Abstract
Several prior studies have highlighted the promise of mesenchymal stem cells (MSCs) as tools for treating myocardial infarction (MI) patients. While MSCs were initially thought to mediate post-MI repair through differentiation and replacement of injured cells, they are now thought to function by releasing exosomes carrying important cargos which can prevent apoptosis and facilitate revascularization in the context of MI. Herein, we comprehensively survey prior preclinical studies examining MSC-derived exosomes (MSC-Exos) utility for the repair of MI-related tissue injury. In total, 24 relevant studies were identified in the PubMed, Web of Science, Embase, and Cochrane Library databases as per the PRISMA guidelines. In most studies, exosome-treated rodents exhibited improved cardiac function and angiogenesis together with decreased apoptotic cell death. MSC-Exos thus offer beneficial therapeutic efficacy when treating MI injury. However, further work will be necessary to standardize experimental preclinical models and to validate these results.
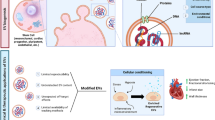
Graphical abstract
This systematic review provides a comprehensive overview of previous preclinical studies on the utility of exosomes derived from mesenchymal stem cells (MSCs) in the repair of myocardial infarction (MI) injury.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a leading cause of global morbidity and mortality, despite major advances in its prevention and treatment in recent years [1]. A primary goal of MI treatment is the salvaging and repair of infarcted myocardial tissue. However, even with rapid coronary intervention, a majority of patients nonetheless experience cardiomyocyte apoptosis, ventricular wall thinning, cavity dilatation, and eventual heart failure (HF) [2]. Many animal studies and clinical trials have explored the efficacy of bone marrow-derived mesenchymal cells (MSCs) as tools to facilitate MI repair [3,4,5,6], yet the efficacy of these cells and the mechanisms governing their purported efficacy remain controversial. These MSCs may function by undergoing differentiation into cell types that can replace those damaged in the context of MI. Other evidence, however, suggests that these MSCs primarily function in a paracrine manner [7, 8], secreting factors including proteins, lipids, nucleic acids, and extracellular vesicles (EVs) that can modulate the biological activity of recipient cells [9,10,11]. EVs are clustered into four major classes based upon their size: exosomes (50–150 nm), microvesicles (100–1,000 nm), large oncosomes (1,000–10,000 nm), and apoptotic bodies (100–5,000 nm) [12], with exosomes being the most thoroughly studied of these particle types.
Exosomes are lipid bilayer-enclosed EVs produced by virtually all cells and are derived from multivesicular bodies that are generated via endosomal membrane invagination [13,14,15]. Exosomes can carry a range of functionally important cargos including proteins, RNAs, and DNA molecules. Multiple online databases including ExoCarta (http://exocarta.org) and Vesiclepedia (http://microvesicles.org) have sought to catalog exosomal cargos in a range of contexts. Exosomes have also been highlighted as important regulators of MI and related processes in recent years, with MSC-derived exosomes (MSC-Exos) offering great promise for treating MI, HF, and dilated cardiomyopathy [16,17,18,19]. Intramyocardial or intravenous exosome delivery has been shown to promote proliferation, suppress apoptosis, disrupt fibrosis, facilitate angiogenesis, and suppress oxidative stress, thereby exerting cardioprotective activity [20].
While there has been substantial variability with respect to the goals and models used in prior analyses of MSC-Exos as regulators of MI, studies generally follow four key steps: exosome isolation and characterization, coronary artery ligation, exosome delivery, and analyses of infarct size and cardiac function. In an effort to more broadly understand the therapeutic value of these stem cell–derived exosomes, the present systematic review was conducted to summarize the findings of prior in vivo analyses assessing the efficacy of MSC-Exos in the treatment of MI.
Materials and Methods
Search Strategy
The PubMed, Web of Science, Embase, and Cochrane Library databases were searched for all studies published as of 2 December 2020. Search terms were as follows: “Mesenchymal stem cell,” “Exosome,” and “Myocardial infarction.”
Inclusion and Exclusion Criteria
Studies eligible for inclusion were (1) studies of the treatment of MI using bone marrow MSC-Exos in animal models, and (2) studies that were described in English. Articles were excluded if they were (1) reviews of meta-analyses, (2) conference abstracts, (3) non-experimental studies or studies lacking complete information, or (4) experimental studies using exosomes derived from cells other than bone marrow MSCs.
Study Quality Evaluation
The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment tool was used to examine the risk of bias associated with included studies [21], with the results of these quality analyses being shown in the Supplementary Material Table 1.
Data Extraction
Data were individually extracted from all studies, including information pertaining to both study design and outcomes. Recorded information included sample size, the sex and species of study model animals, the animal model system utilized, the comparison groups within the study, and the treatment details for individual groups. Treatment-related details included exosome or control sources, treatment concentrations, volume, delivery method, and frequency, and the timing of euthanasia for study animals. Both qualitative and quantitative details regarding study outcomes were noted where possible. The impact of MSC-Exos treatment on cellular apoptosis, proliferation, migration, and autophagy was also assessed. When details were not present within included studies, efforts were made to contact the authors of the original manuscript.
Data Analysis
Study outcomes were primarily evaluated in a qualitative manner owing to a lack of consistent quantitative data necessary to permit pooled analysis.
Results
Literature Search Results
Our initial search strategy initially identified 748 studies, of which 155 were duplicates. Following title and abstract screening, 536 of the remaining studies were excluded, while the remaining 57 were subjected to full-text review, with 24 being retained for inclusion in the final systematic review (Fig. 1).
Systematic Review
Overall, 24 studies were determined to be eligible for inclusion in this systematic review [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Details pertaining to the sample size, gender and speciesused in each study are shown in Table 1. Table 2 compiles the comparison groups used in different studies, while Tables 3 and 4 respectively detail the treatment volume, delivery, frequency of delivery, and timing of euthanasia for animals in the exosome and non-exosome treatment groups. A brief summary of the therapeutic outcomes in the different treatment groups is provided in Table 5. Details of the cells used in each study are shown in Supplementary Material Table 2.
Exosome Sources
All exosomes in the included studies were isolated from bone marrow–derived MSCs. In three of these studies [32, 39, 41], MSCs were purchased from commercial sources, while in one study they were derived from human bone marrow [40]. One study did not report the source of MSCs [37].
Animal Model Establishment
Included studies established rodent models of MI in rats or mice via the ligation of the left anterior descending artery (LAD). Briefly, animals were anesthetized, connected to a ventilator with an orotracheal tube, and left thoracotomy was performed between the 3rd and 4th intercostal space under sterile conditions. The heart was exposed and the LAD was then permanently ligated with a suture [46, 47].
Treatment Delivery
Various studies have shown the intramyocardial or intravenous injection of exosomes to be linked to pro-angiogenic, anti-fibrotic, and/or anti-apoptotic effects in infarcted myocardial tissue. Of the included studies, 20 [22, 24,25,26,27, 30,31,32,33,34,35,36,37,38,39,40,41, 43,44,45] conducted intramyocardial MSC-Exos administration, although the exact sides of injection differed among studies, and included 3–4 sites around the infarcted region, the left ventricular wall, or a site close to the site of arterial ligation. Tail vein injection was the mode of MSC-Exos delivery in 3 studies [28, 29, 42]. In another study [23], exosomes and MSCs were prepared into monolayer cell sheets and then transferred to the infarcted myocardial region.
Echocardiographic Analysis Results
Of the 24 included studies, 21 assessed cardiac function following MSC-Exos treatment [23,24,25,26,27,28,29,30,31,32, 34, 35, 37,38,39,40,41,42,43,44,45]. Echocardiographic analysis indicated that administration of MSC-Exos and exosomes derived from microRNA (miRNA) overexpressed or drug-pretreated MSCs were associated with a reduction in MI-related left ventricular (LV) dilation. These treatments were also related to improvements in left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) relative to other treatments and were linked to lower left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD) values, consistent with the ability of MSC-Exos to enhance cardiac function in animal models of MI.
Histological Analysis
TTC staining of myocardial tissue was performed in 2 studies after MSC-Exos treatment, and the results showed a reduction in myocardial infarct area in rats in the MSC-Exos group compared with the MI group [33, 39]. Histological analysis of infarcted cardiac tissue by HE staining was performed in detail in 8 studies after MSC-Exos treatment [25, 33, 34, 36, 38,39,40, 42]. Samples from MI model groups exhibited extensive cardiomyocyte necrosis, irregularly arranged myocardial cells, fractured myocardial fibers, and extensive inflammatory cell infiltration not present in sham control rodents. In contrast, these changes were ameliorated in the MSC-Exos treatment groups relative to the MI model group. Masson’s trichrome staining of infarcted heart tissues was performed in 17 studies [22,23,24,25,26, 29, 31, 33,34,35, 38,39,40,41,42,43, 45], with collagenous fibers appearing in blue and vascular smooth muscle cells appearing in red. MI model rodents exhibited cardiomyocyte swelling, collagenous fiber disorder, a loss of nuclei, and coagulative necrosis within the infarcted zone that was not evident in sham-operated control animals. MSC-Exos treatment was associated with the abatement of these MI-related pathological changes. In some studies [22,23,24,25,26, 29, 31, 34, 38, 40,41,42,43, 45], quantitative analysis of the LV fibrotic area and the Masson’s trichrome staining results were performed, revealing that MSC-Exos treatment was associated with significantly better outcomes. In analyses conducted by Huang et al. [38], Huang et al. [34], and Sun et al. [40], the collagen area was significantly reduced in therapy-treated groups as evidenced by Sirius-Red staining results.
Xu et al. [36] additionally analyzed macrophage subsets within ischemic cardiac tissues and examined the impact of exosomes derived from pro-inflammatory MSCs on MI-related injury. Through a series of immunohistochemical staining assays, they determined that exosome treatment was associated with reductions in M1 marker (CD11c) staining and with increases in M2 marker (CD206) staining, confirming that LPS-pretreated MSC-Exos significantly enhanced CD206 expression and suppressed CD11c expression relative to that observed in MSC-Exos and PBS control groups. These data suggested that LPS-pretreated MSC-Exos were able to suppress post-MI inflammation at least in part via driving macrophages towards an M2 phenotype in MI model mice.
Western Blot Analysis
Nine studies performed Western blot assays of myocardial tissues [22, 32, 33, 35,36,37, 39, 41, 43]. Zou et al. [33] detected the expressions of Apaf1 and autophagy-associated protein 13 (ATG13) in myocardial tissues of various groups of rats and further confirmed that MSC-Exos could effectively inhibit myocardial tissue damage caused by MI. Li et al. [35] and Xiao et al. [32] examined the expressions of LC3-I, LC3-II, and autophagic fluxes, respectively, and found that MSC-Exos could inhibit cardiomyocyte autophagy in MI rats through the delivery of miRNA. In three other studies [36, 37, 43], MSC-Exos treatment was found to reverse the expression of apoptosis-related proteins such as Bax and Cleaved-caspase 3 in the myocardial tissue of MI rats. Li et al. [39] evaluated the effect of MSC-Exos on myocardial tissue fibrosis, and Western blot results showed that compared with the MI group, the expression levels of collagen-I, collagen-III, and fibronectin were reduced in the MSC-Exos group. Liu et al. [41] found that MSC-Exos treatment significantly reversed the expressions of fission 1 and mitofusin 2, indicating that MSC-Exos treatment was able to inhibit mitochondrial fragmentation. In addition, the expression of Mecp2 gene which was targeted by miR-22 was only examined in the study of Feng et al. [22] to confirm the ability of MSC-Exos to exert cardioprotective effects through miR-22 targeting Mecp2.
Angiogenesis
Following MI-induced injury, cardiomyocyte apoptosis and death occur, and the cardiac tissue remains in a reparative state that can lead to ventricular remodeling and scar formation in the infarcted region, with neovascularization in the infarcted region being particularly important [48,49,50]. In some studies [31, 34, 38, 40], immunofluorescent staining for arterioles and capillaries was performed with antibodies specific for α-SMA and CD31 in order to assess the impact of MSC-Exos treatment on angiogenesis and associated tissue repair. Huang et al. compared the relative effects of exosomes derived from MSCs that were or were not pretreated with atorvastatin (ATV) and found that MI model rats treated with MSC-Exos derived from ATV-pretreated cells exhibited increased arteriole density [38]. Huang et al. also found that arteriole density was significantly higher in animals treated with MSCs and MSC-Exos relative to in animals treated with MSCs alone [34]. Sun et al. observed increased border zone capillary density in ischemic heart tissues from animals treated with exosomes derived from young donors relative to those of animals treated with exosomes from older donors [40]. Zhu et al. also found that arteriole density was enhanced following treatment with exosomes from hypoxia-treated MSCs relative to exosomes isolated from MSCs under normoxic conditions [31]. Kang et al. assessed neovascularization by examining the expression of von Willebrand factor (vWF) and found that CR4-overexpressing MSC-Exos treatment significantly increased numbers of vWF-positive vessels relative to control treatment [23]. Three additional studies assessed angiogenesis in the ischemic border zone using lectin as an immunofluorescent tool for marking endothelial cells [24,25,26]. Sun et al. observed enhanced angiogenesis in animals treated with exosomes derived from HIF-1α-overexpressing MSCs relative to those from control MSCs, suggesting that HIF-1α was able to promote angiogenesis and thereby exert cardioprotective activity [24]. Teng et al. also analyzed rats treated with MSC-Exos and observed significant increases in the numbers of new capillaries relative to those in animals treated with PBS or exosome-depleted culture media [25]. Zhang et al. also showed that animals treated with cardiac stem cells (CSCs) that had been MSC-Exos preconditioned exhibited increased numbers of arterioles and capillaries, suggesting enhanced neovascularization within ischemic heart tissues [26].
These in vivo results were consistent with in vitro data demonstrating that MSC-Exos had positive effects on cell proliferation, migration, and tube formation. In total, seven studies detailed positive impacts of MSC-Exos on cell proliferation, migration, and endothelial cell vessel formation [23,24,25, 30, 31, 38, 40]. Zhang et al. explored the impact of MSC-Exos treatment on CSC angiogenesis in vitro [26]. Together, these data indicate that MSC-Exos can enhance cell proliferation, migration, and tube formation, thereby enhancing neovascularization.
The Anti-apoptotic Activity of MSC-Exos
Suppressing the apoptotic death of myocardial cells after MI is vital to preventing widespread HF [51,52,53]. In total, 17 of the included studies described the effects of MSC-Exos treatment on MI-related cardiomyocyte apoptosis [22, 24, 28, 29, 31,32,33,34, 36,37,38,39,40,41, 43,44,45]. Of these, some studies favored TUNEL staining [22, 28, 29, 31,32,33,34, 36,37,38,39,40,41, 43], revealing a significant reduction in the number of apoptotic cells in the myocardial border zone following MSC-Exos treatment relative to PBS treatment.
In 13 studies, the messenger RNA (mRNA) or miRNAs present within MSC-Exos were evaluated as regulators of post-MI apoptosis [22, 24, 28, 29, 31, 32, 37, 39,40,41, 43,44,45]. Yu et al. [24] found that exosomes derived from MSCs overexpressing GATA-4 were able to effectively enhance myocardial contractile function while reducing infarct size in an MI model, with miR-19a being present at much higher levels in these exosomes. This miRNA was able to function by inhibiting multiple phosphatases. He et al. [28] also determined that GATA-4-MSC-Exos treatment suppressed the apoptotic death of hypoxic cardiomyocytes more effectively than control exosomes, with similar efficacy being observed in vivo. These results were consistent with those of Yu et al. [24], suggesting a similar mechanism of action. Feng et al. [22] also determined that ischemia-pretreated and miR-22-enriched MSC exosomes were able to suppress apoptosis in the ischemic myocardium, with the anti-apoptotic effects of miR-22 being mediated by its ability to target Mecp2 such that in a murine MI model, the administration of these MSC-Exos suppresses infarct size and enhanced myocardial fibrosis.
Consistent with these in vivo results, 11 articles [23, 24, 28, 29, 31, 33, 37, 40, 41, 43, 45] found that MSC-Exos had a positive impact on hypoxia-induced apoptotic death in cardiomyocytes. Additionally, two studies [31, 44] demonstrated the ability of MSC-Exos to inhibit H2O2-induced cardiomyocyte apoptosis. In these reports, MSC-Exos were able to suppress apoptosis-related gene expression, thereby protecting against cardiomyocyte injury.
The Anti-inflammatory, Anti-fibrotic, and Anti-autophagic Properties of MSC-Exos
Inflammation is a key mechanism that governs myocardial damage following MI, with secreted cytokines and chemokines additionally serving to recruit multiple immune cell types to the infarcted region [54,55,56]. Xu et al. [36] found that exosomes derived from MSCs treated with low levels of LPS were better able to promote M2 macrophage polarization and to attenuate post-MI inflammation and cardiomyocyte apoptosis in vitro and in vivo relative to control MSC-Exos. Consistent with these findings, Teng et al. [25] determined that MSC-Exos were able to suppress T cell proliferation in vitro and to inhibit ventricular infiltration by inflammatory cells in vivo.
Three studies explored the impact of MSC-Exos treatment on autophagic activity in the context of MI [32, 33, 35], revealing that exosome treatment was associated with increases in the expression of autophagy-related genes such as ATG13 in H9c2 cells and rats after MI modeling, with these changes coinciding with a reduction in the expression of apoptosis-related genes such as Apaf1 [33]. In other reports [32, 35], the overexpression of miR-301 or miR-125b within MSC-Exos was found to protect against MI by inhibiting autophagy.
In vitro, Shao et al. reported the capacity of MSC-Exos to inhibit the transformation of fibroblast to myofibroblast, which explains why MSC-Exos injection could reduce cardiac fibrosis after MI [27].
Discussion
MI is the most common cardiovascular disease-related cause of death [57]. As cardiomyocytes largely lack the capacity for self-renewal, many researchers have sought to leverage stems cells for cardiac tissue repair. However, stem cell transplantation efforts have exhibited only limited efficacy to date and have been hampered by the relatively limited survival of transplanted cells as well as by immune responses to transplantation [58,59,60]. Cell-free regenerative medicine approaches have evolved rapidly over the last decade, in part owing to the discovery that MSC-Exos are primary mediators of the beneficial paracrine signaling activity of MSCs in the context of regenerative treatment for MI patients, suggesting that these MSC-Exos may represent a viable alternative to direct MSC transplantation [61]. Importantly, these exosomes can carry substantial quantities of proteins and nucleic acid cargos that are internalized by recipient cells, thus allowing for efficient macromolecule delivery. The present systematic review clearly demonstrates that MSC-Exos treatment holds promise as a therapeutic strategy in the context of MI, as evidenced by preclinical findings indicating that these exosomes can enhance cardiac function, suppress myocardial apoptotic cell death, inhibit inflammation, and augment neovascularization following MI. In analyzed studies, these treatment-related effects were shown to be superior to those associated with control treatments with saline solution or MSCs. Overall, these findings thus offer robust evidence for the potency of cell-free regenerative medicine as an approach to MI patient treatment.
MSC-Exos-based Therapeutic Cardiac Regeneration
Many different stem cell types have previously been explored in clinical contexts pertaining to regenerative medicine, including bone marrow mononuclear cells, bone marrow MSCs, embryonic stem cells, cardiac stem cells, and induced pluripotent stem cells [62, 63]. Bone marrow MSCs offer great promise for the treatment of myocardial ischemia owing to the ease with which they can be isolated, cultured, and expanded while maintaining important stem cell properties [64]. Researchers initially hypothesized that transplanted MSCs would be able to differentiate into cardiomyocytes and vascular endothelial cells, but more recent evidence suggests that the majority of transplanted cells die within a month under hypoxic conditions within the ischemic microenvironment, while their differentiation cannot be effectively controlled [65].
Paracrine mechanisms are now thought to be the primary mechanism whereby MSCs exert beneficial effects on target cells, with exosomes being among the most important secreted paracrine factors derived from these cells. Exosomes can interact with nearby cells or can enter into the systemic circulation and can be internalized via endocytosis or fusion with the plasma membrane of target cells, leading to the release of exosomal contents into the cytoplasm [66]. Exosomes are believed to be important mediators of cell–cell communication, and the miRNAs present within many exosomes represent a key form of information that is readily transmitted via this mechanism. Exosomes and miRNAs derived therefrom are important regulators of the function of cardiomyocytes, endothelial cells, vascular smooth muscle cells, and inflammatory cells, thus contributing to the development and progression of MI [67].
The Pro-angiogenic Effects of MSC-Exos Treatment
Exosomes can carry proteins, miRNAs, and other molecules capable of promoting neovascularization after MI. For example, miR-132, miR-221, and miR-130 have been shown to drive vascular smooth muscle cell proliferation, differentiation, migration, and angiogenesis [68,69,70]. These miRNAs primarily impact angiogenesis via the regulation of related signaling molecules and pathways including VEGF, HIF, PTEN, and Akt/eNOS [71]. Ma et al. found that exosomes derived from MSCs overexpressing miR-132 were better able to promote angiogenesis than were exosomes from control MSCs [30]. In contrast, miR-221-3p levels were elevated in MI patients and correlated with troponin and left ventricular systolic function [72]. Recent clinical trials of antisense drugs targeting miR-132 have been conducted in HF patients and have shown promising results, and clinical trials using miRNAs in patients with MI are expected in the near future [73].
There have been relatively few studies to date examining changes in the properties of MSC-Exos following pharmacological intervention. In one report, Huang et al. found that atorvastatin-pretreated MSC-Exos were better able to drive endothelial cell migration, tube formation, and survival relative to control MSC-Exos [38]. Studies of the relative therapeutic impact of administering both MSCs and MSC-Exos or MSCs alone have shown that the combination approach is more efficacious than MSC transplantation in isolation [34]. While the therapeutic benefits of MSC-Exos have been detected in multiple studies, the results may be specific to the particular exosome source used in a given analysis, and all of these findings remain to be replicated in independent reports.
While the exact mechanisms whereby MSC-Exos treatment enhances cardiac outcomes following MI remain to be determined, it is important to note that several of the studies in the present systematic review found that these exosomes functioned through a multi-faceted mechanism associated with the enhancement of proliferation, migration, and angiogenesis coinciding with reductions in autophagic and apoptotic activity.
The Anti-apoptotic Effects of MSC-Exos Treatment
As cardiomyocytes exhibit a very limited capacity for regeneration, MI typically results in extensive cardiomyocyte death, ventricular remodeling, HF, and even mortality in severe cases. In some reports, exosomes derived from genetically modified MSCs were shown to exhibit therapeutic efficacy in the treatment of MI [22, 24, 28, 29, 31, 32, 37, 39,40,41, 43,44,45]. Macrophage migration inhibitory factor (MIF) is an inflammatory cytokine that has been shown to activate the AMPK pathway, leading to the upregulation of SIRT1 and the downregulation of pro-apoptotic p53, thereby suppressing the apoptotic death of exposed cells [74]. For example, Liu et al. found that exosomes derived from MSCs overexpressing MIF were better able to promote post-MI myocardial repair relative to control MSC-Exos [41]. Yu et al. also found that exosomes derived from GATA-4 overexpressing MSCs were able to enhance survival and maintain mitochondrial membrane potential values in hypoxia treated cardiomyocytes in vitro, in addition to enhancing myocardial contractile function and decreasing the infarct size in a murine MI model [24]. They further determined that these GATA-4 overexpressing MSC-Exos contained elevated levels of miR-19a, which was able to suppress many phosphatases and thereby preserve MI.
MSC-Exos containing miR-185, miR-210, miR-22, miR-338, miR-19a/19b, and miR-125b have been reported to inhibit cardiomyocyte apoptosis after MI [22, 29, 39, 43,44,45]. In an animal model of MI, MSC-Exos overexpressing miR-185 inhibited cardiomyocyte apoptosis by suppressing SOCS2 expression [39]; MiR-210 exerted cardioprotective effects by targeting PI3K/AKT and p53 signaling [43]. In clinical trials, miR-22 and miR-185 were reported to be increased in the supernatant after platelet aggregation and disappeared in the thrombus of patients with MI, thus demonstrating their association with thrombosis during MI [75]. In contrast, miR-210 was reported to have contributed to the growth of atherosclerosis and plaque instability [76]. MiR-19b expression was upregulated in plasma from patients with ST-segment elevation MI, while miR-19b expression peaked earlier than troponin in a study of 280 patients, suggesting a possible use as a diagnostic biomarker for MI with ST-segment elevation [77].
Potential Confounders and Limitations
While the above studies highlight the therapeutic promise of MSC-Exos treatment for MI-related tissue injuries, much of this research is in its early stages, and its clinical relevance remains to be defined. All included studies were conducted using rat or mouse models, consistent with a need for further progress focused on large animal studies and subsequent clinical trials.
Many exosome-based phase I/II clinical trials are currently underway, particularly in antitumor therapy [78]. However, clinical trials of exosomes for the treatment of MI have not yet started. Many challenges remain before its clinical application: (1) the isolation of exosomes is complicated and standardized procedures are lacking; (2) the duration of exosome exertion in the infarcted myocardium cannot be assessed; (3) assessment of MSC- Exos in a clinical setting, such as their biodistribution, metabolism, excretion, etc., cannot be performed; (4) lack of guidelines for large-scale production and quality control; and (5) optimal dose/concentration and mode of administration of MSC-Exos remain to be determined. In addition, the safety, tolerability, and efficacy of exosomes for the treatment of MI still need to be elucidated through preclinical studies before entering clinical trials [79, 80].
Despite the formulation of guidelines aimed at standardizing the improving overall reporting pertaining to experimental animal use, such as the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, our analysis underscores the need for improvements in the methodological reporting for many studies [81, 82]. In addition, while the included studies appeared to be methodologically sound, the SYRCLE risk of bias tool suggested all of these studies have an unclear risk of bias for most analyzed domains owing to a lack of clear documentation pertaining to many relevant parameters. As such, there is a clear need for better methodological documentation in future studies in an effort to improve the reliability and credibility of the resultant publications [81, 82].
Owing to a lack of uniformity with respect to the reporting of quantitative results, we were unable to conduct aggregate meta-analyses of the findings of these prior studies. As such, future research efforts will be essential to improve and standardize the statistical analysis and reporting approaches in related studies in order to permit more robust meta-analyses of the underlying data.
Conclusion
In summary, the results of the present systematic review suggest that MSC-Exos offer therapeutic value as a tool for the treatment or inhibition of MI-associated cardiac tissue damage. Animals in the majority of identified studies exhibited exosome treatment-related improvements in cardiac function, together with increased angiogenic activity and suppression of cardiomyocyte apoptosis. This review thus serves as a comprehensive analysis of recent preclinical animal model data on this topic and underscores the therapeutic benefit of MSC-Exos for the treatment of MI injury.
Abbreviations
- ATV:
-
Atorvastatin
- CSCs:
-
Cardiac stem cells
- EVs:
-
Extracellular vesicles
- HF:
-
Heart failure
- LAD:
-
Left anterior descending artery
- lncRNAs:
-
Long non-coding RNAs
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular fractional shortening
- LVESD:
-
Left ventricular end-systolic diameter
- LVEDD:
-
Left ventricular end-diastolic diameter
- Mecp2:
-
Methyl CpG binding protein 2
- MI:
-
Myocardial infarction
- MIF:
-
Macrophage migration inhibitory factor
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MSCs:
-
Mesenchymal stem cells
- MSC-Exos:
-
MSC-derived exosomes
- vWF:
-
von Willebrand factor
References
McCarroll, C. S., He, W., Foote, K., Bradley, A., McGlynn, K., Vidler, F., et al. (2018). Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction. Circulation, 137(1), 57–70. https://doi.org/10.1161/circulationaha.117.028911
Spiliopoulos, S., Koerfer, R., & Tenderich, G. (2016). Acute myocardial infarction complicated by cardiogenic shock: Results of primary percutaneous coronary interventions are insufficient. European Journal of Cardio-Thoracic Surgery, 49(4), 1298. https://doi.org/10.1093/ejcts/ezv331
Shafei, A. E. S., Ali, M. A., Ghanem, H. G., Shehata, A. I., Abdelgawad, A. A., Handal, H. R., et al. (2017). Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. The Journal of Gene Medicine, 19(12), e2995. https://doi.org/10.1002/jgm.2995
Bao, L., Meng, Q., Li, Y., Deng, S., Yu, Z., Liu, Z., et al. (2017). C-Kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. Journal of Cardiac Failure, 23(5), 403–415. https://doi.org/10.1016/j.cardfail.2017.03.002
Dakhlallah, D., Zhang, J., Yu, L., Marsh, C. B., Angelos, M. G., & Khan, M. (2015). MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. Journal of Cardiovascular Pharmacology, 65(3), 241–251. https://doi.org/10.1097/fjc.0000000000000183
Wen, Z., Zheng, S., Zhou, C., Yuan, W., Wang, J., & Wang, T. (2012). Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: MicroRNAs as novel regulators. Journal of Cellular and Molecular Medicine, 16(4), 657–671. https://doi.org/10.1111/j.1582-4934.2011.01471.x
Song, M., Heo, J., Chun, J. Y., Bae, H. S., Kang, J. W., Kang, H., et al. (2014). The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells and Development, 23(6), 654–663. https://doi.org/10.1089/scd.2013.0277
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., & Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplantation, 23(9), 1045–1059. https://doi.org/10.3727/096368913x667709
Bogatcheva, N. V., & Coleman, M. E. (2019). Conditioned medium of mesenchymal stromal cells: A new class of therapeutics. Biochemistry, 84(11), 1375–1389. https://doi.org/10.1134/s0006297919110129
Lelek, J., & Zuba-Surma, E. K. (2020). Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. International Journal of Molecular Sciences, 21(3), 799. https://doi.org/10.3390/ijms21030799
Tsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells, 9(4), 991. https://doi.org/10.3390/cells9040991
Nazarenko, I. (2020). Extracellular vesicles: Recent developments in technology and perspectives for cancer liquid biopsy. Recent Results in Cancer Research, 215, 319–344. https://doi.org/10.1007/978-3-030-26439-0_17
Mignot, G., Roux, S., Thery, C., Ségura, E., & Zitvogel, L. (2006). Prospects for exosomes in immunotherapy of cancer. Journal of Cellular and Molecular Medicine, 10(2), 376–388. https://doi.org/10.1111/j.1582-4934.2006.tb00406.x
Chaput, N., Flament, C., Viaud, S., Taieb, J., Roux, S., Spatz, A., et al. (2006). Dendritic cell derived-exosomes: Biology and clinical implementations. Journal of Leukocyte Biology, 80(3), 471–478. https://doi.org/10.1189/jlb.0206094
Jin, J., & Menon, R. (2018). Placental exosomes: A proxy to understand pregnancy complications. American Journal of Reproductive Immunology, 79(5), e12788. https://doi.org/10.1111/aji.12788
Hoeeg, C., Frljak, S., Qayyum, A. A., Vrtovec, B., Kastrup, J., Ekblond, A., et al. (2020). Efficacy and mode of action of mesenchymal stem cells in non-ischemic dilated cardiomyopathy: A systematic review. Biomedicines, 8(12), 570. https://doi.org/10.3390/biomedicines8120570
Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N., & Volarevic, V. (2019). Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells, 8(12), 1605. https://doi.org/10.3390/cells8121605
Tan, S. J. O., Floriano, J. F., Nicastro, L., Emanueli, C., & Catapano, F. (2020). Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules, 10(5), 707. https://doi.org/10.3390/biom10050707
Harrell, C. R., Jovicic, N., Djonov, V., & Volarevic, V. (2020). Therapeutic use of mesenchymal stem cell-derived exosomes: From basic science to clinics. Pharmaceutics, 12(5), 474. https://doi.org/10.3390/pharmaceutics12050474
Mokhtari, B., Aboutaleb, N., Nazarinia, D., Nikougoftar, M., Razavi Tousi, S. M. T., Molazem, M., et al. (2020). Comparison of the effects of intramyocardial and intravenous injections of human mesenchymal stem cells on cardiac regeneration after heart failure. Iranian Journal of Basic Medical Sciences, 23, 879. https://doi.org/10.22038/ijbms.2020.40886.9660
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., & Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Medical Research Methodology, 14, 43. https://doi.org/10.1186/1471-2288-14-43
Feng, Y., Huang, W., Wani, M., Yu, X., & Ashraf, M. (2014). Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE, 9(2), e88685. https://doi.org/10.1371/journal.pone.0088685
Kang, K., Ma, R., Cai, W., Huang, W., Paul, C., Liang, J., et al. (2015). Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells International, 2015, 659890. https://doi.org/10.1155/2015/659890
Yu, B., Kim, H. W., Gong, M., Wang, J., Millard, R. W., Wang, Y., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360. https://doi.org/10.1016/j.ijcard.2014.12.043
Teng, X., Chen, L., Chen, W., Yang, J., Yang, Z., & Shen, Z. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cellular Physiology and Biochemistry, 37(6), 2415–2424. https://doi.org/10.1159/000438594
Zhang, Z., Yang, J., Yan, W., Li, Y., Shen, Z., & Asahara, T. (2016). Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. Journal of the American Heart Association, 5(1), e002856. https://doi.org/10.1161/jaha.115.002856
Shao, L., Zhang, Y., Lan, B., Wang, J., Zhang, Z., Zhang, L., et al. (2017). MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Research International, 2017, 4150705. https://doi.org/10.1155/2017/4150705
He, J. G., Li, H. R., Han, J. X., Li, B. B., Yan, D., Li, H. Y., et al. (2018). GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Scientific Reports, 8(1), 9047. https://doi.org/10.1038/s41598-018-27435-9
Zhu, L. P., Tian, T., Wang, J. Y., He, J. N., Chen, T., Pan, M., et al. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8(22), 6163–6177. https://doi.org/10.7150/thno.28021
Ma, T., Chen, Y., Chen, Y., Meng, Q., Sun, J., Shao, L., et al. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells International, 2018, 3290372. https://doi.org/10.1155/2018/3290372
Zhu, J., Lu, K., Zhang, N., Zhao, Y., Ma, Q., Shen, J., et al. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artificial Cells, Nanomedicine, and Biotechnology, 46(8), 1659–1670. https://doi.org/10.1080/21691401.2017.1388249
Xiao, C., Wang, K., Xu, Y., Hu, H., Zhang, N., Wang, Y., et al. (2018). Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circulation Research, 123(5), 564–578. https://doi.org/10.1161/circresaha.118.312758
Zou, L., Ma, X., Lin, S., Wu, B., Chen, Y., & Peng, C. (2019). Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Experimental and Therapeutic Medicine, 18(4), 2574–2582. https://doi.org/10.3892/etm.2019.7874
Huang, P., Wang, L., Li, Q., Xu, J., Xu, J., Xiong, Y., et al. (2019). Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Research & Therapy, 10(1), 300. https://doi.org/10.1186/s13287-019-1353-3
Li, Y., Yang, R., Guo, B., Zhang, H., Zhang, H., Liu, S., et al. (2019). Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochemical and Biophysical Research Communications, 514(1), 323–328. https://doi.org/10.1016/j.bbrc.2019.04.138
Xu, R., Zhang, F., Chai, R., Zhou, W., Hu, M., Liu, B., et al. (2019). Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. Journal of Cellular and Molecular Medicine, 23(11), 7617–7631. https://doi.org/10.1111/jcmm.14635
Zhang, C. S., Shao, K., Liu, C. W., Li, C. J., & Yu, B. T. (2019). Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. European Review for Medical and Pharmacological Sciences, 23(15), 6691–6699. https://doi.org/10.26355/eurrev_201908_18560
Huang, P., Wang, L., Li, Q., Tian, X., Xu, J., Xu, J., et al. (2020). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research, 116(2), 353–367. https://doi.org/10.1093/cvr/cvz139
Li, Y., Zhou, J., Zhang, O., Wu, X., Guan, X., Xue, Y., et al. (2020). Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. International Immunopharmacology, 80, 106156. https://doi.org/10.1016/j.intimp.2019.106156
Sun, L., Zhu, W., Zhao, P., Zhang, J., Lu, Y., Zhu, Y., et al. (2020). Down-regulated exosomal MicroRNA-221 - 3p derived from senescent mesenchymal stem cells impairs heart repair. Frontiers in Cell and Developmental Biology, 8, 263. https://doi.org/10.3389/fcell.2020.00263
Liu, X., Li, X., Zhu, W., Zhang, Y., Hong, Y., Liang, X., et al. (2020). Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. Journal of Cellular Physiology, 235(11), 8010–8022. https://doi.org/10.1002/jcp.29456
Sun, J., Shen, H., Shao, L., Teng, X., Chen, Y., Liu, X., et al. (2020). HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Research & Therapy, 11(1), 373. https://doi.org/10.1186/s13287-020-01881-7
Cheng, H., Chang, S., Xu, R., Chen, L., Song, X., Wu, J., et al. (2020). Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Research & Therapy, 11(1), 224. https://doi.org/10.1186/s13287-020-01737-0
Fu, D. L., Jiang, H., Li, C. Y., Gao, T., Liu, M. R., & Li, H. W. (2020). MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. European Review for Medical and Pharmacological Sciences, 24(19), 10107–10117. https://doi.org/10.26355/eurrev_202010_23230
Wang, S., Li, L., Liu, T., Jiang, W., & Hu, X. (2020). miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regenerative Medicine, 15(6), 1749–1759. https://doi.org/10.2217/rme-2019-0136
Cheng, W., Wang, L., Yang, T., Wu, A., Wang, B., Li, T., et al. (2020). Qiliqiangxin capsules optimize cardiac metabolism flexibility in rats with heart failure after myocardial infarction. Frontiers in Physiology, 11, 805. https://doi.org/10.3389/fphys.2020.00805
He, X., Yao, M. W., Zhu, M., Liang, D. L., Guo, W., Yang, Y., et al. (2018). Metformin induces apoptosis in mesenchymal stromal cells and dampens their therapeutic efficacy in infarcted myocardium. Stem Cell Research & Therapy, 9(1), 306. https://doi.org/10.1186/s13287-018-1057-0
Yu, W., Sun, S., Xu, H., Li, C., Ren, J., & Zhang, Y. (2020). TBC1D15/RAB7-regulated mitochondria-lysosome interaction confers cardioprotection against acute myocardial infarction-induced cardiac injury. Theranostics, 10(24), 11244–11263. https://doi.org/10.7150/thno.46883
Zhang, H., Yin, Y., Liu, Y., Zou, G., Huang, H., Qian, P., et al. (2020). Necroptosis mediated by impaired autophagy flux contributes to adverse ventricular remodeling after myocardial infarction. Biochemical Pharmacology, 175, 113915. https://doi.org/10.1016/j.bcp.2020.113915
Walker, B. W., Lara, R. P., Yu, C. H., Sani, E. S., Kimball, W., Joyce, S., et al. (2019). Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials, 207, 89–101. https://doi.org/10.1016/j.biomaterials.2019.03.015
Won, Y. W., Bull, D. A., & Kim, S. W. (2014). Functional polymers of gene delivery for treatment of myocardial infarct. Journal of Controlled Release, 195, 110–119. https://doi.org/10.1016/j.jconrel.2014.07.041
Zeng, Y., Li, J., Wang, H. X., Guo, S. B., Yang, H., Zeng, X. J., et al. (2013). Transcriptional effects of E3 ligase atrogin-1/MAFbx on apoptosis, hypertrophy and inflammation in neonatal rat cardiomyocytes. PLoS ONE, 8(1), e53831. https://doi.org/10.1371/journal.pone.0053831
Kim, S. H., Jeong, J. H., Ou, M., Yockman, J. W., Kim, S. W., & Bull, D. A. (2008). Cardiomyocyte-targeted siRNA delivery by prostaglandin E(2)-Fas siRNA polyplexes formulated with reducible poly(amido amine) for preventing cardiomyocyte apoptosis. Biomaterials, 29(33), 4439–4446. https://doi.org/10.1016/j.biomaterials.2008.07.047
Huang, S., & Frangogiannis, N. G. (2018). Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. British Journal of Pharmacology, 175(9), 1377–1400. https://doi.org/10.1111/bph.14155
Adrover, J. M., Del Fresno, C., Crainiciuc, G., Cuartero, M. I., Casanova-Acebes, M., Weiss, L. A., et al. (2019). A neutrophil timer coordinates immune defense and vascular protection. Immunity, 50(2), 390-402.e310. https://doi.org/10.1016/j.immuni.2019.01.002
Gast, M., Rauch, B. H., Haghikia, A., Nakagawa, S., Haas, J., Stroux, A., et al. (2019). Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovascular Research, 115(13), 1886–1906. https://doi.org/10.1093/cvr/cvz085
Sahoo, S., & Losordo, D. W. (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research, 114(2), 333–344. https://doi.org/10.1161/circresaha.114.300639
Yu, H., Lu, K., Zhu, J., & Wang, J. (2017). Stem cell therapy for ischemic heart diseases. British Medical Bulletin, 121(1), 135–154. https://doi.org/10.1093/bmb/ldw059
Bernstock, J. D., Peruzzotti-Jametti, L., Ye, D., Gessler, F. A., Maric, D., Vicario, N., et al. (2017). Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. Journal of Cerebral Blood Flow and Metabolism, 37(7), 2314–2319. https://doi.org/10.1177/0271678x17700432
Maffioletti, S. M., Noviello, M., English, K., & Tedesco, F. S. (2014). Stem cell transplantation for muscular dystrophy: The challenge of immune response. BioMed Research International, 2014, 964010. https://doi.org/10.1155/2014/964010
Duran, J. M., Makarewich, C. A., Sharp, T. E., Starosta, T., Zhu, F., Hoffman, N. E., et al. (2013). Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circulation Research, 113(5), 539–552. https://doi.org/10.1161/circresaha.113.301202
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., & Perez-Fernandez, R. (2017). Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. International Journal of Molecular Sciences, 18(9), 1852. https://doi.org/10.3390/ijms18091852
Bacakova, L., Zarubova, J., Travnickova, M., Musilkova, J., Pajorova, J., Slepicka, P., et al. (2018). Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - A review. Biotechnology Advances, 36(4), 1111–1126. https://doi.org/10.1016/j.biotechadv.2018.03.011
Nesselmann, C., Ma, N., Bieback, K., Wagner, W., Ho, A., Konttinen, Y. T., et al. (2008). Mesenchymal stem cells and cardiac repair. Journal of Cellular and Molecular Medicine, 12(5b), 1795–1810. https://doi.org/10.1111/j.1582-4934.2008.00457.x
Khasawneh, R. R., Abu-El-Rub, E., Serhan, A. O., Serhan, B. O., & Abu-El-Rub, H. (2019). Cross talk between 26S proteasome and mitochondria in human mesenchymal stem cells’ ability to survive under hypoxia stress. Journal of Physiological Sciences, 69(6), 1005–1017. https://doi.org/10.1007/s12576-019-00720-6
Mead, B., & Tomarev, S. (2017). Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Translational Medicine, 6(4), 1273–1285. https://doi.org/10.1002/sctm.16-0428
Li, N., Rochette, L., Wu, Y., & Rosenblatt-Velin, N. (2019). New insights into the role of exosomes in the heart after myocardial infarction. Journal of Cardiovascular Translational Research, 12(1), 18–27. https://doi.org/10.1007/s12265-018-9831-z
Katare, R., Riu, F., Mitchell, K., Gubernator, M., Campagnolo, P., Cui, Y., et al. (2011). Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation Research, 109(8), 894–906. https://doi.org/10.1161/circresaha.111.251546
Sun, L., Zhang, Y., Zhang, J., Wang, J., & Xing, S. (2020). Atorvastatin improves the proliferation and migration of endothelial progenitor cells via the miR-221/VEGFA axis. Bioscience Reports, 40(11), BSR20193053. https://doi.org/10.1042/BSR20193053
Sayed, D., & Abdellatif, M. (2011). MicroRNAs in development and disease. Physiological Reviews, 91(3), 827–887. https://doi.org/10.1152/physrev.00006.2010
Kir, D., Schnettler, E., Modi, S., & Ramakrishnan, S. (2018). Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis, 21(4), 699–710. https://doi.org/10.1007/s10456-018-9632-7
Coskunpinar, E., Cakmak, H. A., Kalkan, A. K., Tiryakioglu, N. O., Erturk, M., & Ongen, Z. (2016). Circulating miR-221-3p as a novel marker for early prediction of acute myocardial infarction. Gene, 591(1), 90–96. https://doi.org/10.1016/j.gene.2016.06.059
Täubel, J., Hauke, W., Rump, S., Viereck, J., Batkai, S., Poetzsch, J., et al. (2021). Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. European Heart Journal, 42(2), 178–188. https://doi.org/10.1093/eurheartj/ehaa898
Zhang, Y., Zhu, W., He, H., Fan, B., Deng, R., Hong, Y., et al. (2019). Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging, 11(24), 12641–12660. https://doi.org/10.18632/aging.102592
Gidlöf, O., van der Brug, M., Ohman, J., Gilje, P., Olde, B., Wahlestedt, C., et al. (2013). Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood, 121(19), 3908–3917. https://doi.org/10.1182/blood-2012-10-461798 s3901–3926.
Silva, D., Carneiro, F. D., Almeida, K. C., & Fernandes-Santos, C. (2018). Role of miRNAs on the pathophysiology of cardiovascular diseases. Arquivos Brasileiros de Cardiologia, 111(5), 738–746. https://doi.org/10.5935/abc.20180215
Li, L., Li, S., Wu, M., Chi, C., Hu, D., Cui, Y., et al. (2019). Early diagnostic value of circulating microRNAs in patients with suspected acute myocardial infarction. Journal of Cellular Physiology, 234(8), 13649–13658. https://doi.org/10.1002/jcp.28045
Dai, S., Wei, D., Wu, Z., Zhou, X., Wei, X., Huang, H., et al. (2008). Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Molecular Therapy, 16(4), 782–790. https://doi.org/10.1038/mt.2008.1
Dougherty, J. A., Mergaye, M., Kumar, N., Chen, C. A., Angelos, M. G., & Khan, M. (2017). Potential Role of Exosomes in Mending a Broken Heart: Nanoshuttles Propelling Future Clinical Therapeutics Forward. Stem Cells International, 2017, 5785436. https://doi.org/10.1155/2017/5785436
Kishore, R., & Khan, M. (2016). More than tiny sacks: Stem cell exosomes as cell-free modality for cardiac repair. Circulation Research, 118(2), 330–343. https://doi.org/10.1161/circresaha.115.307654
Faggion, C. M., Jr., Diaz, K. T., Aranda, L., Gabel, F., Listl, S., & Alarcón, M. A. (2017). The risk of bias of animal experiments in implant dentistry: A methodological study. Clinical Oral Implants Research, 28(7), e39–e45. https://doi.org/10.1111/clr.12852
du Sert, N. P., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. https://doi.org/10.1371/journal.pbio.3000410
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81973787) and the Postdoctoral Research Foundation of China (2019M660574).
Author information
Authors and Affiliations
Contributions
H.M. and W.T.C. have contributed equally to this work. Theme and design of the research: H.M.; article retrieval: H.M. and L.W.; data extraction: S.Q.C., Y.T., Z.W.L., Y.L., and W.T.C.; verification of data: H.M.; writing of the manuscript: H.M.; critical revision of the manuscript for intellectual content: W.T.C. and M.J.Z.; obtaining funding: M.J.Z. and H.M.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, H., Cheng, W., Wang, L. et al. Mesenchymal Stem Cell Exosomes in the Treatment of Myocardial Infarction: a Systematic Review of Preclinical In Vivo Studies. J. of Cardiovasc. Trans. Res. 15, 317–339 (2022). https://doi.org/10.1007/s12265-021-10168-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10168-y