Abstract
Epitranscriptomics is the emerging field of research that comprises the study of epigenetics changes in RNAs. Progressing development in the field of epigenetics has helped to manage and comprehend human diseases. RNA methylation regulates all aspects of RNA functions, which are involved in the pathogenesis of human diseases. Interestingly, RNA m5C methylation is significantly linked to various types of human disease, including cardiovascular diseases (CVD). The m5C methylation is controlled by m5C regulatory proteins, which act as methyltransferase, demethyltransferase, and RNA-binding protein. Dysregulated expression in m5C regulatory proteins is significantly associated with cardiovascular disease, and these regulatory proteins have crucial roles in biological and cellular functions. This review is mainly focused on the role of RNA m5C modification in CVD and mitochondrial dysfunction. Thus, m5C will contribute to discovering the new diagnostic marker and therapeutic target for CVD.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
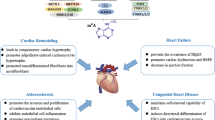
In recent years, the mortality rate has consistently increased in cardiovascular diseases (CVDs) and emerged as the primary cause of death worldwide. It has become a serious threat to humans, especially in the Western world. According to WHO statistics, 17.9 million people die each year as a result of CVD [1]. These multifactorial disorders of the heart and blood vessels include cerebrovascular disease, coronary heart disease, rheumatic heart disease, heart failure, and other conditions. The majority of CVD deaths are caused by heart attacks and strokes, and the majority of deaths occur in people under the age of 70. This disorder affects the structure and functions of the heart and its vessels. Hypertension, diabetes, and obesity are the main risk factors for CVD death. Several risk factors are reported as causative factors for CVD, where the long-term interactions of those risk factors resulted in CVD. Mechanisms underlying CVDs are driven by complex pathophysiology, which is still far from clear. Progressing development in the field of epigenetics has helped to manage and comprehend human diseases, including the prevalence of cardiovascular disorders, based on the role of genes and interactions between the environment and pathophysiological conditions [2]. Significant evidence suggests that human epigenetic patterns are defined by their lifestyle and environment throughout their lives. These patterns are a cellular memory for further environmental exposure [3]. RNA methylation modifications are extremely important in epigenetics, which involves heritable gene expression without alteration of RNA sequences [4]. Epitranscriptomics is an emerging field of biology that is involved in the posttranscriptional modifications of mRNA, rRNA, tRNA, and other RNAs. These modifications are essential and govern the RNA’s functions and fate [5].
RNA is the target for various modifications; the first RNA modification was described in the 1960s, which demonstrated pseudouridine (5-ribosyl uracil). This modification is incorporated into tRNA and rRNA [6]. More than 170 types of RNA modifications were reported, which involved the regulation of RNA expression, structure, splicing, stability, and interactions with RNA-binding proteins [7]. In contrast, the known modifications in DNA are very low, with approximately 20 found in DNA [8]. A vast range of RNA modifications was reported in the two most abundant RNAs, such as rRNA and tRNA. N6-methyladenosine (m6A) is the most common RNA modification in mRNA, which was first described by Desrosiers et al. (1974). 5-methylcytosine (m5C) is a substantial RNA modification that is found in a wide range of RNA species, including human cytoplasmic and mitochondrial mRNA, rRNA, tRNA, enhancer RNA, and noncoding RNA (Fig. 1). The m5C modification was discovered in mRNA in the 1970s [9], and it is most commonly found in tRNA [10]. In DNA as well, m5C is the common epigenetic modification that plays a regulatory role in transcription [11]. According to several recent studies, m5C modification is mostly found in the 5′ and 3′ UTRs of mRNA, with a marked peak in the translational start codon [12, 13]. m5C modifications are detected in various eukaryotic and archaeal tRNAs, except in eubacterial organisms, which do not contain m5C [14]. In various studies, the m5C modification was identified at over 8000 sites in RNAs. In particular, the role of m5C in CVD might help discover the early biomarker and therapeutic targets for preventing high mortality in humans.
Generally, the heart consumes high-energy molecules for its function, so it utilizes large amounts of ATP molecules, which are produced by mitochondria through the oxidative phosphorylation mechanism. Mitochondria are the essential organelles that regulate a variety of key cellular activities, including ATP synthesis and immunological activation. A functioning mitochondrial network is required for cardiovascular function and tolerance to pathological stresses. A considerable amount of evidence suggests that mitochondrial dysfunction plays a significant role in the etiology of several CVD. Over the last two decades, tremendous effort has gone into developing medicines that precisely target mitochondria for the treatment of CVD [15]. Moreover, several previous studies in RNA m5C regulate mitochondrial functions. Thus, in this paper, we have correlated mitochondrial dysfunction and cardiovascular diseases in RNA m5C modification. This review mainly discussed the m5C modifications’ biological significance and their roles in cardiovascular diseases, mitochondrial dysfunction, and other human diseases.
Enzymes Responsible for RNA m5C Modification
Several enzymes are involved in m5C modification (Fig. 2). Most of it is catalyzed by NOL1, NOL2, and SUN enzymes, which are members of the NSUN family in eukaryotes. The DNA methyltransferase homolog DNMT2 is also involved in m5C modification in eukaryotes, except for DNMT2, which catalyzes m5C formation in the C38 position of cytoplasmic t-RNA(Asp, Val, and Gly). The m5C has emerged as an important regulator in various aspects of gene expression, including ribosome assembly, RNA export, translation, and RNA stability [16, 17]. m5C held a privileged position among various modifications, which have been described in various datasets. It involved several RNAs and complex enzymatic machinery for synthesis that was found in all kingdoms of life [18].
Representation of enzymes responsible for RNA m5C modification, a methyl group is attached to the fifth position of the cytosine by m5C writers (methyltransferases) such as DNMT, NSUN family, and TRDM. The erasers (demethyltransferase) such as TET and ALKBH1. The readers (binding proteins) include ALYREF and YBX1
The m5C RNA methyltransferases (RNMTs) either belong to the DNA methyltransferase family such as TRDMT1/DNMT2 or belong to the NSUN family of NOL1/NOP2/ NSUN1-NSUN7 in known mammals [19, 20]. Both the NSUN and DNA methyltransferase families use S-adenosyl-L-methionine (SAM) as a donor for transferring the methyl groups to RNA and their mechanisms [17]. RNA methyltransferases such as NSUN1, NSUN4, and NSUN5 play a role in rRNA methylation. NSUN2, NSUN3, NSUN5, NSUN6, and DNMT2 methylate tRNA, especially NSUN2 and NSUN3, which methylate mitochondrial tRNAs. NSUN3 methylates at position C34 of mt-tRNAMet, and NSUN4 methylates 12 s mitochondrial rRNA, which plays a role in mitochondrial ribosome biogenesis [21]. NSUN7 methylates the enhancer RNA and NSUN2, being the most predominant methyltransferase, and has a role in methylating mRNAs, miRNAs, and lncRNAs [22,23,24,25]. NSUN2 is a well-known RNA methyltransferase that is responsible for various m5C methylations in tRNAs, such as tRNALeu(CAA) at position C34, tRNAGly at positions C40 and and C50, and tRNAAsp, tRNAGly, and tRNAVal at positions C48 and C49 [26, 27]. As has been recently reported, TRDMT1 may also be involved in the methylation of mRNAs [28].
m5C methyltransferases (writers) are included in the DNMT, NSUN, and TRDMT families, which add methyl groups; m5C demethylases (erasers) are included in the TET families; and ALKBH1, which deletes, affecting the methyl groups, and m5C binding proteins (readers) are included in ALYREF and YBX1 (Fig. 2). These are involved in the regulatory functions in RNA splicing, stability, translation, decay, and nuclear export (Fig. 3).
m5C Mapping Approaches
Earlier Dubin and Taylor [9] discovered that the methylation degree of polyA+ RNA is approximately 1.8 methyl groups per 1000 nucleotides or 4–5 methyl groups on average per molecule. Half of the methyl groups were found in standard (internal) linkage, with 10% of the residues as m5C and 40% as m6A. By using developed LC–MS/MS technology, the m5C ratio in human mRNA was reliably calculated to be about 0.02–0.09%, which was similar to the ratio reported by Dubin and Taylor [29]. However, due to the scarcity of sensitive and accurate detection tools, its profile and biological importance remain obscure.
Numerous m5C mapping approaches have been developed to detect the modified nucleotides in both the genome and transcriptome, such as anti-m5C cross-linking and immunoprecipitation (CLIP), Aza-IP, bisulfite sequencing, methylated iCLIP, m5C-RIP sequencing, nanopore sequencing, and TAWO sequencing [29]. Among these techniques, the principle of authenticating RNA m5C can be divided into three categories such as immunoprecipitation-based sequencing which uses m5C-specific antibodies or m5C methyltransferases. The second one is chemical-dependent sequencing which uses bisulfite, 5-azacytidine, or peroxotungstate and, finally, third-generation sequencing which is electronic current signal-based sequencing. These high-throughput sequencing techniques are providing precise m5C landscape contributions to the deciphering of their biological functions.
The Biological Significance of m5C in RNA
Nuclear export is a critical step in the cytoplasmic translation of mRNAs. RNA methylations, particularly m5C, are important in the process of mRNA transport from the nucleus to the cytoplasm [30]. The modified nucleotides in tRNA generally have a role in their structure, and their metabolic stabilization is well characterized [31]. The various physicochemical techniques such as NMR and circular dichroism help to reveal the structural stabilization of RNAs by m5C. Very little is known about m5c residues in rRNA and tRNA that have a specific role during the decoding of mRNA on the ribosome. In eukaryotic tRNALeu, the presence of m5C at position 34 seems to be important for their function in translation, especially the function of suppressor in vivo [32]. m5C in rRNA may also participate in peptidyl transfer and the recognition of tRNA [18]. Recently, an m5C modification at position 841 in 12 s rRNA has been identified, which is implicated by methyltransferase NSUN4, and the 12 s m5C841 modification, which has an important role in ribosome functions such as biogenesis and large subunit assembly [21]. NSUN1 and NSUN5 are involved in rRNA m5C modification; they are destined for cytoplasmic ribosomes. Depletion of m5C modification in the organism can lead to abnormal neuro and brain growth, stress resistance, mitochondrial dysfunction, frustration during gametogenesis and embryogenesis, and cell migration and tumorigenesis. The m5C sites have been discovered to overlap to several degrees with the binding sites of certain regulatory RNA proteins, such as Argonaute [13], or splicing-and mRNA decay-associated factors, such as SRSF3 or UPF [12]. In rRNA, the alteration of m5C levels increases the life span of organisms, including humans, by favoring the translation of stress response-decoding transcripts in order [33]. With the help of angiogenin, the m5C deposition modification protects tRNAs from endonucleolytic cleavage [16]. A recent discovery suggests that the ten-eleven Translocation family enzyme TET2 promotes translation through m5C tRNA methylation in vitro [34]. m5C RNA methylations are closely linked to mRNA stability, as they are involved in the dynamic production and decomposition of mRNAs, as well as maintaining the acute number of mRNAs in various physiological and pathological processes. This all suggests that m5C RNA methylation enzymes are regulating multiple nodes of the central dogma through mRNA and protein translation.
On the other hand, m5C regulators have played a pivotal role in mitochondria function. The NSUN2 has a speculated effect on the translation of mitochondrially encoded proteins, whereas the NSUN3 regulates the mitochondria oxygen consumption and protein synthesis through targeting of mt-tRNA at the m5C 34th site. In human mt-12S rRNA, NSUN4 regulates the translation and mitochondrial ribosome assembly. The m5C demethylase, ALKBH1 regulates the mitochondrial activity by targeting mitochondrial tRNALeu and tRNAMet at the m5C 34th site [29]. All these indicate that the RNA m5C regulators have a significant role in mitochondrial function.
RNA m5C Role in Mitochondrial Dysfunction
In cardiac pathology, mitochondria have a specific role and function [35]. Dysfunctional mitochondria could cause an adverse reaction in CVD, so we hypothesized that mitochondrial dysfunction caused by m5C methyltransferase silently leads to cardiovascular and associated diseases. Human mitochondrial respiration is caused by mt-tRNA nucleotide modification defects [36], and m5C modification in the D-loop region is found in bovine and human mt-RNAs [37]. The NSUN2 gene knockdown in the mouse model reveals that the knockdown mice were smaller in size when compared to wild type, had no significantly different mt-DNA copy number, and played no crucial role in mt-tRNA stability when compared to wild-type mice. Another study reveals that the NSUN4 knockout mouse heart exhibits impaired mitochondrial function and oxidative phosphorylation system (OXPHOS), thus demonstrating a heart failure phenotype (cardiac hypertrophy) and increased mortality [21]. OXPHOS produces 90% of cellular ATP in the human heart; thus, a malfunction in this pathway is a major concern in terms of cardiac energy production. A lack of m5C in the D-loop region of mt-RNA may affect intramitochondrial translation and, as a result, the assembly and function of the OXPHOS complex. NSUN2 is located in mitochondria, which causes mitochondrial tRNA, whereas downregulation of NSUN2 does not affect mt-tRNA stability and OXPHOS [22]. NSUN3 results in low mitochondrial respiration and mitochondrial protein translation [38]. The depletion of m5C modification could cause mitochondrial dysfunction, which also causes drawbacks in the stress response, gametogenesis, and embryogenesis frustration [29]. Overexpressed NSUN4 has a function in ribosomal biogenesis in mammalian mitochondria through methylation of C911 in 12S rRNA assembly [21]. Additionally, m5C methyltransferase NSUN4 regulates mitochondrial biogenesis through the MTERF4-NSUN4 protein complex [39]. Several m5C modifications were observed when sequencing m5C in human and mouse tissue samples. In particular, m5C was significantly increased in the myocardium and skeletal muscles when compared to other organs and tissues. m5C levels were enhanced in mitochondrial-associated genes with high methylation levels, as revealed by enrichment analysis [40]. Aside from that, m5C methylation in mt-tRNA mediated by NSUN3 or ALKBH1 could mediate codon recognition during mt-RNA translation initiation or elongation, thereby influencing mitochondrial function and OXPHOS [41]. Cardiomyocytes consume high energy and depend on mitochondria for their function, so methylation of m5C may play a crucial role in CVD through dysregulation of mitochondrial functional homeostasis and additionally leads to CVD being more vulnerable.
RNA m5C in Cardiovascular Diseases
Many risk factors contribute to causing CVD, where the long-term interactions of those risk factors result in death. Atherosclerosis, hypertension, diabetes, dyslipidemia, nonalcoholic fatty liver disease, and obesity are examples of metabolic diseases that endanger cardiovascular health [42]. Apart from neurological disorders recently, some emerging notable evidence links the activities of both m6A and m5C modifications in the vascular setting. Curcumin increases the expression of DNMT2 in vascular smooth muscle cells. DNMT2-mediated RNA methylation increases RNA stabilization [43]. This result suggests curcumin helps the human cardiovascular system through DNMT2 methyltransferase. Heart failure leads to the loss of cardiac homeostasis, which depends on tight regulation of gene expression. This regulation is controlled by multiple types of RNA molecules, such as mRNAs and noncoding RNAs. Furthermore, DNMT2 and NSUN2 are well-known tRNA m5C-modifying enzymes, which reduce endonucleolytic tRNA cleavage [44].
DNMT2-deficient mice showed impaired cardiac hypertrophy (Fig. 3). The noncoding RNA Rn7sk was identified as a possible defect-mediating mechanism. Rn7sk inhibits its phosphorylation of the C‐terminal domain of RNA polymerases II by the P‐Tefb transcription elongation regulator. The connections between Rn7sk and PTefb were significantly reduced in embryonic stem cells, and meRIP analysis revealed that Rn7sk from DNMT2-deficient hearts had significantly lower cytosine methylation, implying that DNMT2 methylation of Rn5-Rn7sk could regulate its interaction with PTefb. Therefore, overactive P‐Tefb can cause heart hypertrophy and improve transcription [45]. Curiously, DNMT2 has also recently been identified as an active RNA pol II in human leukemia cells with P‐TEFb, NSUN3, and hnRNPK in a complex of phosphoserine 2. The function of DNMT2 is unknown in this complex [46]. Thus, the results indicate that m5C methyl transferase enzymes play a crucial role in multiple CVDs. Therefore, targeting those unique m5C enzymes could be promising therapeutic for CVDs as well as CVDs health.
The knockout of NSUN4 in mice reveals a lack of NSUN4 leads to progressive cardiomyopathy in the heart, which is very essential for embryonic development in mice and mitochondrial translation [21, 39]. NSUN4 knockdown mice exhibit heart defects with mitochondrial dysfunction, which shows that NSUN4 plays a crucial role in controlling the ribosome assembly of mitochondria [21]. Endothelial cells play a key role in CVDs, and m5C methyltransferase NSUN2 upregulates the expression of the ICAM-1 gene, which involves a regulatory process that impairs vascular endothelial inflammation, arteriosclerosis, and hypertension. An increased level of NSUN2 improves ICAM1 translation via increasing the m5C level in ICAM1 which mediates TNF- or homocysteine-induced endothelial inflammatory responses, resulting in leukocyte adhesion to endothelial cells. Inhibition of NSUN2 reverses the vascular endothelial inflammatory response in the mice model [47]. Recently, a study by Yuan et al. found that m5C modification was observed in miR-125b biogenesis; this miRNA is likewise highly linked to atherosclerosis [48]. Furthermore, m5C methylation influences cardiac functions through modification in long noncoding RNAs such as antisense ncRNA in the cyclin-dependent kinase inhibitors locus (ANRIL), which was previously identified as a prognostic marker for myocardial infarction [49]. This indicates that m5C methylation influences both coding RNA and noncoding RNAs in cardiac pathology. The NSUN5 gene is deleted in a Williams-Beuren syndrome patient. It is a rare neurodevelopmental disorder where patients have multiple problems, including cardiovascular abnormalities, developmental delay, and intellectual disability [50]. The m5C methylation level in IL-17A mRNA coding region enhances the elevated IL-17A protein expression in the atherosclerotic mice model [51]. Moreover, recent studies demonstrated that m5c methylation is associated with abdominal aortic aneurysm, it is a risk factor for cardiovascular disease in elder males. The NSUN2 and ALYREF are significantly overexpressed in AAA patient tissue samples with elevated m5C methylation levels [52]. These indicate that m5C methylation has a potential role in cardiovascular pathogenesis. Further research into m5C modification in CVD pathology may aid in the identification of drug targets and early biomarkers.
Conclusion
m5C is a remarkable RNA modification that is involved in various human diseases, including high-mortality diseases such as cardiovascular and several types of cancer. The m5C modification plays a potential role in CVD, which may provide a novel therapeutic for cardiovascular disease and hypertension. Dysregulation of RNA m5C modification may result in a variety of issues for organisms, including mitochondrial dysfunction, and cause different types of human disease. Further studies on the biological function and the role of RNA m5C modification in diseases could help discover early biomarkers. Therefore, RNA m5C and its enzymes could be a great biomarker and promising therapeutic target for the treatment of mitochondrial dysfunction, cardiovascular disease, and other human diseases.
Abbreviations
- Asp:
-
Asparagine
- Aza-IP:
-
5-Azacytidine-mediated RNA immunoprecipitation
- CLIP:
-
Cross-linking and immunoprecipitation
- CVD:
-
Cardiovascular disease
- DNMT2:
-
DNA methyltransferase 2
- DS:
-
Dubowitz syndrome
- Gly:
-
Glycine
- Leu:
-
Leucine
- lncRNA:
-
Long noncoding RNA
- m5C:
-
5-Methylcytosine
- m5C-RIP:
-
5-Methylcytosine-RNA immunoprecipitation
- m6A:
-
N6-Methyladenosine
- Me-RIP:
-
Methylated RNA immunoprecipitation
- Met:
-
Methionine
- miRNA:
-
Micro RNA
- mRNA:
-
Messenger RNA
- mt-tRNA:
-
Mitochondrial transfer RNA
- RNMT:
-
RNA methyl transferase
- rRNA:
-
Ribosomal RNA
- SAM:
-
S-Adenosyl-L-methionine
- TAWO-seq:
-
TET-assisted WO-seq
- TCGA:
-
The Cancer Genome Atlas
- TET family:
-
Ten-eleven translocation methylcytosine dioxygenases
- TNF-α:
-
Tumor necrosis factor-α
- TRDMT1:
-
TRNA aspartic acid methyltransferase 1
- tRNA:
-
Transfer RNA
- UTR:
-
Untranslated region
- Val:
-
Valine
- WHO:
-
World Health Organization
References
Anonymous, The top 10 causes of death by WHO, 2020. Retrieved from; https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54.
Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–9.
Jones P, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–41.
Stellos K. The rise of epitranscriptomic era: implications for cardiovascular disease. Cardiovasc Res. 2017;113:e2–3.
Cohn WE. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J Biol Chem. 2017;235:1488–98.
Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2018;46:303–7.
Korlach J, Turner SW. Going beyond five bases in DNA sequencing. Curr Opin Struct Biol. 2012;22:251–61.
Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–68.
Trixl L, Lusser A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip Rev RNA. 2019;10:e1510.
Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–76.
Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, Lusser A. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18(1):1.
Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–33.
Motorin Y, Lyko F, Helm M. 5-methylcytosine in RNA: detection, enzymatic formation, and biological functions. Nucleic acids Res. 2010;38:1415–30.
Brown DA, Perry JB, Allen ME, et al. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14(4):238–50.
Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5.
Bohnsack KE, Höbartner C, Bohnsack MT. Eukaryotic 5-methylcytosine (m5C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes (Basel). 2019;10(2):102.
Motorin Y, Lyko F, Helm M. 5-methylcytosine in RNA: detection, enzymatic formation, and biological functions. Nucleic acids Res. 2010;38(5):1415–30.
Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, et al. Methylation of tRNA Asp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–8.
Reid R, Greene PJ, Santi DV. Exposition of a family of RNA m5C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27:3138–45.
Metodiev MD, Spåhr H, Loguercio Polosa P, Meharg C, Becker C, Altmueller J, Habermann B, Larsson NG, Ruzzenente B. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110.
Van Haute L, Lee SY, McCann BJ, Powell CA, Bansal D, Vasiliauskaitė L, Garone C, Shin S, Kim JS, Frye M, Gleeson JG, Miska EA, Rhee HW, Minczuk M. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;47(16):8720–33.
Sun Z, Xue S, Xu H, Hu X, Chen S, Yang Z, Yang Y, Ouyang J, Cui H. Effects of NSUN2 deficiency on the mRNA 5-methylcytosine modification and gene expression profile in HEK293 cells. Epigenomics. 2019;11:439–53.
Li Y, Li J, Luo M, Zhou C, Shi X, Yang W, Lu Z, Chen Z, Sun N, He J. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 2018;430:57–66.
Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, Li A, Wang X, Bhattarai DP, Xiao W, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–25.
Brzezicha B, Schmidt M, Makalowska I, Jarmolowski A, Pienkowska J, Szweykowska-Kulinska Z. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Res. 2006;34:6034–43.
Tuorto F, Liebers R, Musch T, Schaefer M, Hofmann S, Kellner S, Frye M, Helm M, Stoecklin G, Lyko F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19:900–5.
Xue S, Xu H, Sun Z, Shen H, Chen S, Ouyang J, Zhou Q, Hu X, Cui H. Depletion of TRDMT1 affects 5-methylcytosine modification of mRNA and inhibits HEK293 cell proliferation and migration. Biochem Biophys Res Commun. 2019;520:60–6.
Chen YS, Yang WL, Zhao YL, Yang YG. Dynamic transcriptomic m5 C and its regulatory role in RNA processing. Wiley Interdiscip Rev RNA. 2021;12(4):e1639.
Li X, Meng Y. Expression and prognostic characteristics of m5 C regulators in low-grade glioma. J Cell Mol Med. 2021;25(3):1383–93.
Helm M. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006;34:721–33.
Strobel MC, Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol Cell Biol. 1986;6:2663–73.
Schosserer M, Minois N, Angerer TB, Amring M, Dellago H, Harreither E, Calle-Perez A, Pircher A, Gerstl MP, Pfeifenberger S, et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat Commun. 2015;6:6158.
Shen H, Ontiveros RJ, Owens MC, Liu MY, Ghanty U, Kohli RM, Liu KF. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J Biol Chem. 2021;296:100087.
Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med. 2018;50(2):121–7.
Lin H, Miyauchi K, Harada T, Okita R, Takeshita E, Komaki H, Fujioka K, Yagasaki H, Goto YI, Yanaka K, et al. CO2-sensitive tRNA modification associated with human mitochondrial disease. Nat Commun. 2018;9:1875.
Suzuki T, Nagao A, Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 2011;45:299–329.
Trixl L, Amort T, Wille A, Zinni M, Ebner S, Hechenberger C, Lusser A. RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cell Mol Life Sci. 2018;75(8):1483–97.
Spåhr H, Habermann B, Gustafsson CM, Larsson NG, Hallberg BM. Structure of the human MTERF4–NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc Natl Acad Sci USA. 2012;109:15253–8.
Huang T, Chen W, Liu J, Gu N, Zhang R. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat Struct Mol Biol. 2019;26(5):380–8.
Kawarada L, Suzuki T, Ohira T, Hirata S, Miyauchi K, Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45(12):7401–15.
Zhang B, Jiang H, Dong Z, Sun A, Ge J. The critical roles of m6A modification in metabolic abnormality and cardiovascular diseases. Genes Dis. 2020;8(6):746–58.
Lewinska A, Wnuk M, Grabowska W, Zabek T, Semik E, Sikora E, Bielak-Zmijewska A. Curcumin induces oxidation-dependent cell cycle arrest mediated by SIRT7 inhibition of rDNA transcription in human aortic smooth muscle cells. Toxicol Lett. 2015;233:227–38.
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, He C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–99.
Ghanbarian H, Wagner N, Polo B, Baudouy D, Kiani J, Michiels JF, Wagner KD. Dnmt2/Trdmt1 as mediator of RNA polymerase II transcriptional activity in cardiac growth. PLoS ONE. 2016;11(6):e0156953.
Cheng JX, Chen L, Li Y, Cloe A, Yue M, Wei J, Vardiman JW. RNA cytosine methylation and methyltransferases mediate chromatin organization and 5-azacytidine response and resistance in leukaemia. Nat Commun. 2018;9(1):1163.
Luo Y, Feng J, Xu Q, Wang W, Wang X. NSun2 deficiency protects endothelium from inflammation via mRNA methylation of ICAM-1. Circ Res. 2016;118(6):944–56.
Yuan S, Tang H, Xing J, et al. Methylation by NSun2 represses the levels and function of microRNA 125b. Mol Cell Biol. 2014;34(19):3630–41.
Yin L, Zhu X, Novák P, Zhou L, Gao L, Yang M, Zhao G, Yin K. The epitranscriptome of long noncoding RNAs in metabolic diseases. Clin Chim Acta. 2021;515:80–9.
Martens MA, Wilson SJ, Reutens DC. Williams syndrome: a critical review of the cognitive, behavioral and neuroanatomical phenotype. J Child Psychol Psychiatry. 2008;14:576–608.
Wang N, Tang H, Wang X, Wang W, Feng J. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys Res Commun. 2017;493(1):94–9. https://doi.org/10.1016/j.bbrc.2017.09.069.
He Y, Zhang H, Yin F, et al. Novel insights into the role of 5-methylcytosine RNA methylation in human abdominal aortic aneurysm. Front Biosci (Landmark Ed). 2021;26(11):1147–65. https://doi.org/10.52586/5016.
Acknowledgements
We acknowledge the contribution of Dr. Anitha Roy, who was involved in the editing and formatting of the manuscript.
Funding
This work was supported by the Science and Engineering Research Board (SERB), Government of India (EEQ/2019/000411).
Author information
Authors and Affiliations
Contributions
K. Balachander undertook literature mining from various reputed databases, drafted the manuscript, and prepared illustrations. Dr. A. Paramasivam and Dr. J. Vijayashree Priyadharsini gave the concept for this article and are responsible for manuscript proofreading and validating the entire manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balachander, K., Priyadharsini, J.V., Roy, A. et al. Emerging Role of RNA m5C Modification in Cardiovascular Diseases. J. of Cardiovasc. Trans. Res. 16, 598–605 (2023). https://doi.org/10.1007/s12265-022-10336-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10336-8