Abstract
Coronary microvascular dysfunction (CMD) has emerged as an important therapeutic target in the contemporary management of ischemic heart disease. However, due to a lack of a reliable traditional “gold standard” test for CMD, optimal treatment remains undefined. The index of microcirculatory resistance (IMR) is an intra-coronary wire-based technique that provides a more reliable and quantitative assessment of CMD and has been increasingly used as a preferred endpoint for evaluating CMD treatment strategies in recent studies. IMR can help diagnose CMD in angina patients with non-obstructive epicardial coronary disease, predict peri-procedural myocardial infarction in stable patients undergoing coronary stenting, and predict long-term prognosis after acute myocardial infarction. Studies of IMR in the setting of non-ST-elevation acute coronary syndromes are still lacking. This review critically appraises the current published literature evaluating targeted therapies for CMD using IMR as the assessment tool and provides insights into evidence gaps in this important field.

The index of microcirculatory resistance has rapidly evolved from a research tool to being the new “gold standard” test for evaluating coronary microvascular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronary arterial tree is comprised of the large epicardial arteries which function as capacitance vessels and the microcirculation (< 500 μm in diameter) which are resistance vessels and responsible for the metabolic regulation of coronary blood flow. Disruption of the coronary microcirculation can therefore lead to impaired myocardial perfusion despite patency of epicardial arteries. Coronary microvascular dysfunction (CMD) is increasingly recognized to play a crucial role in the pathogenesis of myocardial ischemia, and its presence has consistently been shown to portend a worse prognosis [1,2,3]. Consequently, targeted treatment of CMD presents a valuable therapeutic strategy in the contemporary management of ischemic heart disease (IHD).
Direct assessment of CMD has historically been challenging due to the small size of the microcirculation and limited spatial resolution of imaging techniques. Most available tests therefore utilize the indicator-dilution principle to indirectly assess the coronary microcirculation by measuring myocardial blood flow through the entire coronary vascular tree in response to maximal hyperemia. However, this perfusion reserve method is greatly limited by its intrinsic variability due to dependence on hemodynamic perturbations, and lack of specificity for the microcirculation, often resulting in disparate results when different techniques are applied. The absence of a reliable “gold standard” test has contributed to a lack of consensus on the diagnostic criteria of CMD and presents great challenges in evaluating potentially effective therapies [4].
Recently, the index of microcirculatory resistance (IMR), an invasive intra-coronary wire-based technique, has been shown to be a highly reliable and reproducible method for the quantitative assessment of CMD. Unlike other methods that assess myocardial blood flow, the IMR evaluates the minimum achievable microvascular resistance to provide a more specific assessment of microvascular integrity (Fig. 1). In stable coronary artery disease, IMR measured before percutaneous coronary intervention (PCI) is predictive of peri-procedural myocardial infarction (PMI) [5, 6]. In acute coronary syndromes (ACS), IMR measured acutely is predictive of infarct characteristics, left ventricular function recovery, and long-term prognosis [7,8,9,10]. Indeed, the IMR is increasingly considered the new “gold standard” test and a robust endpoint for evaluating CMD treatment strategies. In this review, we analyze the contemporary literature evaluating targeted therapies for CMD using the IMR.
Assessment of the coronary microcirculation. CFR, coronary flow reserve; IMR, index of microcirculatory resistance; Pa, mean proximal arterial pressure; Pd, distal epicardial coronary pressure; Pv, venous pressure. Reprinted from Xu, J et al. Assessing coronary microvascular dysfunction in ischaemic heart disease: little things can make a big difference. Heart, Lung and Circulation. 2020;29 (1):118–27. Copyright (2019), with permission from Elsevier
Principles of the Index of Microcirculatory Resistance
The IMR is a highly reliable and robust parameter for the assessment of CMD, having been shown to significantly correlate with true microvascular resistance derived from absolute coronary flow in open-chested porcine models [11]. It also has the advantages of being independent of variations in hemodynamic state, having low baseline intrinsic variability, high reproducibility [12], and importantly, being independent of epicardial stenoses [11, 13]. Although no large-scale population data exists to define a “normal” IMR value, a cut-off value of < 25 is accepted in stable patients [14].
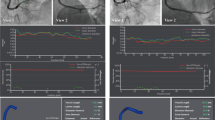
A detailed discussion of the principles of IMR is covered elsewhere [15]. In essence, derivation of IMR is based on two fundamental principles: Ohm’s law and indicator-dilution theory. Using an intra-coronary guide wire with a pressure-temperature micro-sensor near its distal tip acting as a distal thermistor, and the wire shaft acting as a proximal thermistor, standardized volumes (3 mL) of room temperature saline is injected down the coronary artery under maximal hyperemia, and the change in temperature can be recorded and plotted as a coronary thermodilution curve. The IMR is given by the equation: IMR = Pd × Tmn, where Pd is the distal epicardial coronary artery pressure and Tmn is the mean transit time derived from the thermodilution curve (Fig. 2). The main limitation of IMR is the confounding factor of substantial collateral circulation in the presence of severe epicardial stenosis. In these cases, a modified formula should be used to account for the contribution of collateral flow: IMRtrue = Pa × Tmn × [(Pd – Pw)/(Pa – Pw)], where Pa is mean proximal arterial pressure and Pw is the coronary wedge pressure [16]. Alternatively, an empirical mathematically derived formula which does not require coronary wedge pressure measurement can also be used: IMRcalc = Pa × Tmn × [(1.35 × Pd/Pa) – 0.32] [17].
Measuring the index of microcirculatory resistance (IMR) in the catheterization laboratory. The pressure-temperature sensor-tipped wire is placed in the coronary artery. The coronary thermodilution curves are plotted, as shown on the console screen, after injection of 3 mL room temperature saline. The mean transit time (Tmn) at baseline and hyperaemia were averaged from 3 separate injections. IMR is given by hyperemic Tmn × Pd
This review will focus on studies where IMR was used as a tool to guide and evaluate targeted treatment for CMD across the clinical spectrum of IHD. The specific patient population studied for each treatment differs between studies and is clarified in the text and also summarized in Table 1. Treatment therapies will be grouped into pharmacological and non-pharmacological.
Pharmacological Therapies
Statins
CMD can occur in the context of PCI due to a combination of distal micro-embolization of atheromatous debris, platelet activation, and inflammatory reactions and is a major mechanism for PMI [18]. Apart from their lipid-lowering properties, the HMG-CoA reductase inhibitors (statins) also have a number of beneficial cardiovascular pleiotropic effects, and statin pre-treatment has consistently been shown to reduce incidences of PMI across the clinical spectrum of IHD [19,20,21]. With the advent of IMR studies, this reduction in PMI has been recognized to be largely attributable to a targeted effect on PCI-related CMD, despite the variable pre-treatment regimes, small patient numbers, and lack of multi-center data in these studies.
A randomized trial of 80 patients with stable angina showed that, compared with controls, pravastatin pre-treatment (20 mg/day for 4 weeks) prior to elective PCI was associated with a significantly lower post-PCI IMR (median: pre-treatment 12.6 vs no pre-treatment 17.6, p = 0.007) [22]. Similarly, a randomized study of 84 stable patients undergoing elective PCI showed that higher-dose atorvastatin pre-treatment (40 mg/day for 7 days) resulted in a significantly lower post-PCI IMR (mean: high dose 16.5 vs low dose 31.2, p < 0.001) as well as troponin I levels (mean: 0.11 vs 0.16 ng/mL, respectively, p < 0.001), compared with lower-dose atorvastatin (20 mg/day for 7 days) [23].
Findings in ACS patients were comparable. A randomized study of 77 patients with non-ST-elevation acute coronary syndrome (NSTEACS) showed that pre-loading with higher-dose atorvastatin (80 mg 12–24 h and further 40 mg 2 h before PCI) led to a significant lower post-PCI IMR (mean: high dose 14.1 vs low dose 19.2, p = 0.003) and creatine kinase myocardial band levels (median: 1.4 vs 4.0 ng/mL, respectively, p = 0.002), compared with lower-dose atorvastatin (10 mg 12–24 h before PCI) [24].
Whether statin therapy has a beneficial effect on microvascular function in patients who are not undergoing PCI is still unclear. A single-center randomized study of 66 women with suspected ischemic chest pain and non-obstructive epicardial coronary disease showed that treatment with rosuvastatin 20 mg for 6 months, compared with placebo, did not significantly influence IMR values (mean: rosuvastatin 14.2 vs placebo 14.4, p = 0.55) [25]. It should be noted that the mean IMR was very low in both groups at baseline (16.5 and 14.6) suggesting that there was no significant CMD to begin with, and therefore, it would be difficult to prove treatment benefit, particularly given the small sample size. Studies with statins involving patients with elevated baseline IMR are needed.
P2Y12 Receptor Inhibitors
Ticagrelor, prasugrel, and cangrelor are newer generation platelet adenosine diphosphate P2Y12 receptor inhibitors that provide more rapid, potent, and consistent inhibition of platelet activity than clopidogrel. While these agents significantly reduced ischemic events compared with clopidogrel, only ticagrelor was superior to clopidogrel in preventing cardiovascular as well as all-cause mortality in ACS patients [26,27,28]. It has been suggested that P2Y12-independent effects of ticagrelor, specifically its inhibition of cellular uptake of adenosine, may account for its additional mortality benefits beyond platelet inhibition [29]. Indeed, ticagrelor has been shown to dose dependently augment adenosine-induced coronary blood flow in healthy subjects [30].
IMR was utilized in this context in a randomized trial of 76 patients with hemodynamically stable ST-elevation myocardial infarction (STEMI). The study showed that a 180 mg loading dose of ticagrelor before primary PCI led to a significantly lower IMR immediately post-PCI compared with 600 mg loading dose of clopidogrel (mean: ticagrelor 22.2 vs clopidogrel 34.4, p = 0.005), although this did not translate to a reduction in infarct size at 3 months [31]. This benefit however was not seen when ticagrelor was compared with cangrelor in a randomized trial of 100 STEMI patients undergoing primary PCI, where the immediate post-PCI IMR measured in 90 patients was similar between both groups (median: 28 vs 30, respectively, p = 0.52), as was the final infarct size at 12 weeks, which was measured in 75 patients [32]. Similarly, in a randomized study of 110 STEMI patients, maintenance ticagrelor therapy was not superior to prasugrel with respect to IMR (median: 21 vs 18, respectively, p = 0.08), infarct size, and plasma adenosine concentrations at 1 month [33]. Data for prasugrel is scarce in contrast, with only a small randomized study of 40 stable patients showing a lower post-PCI IMR with prasugrel compared with clopidogrel (mean: 17 vs 26, respectively, p = 0.007) [34].
More data is evidently needed before ticagrelor can be established as a potential maintenance therapy to target CMD. Results from several ongoing trials are eagerly awaited (Table 2).
Intra-coronary Low-Dose Fibrinolytics
Disruption of the coronary microvasculature may occur in the setting of acute myocardial infarction due to factors such as micro-embolization and fibrin and platelet aggregation, resulting in microvascular obstruction and lack of myocardial perfusion despite restoration of epicardial flow. Fibrinolytic agents may counteract many of these factors and therefore may improve microvascular perfusion.
In a randomized pilot study of 41 patients with first-presentation STEMI undergoing primary PCI, administration of low-dose intra-coronary streptokinase (250 kU) immediately post-PCI significantly reduced IMR measured 2 days later, without any observed increase in the rate of bleeding complications, compared with standard care (mean: 16.29 vs 32.49, respectively, p < 0.001) [35]. This observation was confirmed in a larger subsequent study of 95 STEMI patients, where the same dose of intra-coronary streptokinase given immediately post-primary PCI not only improved IMR at 2 days compared with controls (mean: 20.2 vs 34.2, respectively, p < 0.001) but also reduced infarct size at 6 months (22.7% vs 32.9%, respectively, p = 0.003) [36]. Notably, concomitant peripheral glycoprotein IIb/IIIa inhibitor was used in all patients in both studies.
Indeed, a more recent study reflective of contemporary PCI practices, where 440 STEMI patients undergoing primary PCI were randomized to low-dose intra-coronary alteplase (10 mg or 20 mg) or placebo after reperfusion and before stent implantation, was stopped early due to futility based on a pre-specified analysis [37]. However, the primary outcome in this study was cardiac magnetic resonance imaging (CMR)-derived microvascular obstruction, rather than IMR. Hopefully, there will be some clarification on the benefit of this treatment strategy in a large upcoming IMR-based trial.
Glycoprotein IIb/IIIa Inhibitors
Intra-coronary bolus administration of glycoprotein IIb/IIIa inhibitors may also improve microvascular perfusion in a manner analogous to fibrinolytic agents. In a study of 49 STEMI patients undergoing primary PCI, a single intra-coronary tirofiban bolus-only strategy resulted in similar IMR, measured 4–5 days post-PCI, compared with the standard intravenous bolus and maintenance infusion strategy [38]. Since this was not a placebo-controlled trial, and there was no baseline IMR measured in this study, whether this treatment strategy was beneficial is uncertain. A more recent randomized study of 62 patients undergoing delayed PCI between 1 and 2 weeks after acute myocardial infarction showed that an intra-coronary bolus injection of 10 μg/kg tirofiban resulted in a significantly lower post-PCI IMR compared with placebo (mean: 16.75 vs 23.63, respectively, p = 0.008) [39]. However, in this study, the mean IMR values were quite low in both groups. Further studies are needed before firm conclusions can be made regarding this strategy.
Nicorandil
Nicorandil is a vasodilator that exerts a unique hybrid pharmacologic effect as both a nitrate and an adenosine triphosphate-sensitive potassium channel activator [40]. It has a number of potentially beneficial effects on the microcirculation including preferential vasodilation of microvessels < 100 μm and reduction in endothelial dysfunction, oxidative stress, and inflammation [41]. In a randomized study of 62 stable patients undergoing elective PCI, intravenous nicorandil (6 mg bolus before PCI and 6 mg/h infusion for 24 h after PCI) led to a significantly lower IMR immediately post-PCI compared with controls (mean: 17.9 vs 25.4, respectively, p < 0.05) [42]. In a small non-randomized study of 32 STEMI patients, intra-coronary nicorandil 12 mg bolus administered after primary PCI has also been shown to lead to a lower post-PCI IMR [43].
Additional intra-coronary administration of nicorandil on top of baseline intravenous infusion may show an additive beneficial effect in STEMI patients. In a randomized study of 40 first-presentation STEMI patients, all patients initially received baseline intravenous nicorandil on presentation (0.067 mg/kg bolus before PCI followed by 1.67 μg/kg/min infusion for 24 h). Patients randomized to receive additional intra-coronary nicorandil 2 mg immediately after primary PCI had a lower IMR post-PCI (median: baseline 27.7, post-nicorandil 18.7, p < 0.0001) compared with controls who received only intra-coronary saline (median: baseline 24.3, post-saline 23.8, p = 0.82), with a preferential effect on those with elevated IMR of ≥ 21 [44].
Other Agents
The direct thrombin inhibitor bivalirudin was compared with a multi-center randomized trial against unfractionated heparin in 64 STEMI patients undergoing primary PCI. This study demonstrated a lower post-PCI IMR with bivalirudin treatment compared with heparin (mean: 43.5 vs 68.7, respectively, p = 0.014) [45]. The high IMR values suggest significant CMD in enrolled patients and therefore potential for this to translate to a demonstrable clinical benefit. Unfortunately, the study was terminated early due to expected futility to meet its primary endpoint of infarct size by CMR.
Nitroprusside, a vasodilator which is a direct nitric oxide donor, has also been hypothesized to exert a beneficial effect on the microcirculation similar to nicorandil. This was investigated in a small prospective observational study of 18 STEMI patients with a post-PCI IMR ≥ 30, whereby intra-coronary nitroprusside 100 μg resulted in an immediate reduction in mean IMR from 76 to 45 (p = 0.0006) [46]. Unfortunately, in addition to its non-randomized design, the study was significantly confounded by the fact that 66.7% of study patients also received nicorandil prior to nitroprusside, and therefore, the results should be interpreted with caution.
Intra-coronary administration of the angiotensin-converting enzyme inhibitor enalaprilat has been tested against placebo in a single-center randomized study of 40 stable patients undergoing elective PCI. This showed that enalaprilat 50 μg not only reduced the pre-PCI IMR shortly after administration (mean: baseline 27 vs after enalaprilat 19, p < 0.0001), it also resulted in a significantly lowered IMR post-PCI IMR compared to placebo (mean: 15 vs 33, respectively, p < 0.001) [47].
Non-pharmacological Therapies
Pressure Controlled Intermittent Coronary Sinus Occlusion
Controlled intermittent increases in coronary sinus pressure, using a balloon-tipped catheter, may have beneficial effects on myocardial perfusion through promotion of retrograde perfusion of hypoperfused or ischemic myocardium [48]. This strategy, pressure-controlled intermittent coronary sinus occlusion, or PICSO, was examined in a non-randomized prospective study, where 25 patients with acute anterior STEMI and an IMR of > 40 after primary PCI were given this treatment and compared with historical controls in a parallel study. PICSO treatment resulted in a lower mean IMR at 24–48 h compared with controls (mean: 24.8 vs 45, respectively, p < 0.001) and a reduced infarct size by CMR at 6 months (26% vs 33%, respectively, p = 0.006) [49]. Randomized trial data is eagerly awaited.
Aspiration Thrombectomy and Distal Embolization Prevention Strategies
Distal thrombus embolization after infarct PCI is another possible mechanism of microvascular disruption and subsequent myocardial hypoperfusion, with the associated consequences of larger infarct size and worse prognosis [50]. Manual thrombus aspiration may reduce this distal embolization, but routine application of current techniques and devices does not improve mortality and may even be associated with elevated stroke risk [51]. Nevertheless, the studies have consistently signaled an improvement in perfusion with aspiration thrombectomy, albeit with subjective angiographic and electrocardiographic measures.
It is in this context that the IMR, being a more objective and quantitative measure of microvascular function and myocardial perfusion, was utilized as the primary endpoint in a randomized study of 63 STEMI patients, where adjunctive aspiration thrombectomy led to a lower IMR post-PCI compared to routine primary PCI (mean: 23.5 vs 34.2, respectively, p = 0.018) [52]. However, subsequent studies have suggested that aspiration thrombectomy may be no better than intra-coronary glycoprotein IIb/IIIa inhibitors [53] and even balloon angioplasty [54] and may be inferior to intra-coronary fibrinolytics [55, 56], with respect to the IMR after primary PCI for STEMI. Given the conflicting data and particularly the potential for increased risk of stroke, routine aspiration thrombectomy cannot currently be recommended as a targeted treatment strategy for CMD in the setting of STEMI.
Dedicated distal embolic protection devices have also failed to show efficacy in native coronary artery PCI, with one study of 626 STEMI patients suggesting that it may not improve microvascular perfusion, although this was based on electrocardiographic ST segment resolution [57]. IMR data is scarce, but a small randomized study of 36 acute anterior STEMI patients did in fact show that distal protection resulted in a significantly lower IMR post-PCI than standard primary PCI without distal protection (mean: 26.6 vs 37.2, respectively, p = 0.03) [58].
Compared with conventional PCI with balloon pre-dilatation, direct stenting is also thought to reduce distal embolization, based on observations of lower rates of PMI with this strategy [59]. This hypothesis was tested in a randomized study of 50 stable patients undergoing elective PCI, which showed that direct stenting was associated with a significantly lower post-PCI IMR (mean: direct stenting 13 vs conventional PCI 24, p < 0.01) as well as troponin T values, compared with conventional PCI [60]. This has yet to be tested in ACS patients.
Lifestyle Measures
Cigarette smoking cessation is undeniably beneficial to the patient for a range of reasons, not the least of which is an improvement in peripheral [61] and coronary endothelial function [62]. With regard to CMD, an observational study of 97 stable patients showed that baseline IMR was higher in smokers compared with non-smokers, and smoking was an independent predictor of increased IMR on multivariate analysis (odds ratio 3.14, 95% confidence interval 1.19–8.80, p = 0.02) [63]. Currently, however, there are no randomized data on the specific effects of smoking cessation on CMD.
Microvascular complications are well recognized in diabetes. Not surprisingly, coronary endothelial dysfunction and attenuated coronary vasodilator capacity have been demonstrated in diabetic patients [64]. In an observational IMR study of 56 patients, diabetic patients with stable coronary disease had higher mean IMR than non-diabetic patients (mean: 27 vs 16, respectively, p = 0.009), with a significant positive correlation found between the mean IMR and HbA1c, suggesting worse CMD with poor glycemic control [65]. Furthermore, dyslipidemia, hypertension, and obesity were also found to be associated with higher IMR in diabetic patients, with metformin having a protective effect.
Despite the lack of direct randomized evidence that smoking cessation, weight loss, blood pressure control, and exercise improves CMD, these observations suggest that the benefits of lifestyle modifications are far reaching and should be recommended in all patients with CMD, as stated in the latest European guidelines [14].
Gaps in Evidence
Despite the numerous randomized studies evaluating targeted treatment for CMD using IMR as a marker, most are limited by small sample sizes, and study endpoints have largely been restricted to immediate- or short-term improvements in IMR and, in a few studies, infarct size. Although many studies mentioned earlier demonstrated lower post-procedure IMR with treatment targeted at the microvasculature, the lack of data on pre-procedure IMR, and hence its change with therapy, has limited the validity of the observations. It may be difficult with the currently available data to decide on the value of such therapies with regard to protection against CMD in the setting of PCI. Furthermore, data on key clinical endpoints such as cardiovascular death, heart failure, and quality of life are lacking, and whether improvements seen in IMR are maintained at longer-term follow-up is also still undetermined.
It has been shown that in STEMI patients undergoing primary PCI, coronary stent implantation itself significantly improved the immediate post-PCI IMR compared with pre-PCI [66]. This presents an important treatment confounder that may be overlooked in non-randomized studies and again highlights the importance of obtaining a pre-PCI IMR. Furthermore, in randomized studies where pharmacological agents were studied, it may be important to understand the pharmacokinetic properties of these drugs, since the immediate post-PCI IMR may reflect improved perfusion due to stent implantation, before the drugs had time to work.
In patients with vascular risk factors, IMR has been shown to be different between anterior and posterior coronary microvascular beds [65], but there is no information on potential differential effects of therapies in patients with CMD. Studies involving PCI, for instance, only examine the IMR in target arteries, and it is unclear if non-target arteries would also benefit from treatment.
To date, most IMR studies have been in the setting of stable coronary disease and STEMI, and its role in NSTEACS is therefore less clearly defined, despite it being the most common form of ACS presentation [67]. Indeed, a recent study confirmed that the post-PCI IMR in NSTEACS patients is also an independent predictor of major adverse cardiac events at over 20-month follow up [10]. While aspiration thrombectomy has not been shown to improve CMR-derived microvascular obstruction and infarct size in NSTEACS [68], there is currently no published data using IMR endpoints. Similarly, there is a paucity of IMR studies in patients with non-obstructive epicardial disease. In the British Heart Foundation Coronary Microvascular Angina (BHF CorMicA) study, 151 patients with angina and non-obstructive epicardial disease were randomized to standard care versus stratified medical therapy, whereby treatment was tailored according to the diagnosis of vasospastic angina (assessed using coronary reactivity testing) or microvascular angina (assessed using IMR) [69]. Notably, the primary outcome of this study was qualitative, in the form of patient-reported questionnaires, and therefore whether the treatment improved IMR is not known. Given the prevalence of these conditions, there is a pressing need for studies of IMR-directed therapies in these settings.
Finally, there have been many other small-scale treatment studies for CMD with a variety of pharmacological and non-pharmacological strategies not described here [4]. However, these studies used perfusion reserve methods, such as coronary flow reserve, to assess CMD. Given the limitations of such methods, it would be prudent to investigate the feasibility of these treatments using IMR before conclusions can be drawn. In addition, studies of aspirin and traditional anti-anginal agents such as beta-blockers, calcium channel blockers, and nitrates are lacking and would be worth investigating.
Conclusions
Numerous studies have consistently demonstrated the value of IMR in prognostication as well as in the evaluation of targeted therapies across the spectrum of ischemic heart disease (Table 1). Pharmacological therapies have been the focus of the majority of studies to date, with statins in particular demonstrating more consistent and promising results. With regard to the non-pharmacological therapies, PICSO is a novel therapy that has shown promise in a non-randomized study setting. There are many limitations in the studies reviewed and considerable gaps in evidence in the field. Future research should focus on addressing these knowledge gaps, as well as assessing for longer term benefits and hard clinical endpoints. Results from several ongoing randomized trials are expected in the coming years and are an exciting space to watch. Given the advantages of using IMR as the current preferred tool for evaluating CMD, not only will it provide novel insights into the assessment and treatment of CMD, it is foreseeable that optimal therapy will become more defined for this important condition in the near future.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CMD:
-
Coronary microvascular dysfunction
- CMR:
-
Cardiac magnetic resonance imaging
- IHD:
-
Ischemic heart disease
- IMR:
-
Index of microcirculatory resistance
- PCI:
-
Percutaneous coronary intervention
- PMI:
-
Peri-procedural myocardial infarction
- NSTEACS:
-
Non-ST-elevation acute coronary syndrome
- PICSO:
-
Pressure-controlled intermittent coronary sinus occlusion
- STEMI:
-
ST-elevation myocardial infarction
References
Britten, M. B., Zeiher, A. M., & Schachinger, V. (2004). Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coronary Artery Disease, 15(5), 259–264.
Pepine, C. J., Anderson, R. D., Sharaf, B. L., Reis, S. E., Smith, K. M., Handberg, E. M., et al. (2010). Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. Journal of the American College of Cardiology, 55(25), 2825–2832.
Sara, J. D., Widmer, R. J., Matsuzawa, Y., Lennon, R. J., Lerman, L. O., & Lerman, A. (2015). Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC. Cardiovascular Interventions, 8(11), 1445–1453.
Marinescu, M. A., Loffler, A. I., Ouellette, M., Smith, L., Kramer, C. M., & Bourque, J. M. (2015). Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC: Cardiovascular Imaging, 8(2), 210–220.
Layland, J. J., Whitbourn, R. J., Burns, A. T., Somaratne, J., Leitl, G., Macisaac, A. I., et al. (2012). The index of microvascular resistance identifies patients with periprocedural myocardial infarction in elective percutaneous coronary intervention. Heart., 98(20), 1492–1497.
Ng, M. K., Yong, A. S., Ho, M., Shah, M. G., Chawantanpipat, C., O'Connell, R., et al. (2012). The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circulation. Cardiovascular Interventions, 5(4), 515–522.
Fearon, W. F., Shah, M., Ng, M., Brinton, T., Wilson, A., Tremmel, J. A., et al. (2008). Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. Journal of the American College of Cardiology, 51(5), 560–565.
Lim, H. S., Yoon, M. H., Tahk, S. J., Yang, H. M., Choi, B. J., Choi, S. Y., et al. (2009). Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. European Heart Journal, 30(23), 2854–2860.
Fearon, W. F., Low, A. F., Yong, A. S., McGeoch, R., Berry, C., Shah, M. G., et al. (2013). Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation., 127(24), 2436–2441.
Murai, T., Yonetsu, T., Kanaji, Y., Usui, E., Hoshino, M., Hada, M., et al. (2018). Prognostic value of the index of microcirculatory resistance after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Catheterization and Cardiovascular Interventions, 00, 1–12.
Fearon, W. F., Balsam, L. B., Farouque, H. M., Caffarelli, A. D., Robbins, R. C., Fitzgerald, P. J., et al. (2003). Novel index for invasively assessing the coronary microcirculation. Circulation., 107(25), 3129–3132.
Ng, M. K., Yeung, A. C., & Fearon, W. F. (2006). Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation., 113(17), 2054–2061.
Aarnoudse, W., Fearon, W. F., Manoharan, G., Geven, M., van de Vosse, F., Rutten, M., et al. (2004). Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation., 110(15), 2137–2142.
Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., et al. (2020). 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal, 41(3), 407–477.
Xu, J., Lo, S., Juergens, C. P., & Leung, D. Y. (2020). Assessing coronary microvascular dysfunction in Ischaemic heart disease: Little things can make a big difference. Heart, Lung & Circulation, 29(1), 118–127.
Fearon, W. F., Aarnoudse, W., Pijls, N. H., De Bruyne, B., Balsam, L. B., Cooke, D. T., et al. (2004). Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: Experimental validation. Circulation., 109(19), 2269–2272.
Yong, A. S., Layland, J., Fearon, W. F., Ho, M., Shah, M. G., Daniels, D., et al. (2013). Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC. Cardiovascular Interventions, 6(1), 53–58.
Prasad, A., & Herrmann, J. (2011). Myocardial infarction due to percutaneous coronary intervention. The New England Journal of Medicine, 364(5), 453–464.
Briguori, C., Colombo, A., Airoldi, F., Violante, A., Focaccio, A., Balestrieri, P., et al. (2004). Statin administration before percutaneous coronary intervention: Impact on periprocedural myocardial infarction. European Heart Journal, 25(20), 1822–1828.
Pasceri, V., Patti, G., Nusca, A., Pristipino, C., Richichi, G., Di Sciascio, G., et al. (2004). Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: Results from the ARMYDA (atorvastatin for reduction of MYocardial damage during angioplasty) study. Circulation., 110(6), 674–678.
Patti, G., Pasceri, V., Colonna, G., Miglionico, M., Fischetti, D., Sardella, G., et al. (2007). Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: Results of the ARMYDA-ACS randomized trial. Journal of the American College of Cardiology, 49(12), 1272–1278.
Fujii, K., Kawasaki, D., Oka, K., Akahori, H., Iwasaku, T., Fukunaga, M., et al. (2011). The impact of pravastatin pre-treatment on periprocedural microcirculatory damage in patients undergoing percutaneous coronary intervention. JACC. Cardiovascular Interventions, 4(5), 513–520.
He, G. X., & Tan, W. (2013). High-dose atorvastatin pretreatment could diminishes microvascular impairment in patients undergoing elective percutaneous coronary intervention. Journal of Geriatric Cardiology, 10(4), 355–360.
Lee, B. K., Koo, B. K., Nam, C. W., Doh, J. H., Chung, W. Y., Cho, B. R., et al. (2016). Does pre-treatment with high dose atorvastatin prevent microvascular dysfunction after percutaneous coronary intervention in patients with acute coronary syndrome? Korean Circualtion Journal., 46(4), 472–480.
Solberg, O. G., Stavem, K., Ragnarsson, A., Beitnes, J. O., Skardal, R., Seljeflot, I., et al. (2019). Index of microvascular resistance to assess the effect of rosuvastatin on microvascular function in women with chest pain and no obstructive coronary artery disease: A double-blind randomized study. Catheterization and Cardiovascular Interventions, 94(5), 660–668.
Wallentin, L., Becker, R. C., Budaj, A., Cannon, C. P., Emanuelsson, H., Held, C., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine, 361(11), 1045–1057.
Wiviott, S. D., Braunwald, E., McCabe, C. H., Montalescot, G., Ruzyllo, W., Gottlieb, S., et al. (2007). Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine, 357(20), 2001–2015.
Bhatt, D. L., Stone, G. W., Mahaffey, K. W., Gibson, C. M., Steg, P. G., Hamm, C. W., et al. (2013). Effect of platelet inhibition with cangrelor during PCI on ischemic events. The New England Journal of Medicine, 368(14), 1303–1313.
Cattaneo, M., Schulz, R., & Nylander, S. (2014). Adenosine-mediated effects of ticagrelor: Evidence and potential clinical relevance. Journal of the American College of Cardiology, 63(23), 2503–2509.
Wittfeldt, A., Emanuelsson, H., Brandrup-Wognsen, G., van Giezen, J. J., Jonasson, J., Nylander, S., et al. (2013). Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. Journal of the American College of Cardiology, 61(7), 723–727.
Park, S. D., Lee, M. J., Baek, Y. S., Kwon, S. W., Shin, S. H., Woo, S. I., et al. (2016). Randomised trial to compare a protective effect of clopidogrel versus TIcagrelor on coronary microvascular injury in ST-segment elevation myocardial infarction (CV-TIME trial). EuroIntervention., 12(8), e964–ee71.
Ubaid, S., Ford, T. J., Berry, C., Murray, H. M., Wrigley, B., Khan, N., et al. (2019). Cangrelor versus ticagrelor in patients treated with primary percutaneous coronary intervention: Impact on platelet activity, myocardial microvascular function and infarct size: A randomized controlled trial. Thrombosis and Haemostasis, 119(7), 1171–1181.
van Leeuwen, M. A. H., van der Hoeven, N. W., Janssens, G. N., Everaars, H., Nap, A., Lemkes, J. S., et al. (2019). Evaluation of microvascular injury in revascularized patients with ST-segment-elevation myocardial infarction treated with ticagrelor versus prasugrel. Circulation., 139(5), 636–646.
Mangiacapra, F., Di Gioia, G., Pellicano, M., Di Serafino, L., Bressi, E., Peace, A. J., et al. (2016). Effects of prasugrel versus clopidogrel on coronary microvascular function in patients undergoing elective PCI. Journal of the American College of Cardiology, 68(2), 235–237.
Sezer, M., Oflaz, H., Goren, T., Okcular, I., Umman, B., Nisanci, Y., et al. (2007). Intracoronary streptokinase after primary percutaneous coronary intervention. The New England Journal of Medicine, 356(18), 1823–1834.
Sezer, M., Cimen, A., Aslanger, E., Elitok, A., Umman, B., Bugra, Z., et al. (2009). Effect of intracoronary streptokinase administered immediately after primary percutaneous coronary intervention on long-term left ventricular infarct size, volumes, and function. Journal of the American College of Cardiology, 54(12), 1065–1071.
McCartney, P. J., Eteiba, H., Maznyczka, A. M., McEntegart, M., Greenwood, J. P., Muir, D. F., et al. (2019). Effect of low-dose intracoronary alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with acute myocardial infarction: A randomized clinical trial. JAMA., 321(1), 56–68.
Kirma, C., Erkol, A., Pala, S., Oduncu, V., Dundar, C., Izgi, A., et al. (2012). Intracoronary bolus-only compared with intravenous bolus plus infusion of tirofiban application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Catheterization and Cardiovascular Interventions, 79(1), 59–67.
Sun, Z., Zeng, J., & Huang, H. (2016). Intracoronary injection of tirofiban prevents microcirculation dysfunction during delayed percutaneous coronary intervention in patients with acute myocardial infarction. International Journal of Cardiology, 208, 137–140.
Taira, N. (1989). Nicorandil as a hybrid between nitrates and potassium channel activators. The American Journal of Cardiology., 63(21), J18–J24.
Ishibashi, Y., Takahashi, N., Tokumaru, A., Karino, K., Sugamori, T., Sakane, T., et al. (2008). Effects of long-term nicorandil administration on endothelial function, inflammation, and oxidative stress in patients without coronary artery disease. Journal of Cardiovascular Pharmacology, 51(3), 311–316.
Hirohata, A., Yamamoto, K., Hirose, E., Kobayashi, Y., Takafuji, H., Sano, F., et al. (2014). Nicorandil prevents microvascular dysfunction resulting from PCI in patients with stable angina pectoris: A randomised study. EuroIntervention., 9(9), 1050–1056.
Kostic, J., Djordjevic-Dikic, A., Dobric, M., Milasinovic, D., Nedeljkovic, M., Stojkovic, S., et al. (2015). The effects of nicorandil on microvascular function in patients with ST segment elevation myocardial infarction undergoing primary PCI. Cardiovascular Ultrasound, 13, 26.
Ito, N., Nanto, S., Doi, Y., Sawano, H., Masuda, D., Yamashita, S., et al. (2010). High index of microcirculatory resistance level after successful primary percutaneous coronary intervention can be improved by intracoronary administration of nicorandil. Circulation Journal, 74(5), 909–915.
van Geuns, R. J., Sideris, G., Van Royen, N., El Mahmoud, R., Diletti, R., Bal Dit Sollier, C., et al. (2017). Bivalirudin infusion to reduce ventricular infarction: The open-label, randomised bivalirudin infusion for ventricular infArction limitation (BIVAL) study. EuroIntervention., 13(5), e540–e5e8.
Morimoto, K., Ito, S., Nakasuka, K., Sekimoto, S., Miyata, K., Inomata, M., et al. (2012). Acute effect of sodium nitroprusside on microvascular dysfunction in patients who underwent percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. International Heart Journal, 53(6), 337–340.
Mangiacapra, F., Peace, A. J., Di Serafino, L., Pyxaras, S. A., Bartunek, J., Wyffels, E., et al. (2013). Intracoronary enalaPrilat to reduce MICROvascular damage during percutaneous coronary intervention (ProMicro) study. Journal of the American College of Cardiology, 61(6), 615–621.
De Maria, G. L., Kassimis, G., Raina, T., & Banning, A. P. (2016). Reconsidering the back door approach by targeting the coronary sinus in ischaemic heart disease. Heart., 102(16), 1263–1269.
De Maria, G. L., Alkhalil, M., Borlotti, A., Wolfrum, M., Gaughran, L., Dall'Armellina, E., et al. (2018). Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford acute myocardial infarction - pressure-controlled intermittent coronary sinus occlusion study (OxAMI-PICSO study). EuroIntervention., 14(3), e352–e359.
Henriques, J. P., Zijlstra, F., Ottervanger, J. P., de Boer, M. J., Van ‘t Hof, A. W., Hoorntje, J. C., et al. (2002). Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. European Heart Journal, 23(14), 1112–1117.
Jolly, S. S., James, S., Dzavik, V., Cairns, J. A., Mahmoud, K. D., Zijlstra, F., et al. (2017). Thrombus aspiration in ST-segment-elevation myocardial infarction: An individual patient meta-analysis: Thrombectomy trialists collaboration. Circulation., 135(2), 143–152.
Woo, S. I., Park, S. D., Kim, D. H., Kwan, J., Shin, S. H., Park, K. S., et al. (2014). Thrombus aspiration during primary percutaneous coronary intervention for preserving the index of microcirculatory resistance: A randomised study. EuroIntervention., 9(9), 1057–1062.
Ahn, S. G., Lee, S. H., Lee, J. H., Lee, J. W., Youn, Y. J., Ahn, M. S., et al. (2014). Efficacy of combination treatment with intracoronary abciximab and aspiration thrombectomy on myocardial perfusion in patients with ST-segment elevation myocardial infarction undergoing primary coronary stenting. Yonsei Medical Journal, 55(3), 606–616.
Hoole, S. P., Jaworski, C., Brown, A. J., McCormick, L. M., Agrawal, B., Clarke, S. C., et al. (2015). Serial assessment of the index of microcirculatory resistance during primary percutaneous coronary intervention comparing manual aspiration catheter thrombectomy with balloon angioplasty (IMPACT study): A randomised controlled pilot study. Open Heart., 2(1), e000238.
Xiao, Y., Fu, X., Wang, Y., Fan, Y., Wu, Y., Wang, W., et al. (2019). Effects of different strategies on high thrombus burden in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary catheterization. Coronary Artery Disease, 30(8), 555–563.
Fu, Y., Gu, X. S., Hao, G. Z., Jiang, Y. F., Fan, W. Z., Fan, Y. M., et al. (2019). Comparison of myocardial microcirculatory perfusion after catheter-administered intracoronary thrombolysis with anisodamine versus standard thrombus aspiration in patients with ST-elevation myocardial infarction. Catheterization and Cardiovascular Interventions, 93(S1), 839–845.
Kelbaek, H., Terkelsen, C. J., Helqvist, S., Lassen, J. F., Clemmensen, P., Klovgaard, L., et al. (2008). Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: The drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. Journal of the American College of Cardiology, 51(9), 899–905.
Ito, N., Nanto, S., Doi, Y., Kurozumi, Y., Tonomura, D., Natsukawa, T., et al. (2011). Distal protection during primary coronary intervention can preserve the index of microcirculatory resistance in patients with acute anterior ST-segment elevation myocardial infarction. Circulation Journal, 75(1), 94–98.
Piscione, F., Piccolo, R., Cassese, S., Galasso, G., D'Andrea, C., De Rosa, R., et al. (2010). Is direct stenting superior to stenting with predilation in patients treated with percutaneous coronary intervention? Results from a meta-analysis of 24 randomised controlled trials. Heart., 96(8), 588–594.
Cuisset, T., Hamilos, M., Melikian, N., Wyffels, E., Sarma, J., Sarno, G., et al. (2008). Direct stenting for stable angina pectoris is associated with reduced periprocedural microcirculatory injury compared with stenting after pre-dilation. Journal of the American College of Cardiology, 51(11), 1060–1065.
Johnson, H. M., Gossett, L. K., Piper, M. E., Aeschlimann, S. E., Korcarz, C. E., Baker, T. B., et al. (2010). Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. Journal of the American College of Cardiology, 55(18), 1988–1995.
Hosokawa, S., Hiasa, Y., Miyazaki, S., Ogura, R., Miyajima, H., Ohara, Y., et al. (2008). Effects of smoking cessation on coronary endothelial function in patients with recent myocardial infarction. International Journal of Cardiology, 128(1), 48–52.
Miyazaki, T., Ashikaga, T., Ohigashi, H., Komura, M., Kobayashi, K., & Isobe, M. (2015). Impact of smoking on coronary microcirculatory resistance in patients with coronary artery disease. International Heart Journal, 56(1), 29–36.
Prior, J. O., Quinones, M. J., Hernandez-Pampaloni, M., Facta, A. D., Schindler, T. H., Sayre, J. W., et al. (2005). Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation., 111(18), 2291–2298.
Leung, M., & Leung, D. Y. (2016). Coronary microvascular function in patients with type 2 diabetes mellitus. EuroIntervention., 11(10), 1111–1117.
De Maria, G. L., Cuculi, F., Patel, N., Dawkins, S., Fahrni, G., Kassimis, G., et al. (2015). How does coronary stent implantation impact on the status of the microcirculation during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction? European Heart Journal, 36(45), 3165–3177.
Amsterdam, E. A., Wenger, N. K., Brindis, R. G., Casey Jr., D. E., Ganiats, T. G., Holmes Jr., D. R., et al. (2014). 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Journal of the American College of Cardiology, 64(24), e139–e228.
Thiele, H., de Waha, S., Zeymer, U., Desch, S., Scheller, B., Lauer, B., et al. (2014). Effect of aspiration thrombectomy on microvascular obstruction in NSTEMI patients: The TATORT-NSTEMI trial. Journal of the American College of Cardiology, 64(11), 1117–1124.
Ford, T. J., Stanley, B., Good, R., Rocchiccioli, P., McEntegart, M., Watkins, S., et al. (2018). Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. Journal of American College of Cardiology, 72(23 Pt A), 2841–2855.
Funding
Dr. James Xu is funded by a post-graduate scholarship from the Australian Government Research Training Program (RTP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. James Xu has received minor research grants from Abbott. Dr. Sidney Lo has received minor research grants from Abbott. The other authors have no conflict of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Associate Editor Angela Taylor oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Lo, S., Juergens, C.P. et al. Impact of Targeted Therapies for Coronary Microvascular Dysfunction as Assessed by the Index of Microcirculatory Resistance. J. of Cardiovasc. Trans. Res. 14, 327–337 (2021). https://doi.org/10.1007/s12265-020-10062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10062-z