many diseases, including cancer and other non-neoplastic diseases [33, 34]. Higher cytoplasmic expression of the phosphorylated form of FoxO3a (Ser253) was observed in prostate cancer patients, leading to increased cell proliferation [35]. Under normal circumstances, FoxO3a is localized in the nucleus and binds to DNA. After phosphorylation, FoxO3a interacts with 14–3-3 protein and then is transported to the cytoplasm, thus inhibiting the transcriptional activity of FoxO3a [34]. Many regulatory pathways have been identified to be involved in the regulation of FoxO3a, including the AKT pathway [34]. In the heart, phosphorylated form of AKT can further phosphorylate FoxO3a, leading to its translocation to the nucleus and protection against myocardial apoptosis [16]. FoxO3a phosphorylation was reported to cause transcriptional inhibition of apoptosis-related genes BIM and PUMA and subsequently inhibit cell apoptosis [17, 36, 37]. In the present study, we showed that Dan treatment was effective to activate the AKT/FoxO3a pathway and downregulated the pro-apoptotic genes BIM and PUMA in the myocardial I/RI mouse model and in the OGD/R-induced NRCM apoptosis model. In NRCM co-treated with Dan and AKT inhibitor MK2206 under OGD/R condition, we further found that Dan-induced FoxO3a phosphorylation was abolished by AKT inhibition. Meanwhile, the downregulated BIM and PUMA expressions in the Dan treatment group was also reversed by AKT inhibition. Collectively, these data indicate that Dan treatment can activate the AKT/FoxO3a pathway, leading to downregulation of the pro-apoptotic genes BIM and PUMA thus inhibiting myocardial apoptosis.
In conclusion, our study reveals the protective effect of Dan treatment against cardiac remodeling and dysfunction at long term after myocardial I/RI, and clarifies that Dan treatment is effective to inhibit myocardial apoptosis through activating AKT/FoxO3a pathway and downregulating apoptosis-related genes BIM and PUMA in the myocardium. Our findings expand the theoretical basis of the protective effect of Dan and suggest that Dan treatment may serve as a new strategy for long term protection against myocardial I/RI.
Data Availability
All data generated or analysed during this study are included in this article and its supplementary information files.
References
Starz C, Hardtner C, Mauler M, Dufner B, Hoppe N, Krebs K, et al. Elevated platelet-leukocyte complexes are associated with, but dispensable for myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2022;117(1):61. https://doi.org/10.1007/s00395-022-00970-3.
Li X, Liu M, Sun R, Zeng Y, Chen S, Zhang P. Protective approaches against myocardial ischemia reperfusion injury. Exp Ther Med. 2016;12(6):3823–9. https://doi.org/10.3892/etm.2016.3877.
Florea VG, Mareyev VY, Samko AN, Orlova IA, Coats AJ, Belenkov YN. Left ventricular remodelling: common process in patients with different primary myocardial disorders. Int J Cardiol. 1999;68(3):281–7. https://doi.org/10.1016/s0167-5273(98)00362-3.
Yao H, Xie Q, He Q, Zeng L, Long J, Gong Y, et al. Pretreatment with Panaxatriol Saponin Attenuates Mitochondrial Apoptosis and Oxidative Stress to Facilitate Treatment of Myocardial Ischemia-Reperfusion Injury via the Regulation of Keap1/Nrf2 Activity. Oxid Med Cell Longev. 2022;2022:9626703. https://doi.org/10.1155/2022/9626703.
Castaldo P, Macri ML, Lariccia V, Matteucci A, Maiolino M, Gratteri S, et al. Na(+)/Ca(2+) exchanger 1 inhibition abolishes ischemic tolerance induced by ischemic preconditioning in different cardiac models. Eur J Pharmacol. 2017;794:246–56. https://doi.org/10.1016/j.ejphar.2016.11.045.
Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35(7):709–18. https://doi.org/10.1016/s0022-2828(03)00135-4.
Bil-Lula I, Lin HB, Bialy D, Wawrzynska M, Diebel L, Sawicka J, et al. Subthreshold nitric oxide synthase inhibition improves synergistic effects of subthreshold MMP-2/MLCK-mediated cardiomyocyte protection from hypoxic injury. J Cell Mol Med. 2016;20(6):1086–94. https://doi.org/10.1111/jcmm.12827.
Wang SH, Wang J, Li J. Efficacy assessment of treating patients with coronary heart disease angina of phlegm and stasis mutual obstruction syndrome by Danlou tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(8):1051–5.
Wang L, Mao S, Qi JY, Ren Y, Guo XF, Chen KJ, et al. Effect of Danlou Tablet () on peri-procedural myocardial injury among patients undergoing percutaneous coronary intervention for non-ST elevation acute coronary syndrome: A study protocol of a multicenter, randomized, controlled trial. Chin J Integr Med. 2015;21(9):662–6. https://doi.org/10.1007/s11655-015-2284-1.
Tang JJ, Li GX, Liu ZG, Yi R, Yu D, Zhang YB, et al. Danlou Tablet Improves Chronic Intermittent Hypoxia-Induced Dyslipidemia and Arteriosclerosis by HIF-1alpha-Angptl4 mRNA Signaling Pathway. Chin J Integr Med. 2022;28(6):509–17. https://doi.org/10.1007/s11655-020-3255-8.
Guo LL, Wang J, Lin F, He YX. Effect of danlou tablet on arrhythmia model rats induced by transient myocardial ischemia/ reperfusion. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(9):1125–9.
Wei M, Guo M, Meng X, Li L, Wang H, Zhang M, et al. PPARgamma Mediates the Cardioprotective Roles of Danlou Tablet After Acute Myocardial Ischemia-Reperfusion Injury. Front Cardiovasc Med. 2022;9:858909. https://doi.org/10.3389/fcvm.2022.858909.
Qi JY, Wang L, Gu DS, Guo LH, Zhu W, Zhang MZ. Protective Effects of Danlou Tablet () against Murine Myocardial Ischemia and Reperfusion Injury In Vivo. Chin J Integr Med. 2018;24(8):613–20. https://doi.org/10.1007/s11655-016-2448-7.
Bei Y, Lu D, Bar C, Chatterjee S, Costa A, Riedel I, et al. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection. Mol Ther. 2022;30(4):1675–91. https://doi.org/10.1016/j.ymthe.2022.01.031.
Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95. https://doi.org/10.1016/j.cmet.2015.02.014.
Bei Y, Pan LL, Zhou Q, Zhao C, Xie Y, Wu C, et al. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019;17(1):42. https://doi.org/10.1186/s12916-019-1268-y.
Zou Z, Liu B, Zeng L, Yang X, Huang R, Wu C, et al. Cx43 Inhibition Attenuates Sepsis-Induced Intestinal Injury via Downregulating ROS Transfer and the Activation of the JNK1/Sirt1/FoxO3a Signaling Pathway. Mediators Inflamm. 2019;2019:7854389. https://doi.org/10.1155/2019/7854389.
Miao J, Zhou XB, Mao W, Chen J, Xu XM. Effects of Xuefu Zhuyu Granule and Danlou Tablet on Anti-atherosclerosis Rats and Potential Mechanisms. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36(1):80–4.
Rong Y, Wu Q, Tang J, Liu Z, Lv Q, Ye X, et al. Danlou Tablet May Alleviate Vascular Injury Caused by Chronic Intermittent Hypoxia through Regulating FIH-1, HIF-1, and Angptl4. Evid Based Complement Alternat Med. 2022;2022:4463108. https://doi.org/10.1155/2022/4463108.
Li L, Qu Y, Mao M, Xiong Y, Mu D. The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1alpha in the developing rat brain after hypoxia-ischemia. Brain Res. 2008;1197:152–8. https://doi.org/10.1016/j.brainres.2007.12.059.
Ghigo A, Li M. Phosphoinositide 3-kinase: friend and foe in cardiovascular disease. Front Pharmacol. 2015;6:169. https://doi.org/10.3389/fphar.2015.00169.
Ciuffreda L, Falcone I, Incani UC, Del Curatolo A, Conciatori F, Matteoni S, et al. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul. 2014;56:66–80. https://doi.org/10.1016/j.jbior.2014.07.002.
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29(12):1903–13. https://doi.org/10.1038/jcbfm.2009.102.
Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, et al. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ. 2007;14(5):1060–2. https://doi.org/10.1038/sj.cdd.4402095.
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–7. https://doi.org/10.1161/01.cir.101.6.660.
McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279(6):4782–93. https://doi.org/10.1074/jbc.M310405200.
Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22(11):2531–43. https://doi.org/10.1210/me.2008-0265.
Liu Y, Ao X, Jia Y, Li X, Wang Y, Wang J. The FOXO family of transcription factors: key molecular players in gastric cancer. J Mol Med (Berl). 2022;100(7):997–1015. https://doi.org/10.1007/s00109-022-02219-x.
Chen YF, Pandey S, Day CH, Chen YF, Jiang AZ, Ho TJ, et al. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J Cell Physiol. 2018;233(4):3660–71. https://doi.org/10.1002/jcp.26235.
McGowan SE, McCoy DM. Platelet-derived growth factor-A regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a. Respir Res. 2013;14(1):68. https://doi.org/10.1186/1465-9921-14-68.
Fluteau A, Ince PG, Minett T, Matthews FE, Brayne C, Garwood CJ, et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci Lett. 2015;609:11–7. https://doi.org/10.1016/j.neulet.2015.10.001.
McClelland Descalzo DL, Satoorian TS, Walker LM, Sparks NR, Pulyanina PY, ZurNieden NI. Glucose-Induced Oxidative Stress Reduces Proliferation in Embryonic Stem Cells via FOXO3A/beta-Catenin-Dependent Transcription of p21(cip1). Stem Cell Reports. 2016;7(1):55–68. https://doi.org/10.1016/j.stemcr.2016.06.006.
Orea-Soufi A, Paik J, Braganca J, Donlon TA, Willcox BJ, Link W. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol Sci. 2022;43(12):1070–84. https://doi.org/10.1016/j.tips.2022.09.010.
Marchelek-Mysliwiec M, Nalewajska M, Turon-Skrzypinska A, Kotrych K, Dziedziejko V, Sulikowski T, et al. The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus. Int J Mol Sci. 2022; 23(19). https://doi.org/10.3390/ijms231911611.
Shukla S, Shukla M, Maclennan GT, Fu P, Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int J Oncol. 2009;34(6):1613–20. https://doi.org/10.3892/ijo_00000291.
Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y, Guo JP, et al. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin. 2013;34(11):1386–96. https://doi.org/10.1038/aps.2013.91.
Xue M, Joo YA, Li S, Niu C, Chen G, Yi X, et al. Metallothionein Protects the Heart Against Myocardial Infarction via the mTORC2/FoxO3a/Bim Pathway. Antioxid Redox Signal. 2019;31(5):403–19. https://doi.org/10.1089/ars.2018.7597.
Acknowledgements
This work was supported by the grants from National Key Research and Development Program of China (2017YFC1700401 to YB and MZ), National Natural Science Foundation of China (81970335 and 82170285 to YB), and Science and Technology Commission of Shanghai Municipality (22ZR1423100 to JJ, and 23010500300 and 21SQBS00100 to YB).
Author information
Authors and Affiliations
Contributions
L Li, W Qi, Y Zhu, M Yin, C Chen, M Wei, Z Huang, and Z Su performed the experiments and analyzed the data. M Zhang provided technical assistance. Y Bei and J Jiang designed the study, instructed the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Editor-in-Chief Enrique Lara-Pezzi oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Qi, W., Zhu, Y. et al. Danlou Tablet Protects Against Cardiac Remodeling and Dysfunction after Myocardial Ischemia/Reperfusion Injury through Activating AKT/FoxO3a Pathway. J. of Cardiovasc. Trans. Res. 16, 803–815 (2023). https://doi.org/10.1007/s12265-023-10365-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10365-x