Abstract
Spinal cord stimulation (SCS)-induced analgesia was characterized, and its underlying mechanisms were examined in a spared nerve injury model of neuropathic pain in rats. The analgesic effect of SCS with moderate mechanical hypersensitivity was increased with increasing stimulation intensity between the 20% and 80% motor thresholds. Various frequencies (2, 15, 50, 100, 10000 Hz, and 2/100 Hz dense-dispersed) of SCS were similarly effective. SCS-induced analgesia was maintained without tolerance within 24 h of continuous stimulation. SCS at 2 Hz significantly increased methionine enkephalin content in the cerebrospinal fluid. The analgesic effect of 2 Hz was abolished by μ or κ opioid receptor antagonist. The effect of 100 Hz was prevented by a κ antagonist, and that of 10 kHz was blocked by any of the μ, δ, or κ receptor antagonists, suggesting that the analgesic effect of SCS at different frequencies is mediated by different endorphins and opioid receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord stimulation (SCS) is a neuromodulation technique that delivers electrical impulses to the spinal cord via electrodes placed in the epidural space. It has been implemented for more than 50 years for the treatment of chronic neuropathic and intractable pain [1].
It is estimated that ~50,000 chronic pain patients receive SCS therapy worldwide each year [2]. The mechanism of paresthesia-based SCS is based on the gate control theory of pain modulation proposed by Melzack and Wall. It has been postulated that electrical impulses activate large-diameter fibers (Aβ) which block the noxious information transmitted to the brain by small-diameter fibers [3, 4]. According to gate control theory, the interaction between large- and small-diameter primary sensory fibers presumably occurs in the same segment of the spinal cord, therefore, the SCS site and dorsal root ganglia (DRG) of the primary sensory neurons that transmit pain signals should be in the same segment or a higher segment. Other investigators have provided evidence that supraspinal regions are also involved in SCS-induced analgesia [5,6,7]. The results of many studies have shown that a number of central nervous system (CNS)-derived neurotransmitters, such as gamma-aminobutyric acid [8], serotonin [5, 9], cannabinoid 1 receptors [10, 11], and opioid peptides [12,13,14] are involved in SCS-induced analgesia.
We are interested in the neurochemical mechanisms of acupuncture, electroacupuncture (EA), and the related technique of transcutaneous electrical acupoint stimulation (TEAS). Our previous findings showed that EA induces analgesia by activating the endogenous opioid system in a frequency-dependent manner: EA at 2 Hz selectively increases the gene expression and release of enkephalin in the brain, which binds to the μ-opioid receptor (MOR) and the δ-opioid receptor (DOR) to activate the descending inhibitory pathway, resulting in an analgesic effect. In contrast, EA at 100 Hz selectively increases the gene expression and release of dynorphin in the spinal cord and activates the κ-opioid receptor (KOR) [15,16,17]. We wondered whether SCS also induces analgesia by activating the endogenous opioid system, since primary afferent fibers can be activated either by EA at peripheral nerve terminals or by SCS at the dorsal root and/or dorsal horn. Several lines of evidence support this hypothesis. For example, pain relief and elevation of β-endorphin in cerebrospinal fluid (CSF) occur concomitantly in patients following SCS [14]. Animal experiments have demonstrated that the analgesic effect produced by 4-Hz SCS is blocked by the MOR antagonist naloxone, while that of 60-Hz SCS is blocked by the DOR-specific antagonist naltrindole [13]. Following SCS at 50 Hz, both the pain threshold and the dynorphin A (1–13) level in the spinal cord are elevated [12].
The involvement of endogenous opioid peptides in SCS analgesia is strongly indicated by these studies. However, the types of opioid receptors engaged in SCS at different frequencies of SCS have not yet been fully elucidated. Whether EA and SCS share the same or similar neurochemical mechanisms needs to be investigated. In addition, the analgesic effect of SCS with very high frequencies such as 10 kHz is well received by patients, but the mechanism is unclear.
This study was carried out to determine (1) whether SCS produces analgesia in the rat spared nerve injury (SNI) model of neuropathic pain and the characteristics of the pain-relieving profile regarding the frequency specificity of SCS and (2) whether endogenous opioid peptides and their corresponding receptors are involved, especially in the SCS at 10 kHz.
Materials and Methods
Animals
One hundred thirty-eight male Sprague Dawley rats weighing 160 ± 10 g were given rodent chow and water ad libitum and housed 1–4 per cage. The room temperature was maintained at 20–25 °C with ~50% humidity and on a 12:12 h light-dark cycle (lights on at 07:00). All animal experimental procedures were approved by the Animal Care and Use Committee of Peking University (Ethics Approval ID: LA2020434) and in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Spared Nerve Injury (SNI)
A modified SNI neuropathic pain model was used. Rats were anesthetized with 2%–3% isoflurane. The tibial and common peroneal nerves of the left hind limb were lightly ligated with 4–0 silk, and the sural nerve was left intact, similar to the model described by Decosterd and Woolf [18]. The overlying muscle and skin were sutured and closed.
Implantation of SCS Lead
Three days after SNI surgery (Fig. 1A), rats demonstrating mechanical hypersensitivity underwent laminectomy at the L1 and T12 levels, which corresponds to the L4–L6 lumbar spinal cord region. An SCS lead specifically designed for rats (Beijing PINS Medical Co., Ltd., China) contained three contact points spaced 2 mm apart (Fig. 2A). This lead was inserted epidurally in the rostral direction and fixed with sutures to muscle and ligament. The wound was closed with suture, and the distal end of the lead was tunneled to exit the skin at the base of the neck and fixed to the head for later connection to an external neurostimulator (T902, Beijing PINS Medical Co., Ltd., China). Stimulation at 2 Hz was applied, and motor contraction of the mid-lower trunk, ipsilateral hind limb, or tail was obtained if the electrode was properly placed.
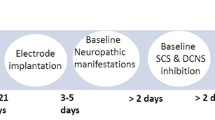
Experimental design. A Animal preparation for the SNI model and implantation of the SCS electrode and intrathecal catheter. B Procedure for 30-min SCS. C SCS at different intensities. D Short-term SCS at different frequencies and collection of CSF. E 24-h SCS at different frequencies and collection of CSF. SCS, spinal cord stimulation; PWT, paw withdrawal threshold; SNI, spared nerve injury; MT, motor threshold; CSF, cerebrospinal fluid.
Illustration of lead implantation and X-ray verification of the lead location. A Lead dimensions and the location of the implanted lead. B Location of the implanted electrode in relation to the vertebrae and ribs verified by X-ray. The T13–L1 vertebrae are identified by counting the ribs: the 13th rib is rostrally attached to the corpus of the 13th thoracic vertebra.
The impedance and motor threshold (MT) of all animals were tested before the study. Impedance represents the functional status of the electrode in the spinal epidural space, 0–1 kΩ suggested that there was a short-circuit between two contacts, 1–10 kΩ implied the electrode was in working condition, and 1–10 MΩ represented an open-circuit condition between the two contacts. Measured before SCS, the mean impedance was 3.99 ± 0.11 kΩ (Fig. 3A) 3 days after electrode implantation. It increased to ~6 kΩ 11 days later and was maintained at this level. MT was determined by slowly increasing the amplitude of the SCS (in 0.01-mA increments) with a 2 Hz, 200 μs pulse-width from zero until muscle contraction was observed in the mid-lower trunk or hind limb.
Characterization of the analgesic effect of SCS in a rat model of neuropathic pain. A, B Time courses for changes in impedance A and motor threshold B after SCS electrode implantation. C1 The 50% PWT in rats with severe and moderate mechanical hypersensitivity during and after 30-min 50-Hz spinal cord stimulation. C2 The mean 50% PWT calculated from C1. Pre-SCS, 50% PWT at 0 min in C1; Dur-SCS, mean 50% PWT at 15 and 30 min in C1; Post-SCS, 50% PWT at 60 min in C1. Repeated ANOVA with Dunnett's multiple comparisons test; **P < 0.01 vs Pre-SCS; ###P < 0.001 vs 50% PWT in Dur-SCS between moderate and severe mechanical hypersensitivity.
Implantation of Intrathecal Catheter
After lead implantation, the spinal canal was punctured at the L5 or L6 levels with a 23-gauge needle. A 32-gauge PE–10 catheter (Smiths Medical ASD, Inc., USA) was introduced into the subarachnoid space and advanced rostrally 3.5–4 cm to reach the lumbar enlargement. The catheter was fixed to the fascia, and its distal end was tunneled subcutaneously and then attached to the skin between the scapulae for intrathecal administration of drugs. To physiologically ascertain the proper position of the catheter tip, rats were dissected after sacrifice. The end of the catheter was located at the L4–L6 levels of the subarachnoid space in 99% of the rats.
Assessment of Mechanical Hypersensitivity
The paw withdrawal threshold (PWT) of rats was tested as described by Dixon [19]. Rats were placed in a plastic cage with a wire mesh bottom which allowed full access to the paws to acclimate for ~30 min until cage exploration and major grooming activities ceased. The area tested was the lateral surface of the hind paw in the sural nerve distribution. PWT was tested using calibrated von Frey filaments with bending forces ranging from 0.4 to 15.0 g (8 filaments: 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0, and 15.0 g). Based on Dixon’s up-down method, testing was initiated with a 2.0 g filament in the middle of the series. A withdrawal was determined based on whether the rat lifted its paw. If a positive withdrawal occurred, then an additional decreasing force was applied. If a negative withdrawal occurred, then an increased force was applied. The PWT was calculated using the 50% PWT method described by Chaplan et al. [20].
30-min SCS Procedure
The animals began to receive SCS seven days after SNI surgery (Fig. 1A). The contacts located in the T13–L1 vertebrae visualized by X-ray were chosen to serve as an anode and a cathode (Fig. 2B). Any two among the three electrodes were selected to cover an area containing the T13–L1 segments. Generally, the middle contact was set as the cathode. The rats were placed on the wire mesh for ~30 min for acclimation, and then the baseline 50% PWT was determined. After that, a 30-min SCS was implemented; the 50% PWT was obtained at 15 min and 30 min after the SCS was started, and again at 15 min and 30 min after the SCS was terminated (Fig. 1B).
Inclusion and Exclusion Criteria
SNI rats were excluded from the main studies if the baseline 50% PWT was < 1 g or >6 g. However, animals with 50% PWT < 1 g were analyzed as a subgroup with severe mechanical hypersensitivity. Animals in which the electrodes did not cover the T13–L1 levels verified by X-ray were also excluded.
Experimental Design
SCS at Different Levels of Mechanical Hypersensitivity
The animals were divided into the following groups: group 1 – severe (50% PWT value < 1 g, n = 4) and group 2 – moderate (50% PWT between 1 and 6 g, n = 4). Stimulation at 50 Hz with a 200-μs pulse width (biphasic symmetrical square waveform in constant-current mode), and an 80% MT was used.
SCS at Different Intensities (20%, 40%, 60%, and 80% MT)
Animals with moderate mechanical hypersensitivity were randomized into the following groups: group 1 – control (No-SCS) (n = 7); group 2 – SCS (n = 7). Animals in group 2 underwent stimulation with intensity set at 20%, 40%, 60%, and 80% MT (Fig. 1C). SCS at 50 Hz and a 200 μs pulse width (biphasic symmetrical square waveform in constant-current mode) was used.
Short-term SCS at Different Frequencies (2, 15, 50, 100, and 2/100 Hz, and 10 kHz)
The animals were divided into the following groups: group 1 – control (No-SCS) (n = 9); group 2 – SCS (n = 11). Multiple frequencies were tested in each animal with a 24-h washout period between two experiments. To avoid bias, the sequence of the frequencies tested was arranged in such a way that 2 rats received SCS in the sequence 2, 15, 50, 100 Hz, 10 kHz, and 2/100 Hz (dense-dispersed waveform with alternating frequencies between 2 and 100 Hz, for 3 s each [17]) (Fig. 1D); 2 rats with 15, 50, 100 Hz, 10 kHz, 2/100, and 2 Hz; 2 rats with 50, 100 Hz, 10 kHz, 2/100, 2, and 15 Hz; 2 rats with 100 Hz, 10 kHz, 2/100, 2, 15, and 50 Hz; 2 rats with 10 kHz, 2/100, 2, 15, 50, and 100 Hz; and 1 rat with 2/100, 2, 15, 50, 100 Hz, and 10 kHz. Stimulation at 80% MT, a 200 μs (30 μs at 10 kHz) pulse width (biphasic symmetrical square waveform in constant-current mode) was used in the frequency study.
Continuous SCS for 24 h at Different Frequencies (2, 50, 100 Hz, and 10 kHz)
The animals were randomized into the following groups: group 1 – control (No-SCS), (n = 6); group 2 – SCS, (n = 6). Multiple frequencies were tested in each rat with a 24-h washout period between any two frequencies tested. To avoid bias, the sequence of the frequencies tested was arranged in such a way that 2 rats received SCS in the sequence 2, 50, 100 Hz, and 10 kHz (Fig. 1E); 2 rats with 100 Hz, 10 kHz, 2, and 50 Hz; and 2 rats with 10 kHz, 2, 50, and 100 Hz.
The rats were placed in the testing cage with wire mesh bottom 30 min before study for acclimation, then the baseline 50% PWT was determined. After that, 24-h SCS at 80% MT, 200 μs (30 μs in 10 kHz) pulse width (biphasic symmetrical square waveform in constant-current mode) was applied, and the 50% PWT was measured every 2 h during 24-h continuous SCS.
Experimental Design for Selective Opioid Receptor Antagonists
The opioid receptor antagonists D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP, ab120078, Abcam, USA) and nor-binaltorphimine (Nor-BNI, P5296, Sigma, USA) were freshly dissolved in saline. Naltrindole (NTI, B6429, APExBIO, USA) was freshly dissolved in 10% dimethylsulfoxide. All drug solutions were pre-warmed to 37°C before administration. The drugs administered i.t. were followed by 10 μL pre-warmed saline to flush the catheter. Antagonists were injected 30 min before (2, 100 Hz, and 10 kHz) SCS at 11, 12, 13, 14, 15, and 16 days after SNI surgery, with a 24 h washout period between any two frequencies tested. The 50% PWT was obtained with 80% MT current and 200 μs pulse width (30 μs at 10 kHz).
The treatment of each experimental group is summarized in Table 1. The rats in the CTOP experiment were divided into three groups: group 1 – naive (no-SNI, n = 9); group 2 – control (SNI, no-SCS, n = 9), and group 3 – SCS (SNI, SCS, n = 9). To avoid bias, the sequence of the frequencies tested was arranged in such a way that 3 rats received SCS in the sequence 2, 100 Hz and 10 kHz (Table 2); 3 rats with 100 Hz, 10 kHz and 2 Hz; and 3 rats with 10 kHz, 2, and 100 Hz.
The rats in the NTI experiment were divided into three groups: group 1 – naive (no-SNI, n = 9); group 2 – control (SNI, no-SCS, n = 11), and group 3 – (SNI, SCS, n = 10). To avoid bias, the sequence of the frequencies tested was arranged in such a way that 3 rats received SCS in the sequence 2, 100 Hz, and 10 kHz (Table 3); 4 rats with 100 Hz, 10 kHz, and 2 Hz; and 3 rats with 10 kHz, 2, and 100 Hz.
The rats in the Nor-BNI experiment were divided into three groups: group 1 – naive (no-SNI, n = 10); group 2 – control (SNI, no-SCS, n = 11), and group 3 – SCS (SNI, SCS, n = 10). To avoid bias, the sequence of the frequencies was arranged in such a way that 3 rats received SCS in the sequence 2, 100 Hz, and 10 kHz (Table 4); 3 rats with 100 Hz, 10 kHz, and 2 Hz; and 4 rats with 10 kHz, 2, and 100 Hz.
Enzyme Linked Immunosorbent Assay (ELISA)
Cerebrospinal fluid (CSF) from the medulla oblongata pool was collected immediately after 30 min SCS under anesthesia. The concentration of methionine enkephalin (Met-enk) was determined following the instructions with a commercially-available ELISA kit (MBS756126, MyBiosource, Inc., San Diego, USA).
Statistics
IBM SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA) were used for statistical analyses and drawing graphs. For comparisons, repeated measures analysis of variance was used with Dunnett’s multiple comparison. The distribution of the data was examined by the Shapiro-Wilk normality test. The non-normally distributed data was analyzed by nonparametric tests (Kruskal-Wallis test and Dunn's multiple comparisons test). For all data, the results are expressed as the mean ± SEM, and P < 0.05 (two-tailed) was considered statistically significant.
Results
Determination of Motor Threshold of SCS
The MT in each rat was assessed by gradually increasing the stimulation intensity from zero mA with a 2 Hz frequency and 200 μs pulse width. The mean MT of rats under the experimental conditions was between 0.12 and 0.17 mA (Fig. 3B).
SCS Analgesia and Severity of Mechanical Hypersensitivity
The results from previous studies suggest that the analgesic effect of SCS is affected by the severity of mechanical hypersensitivity of the model [23, 24]. The analgesic effect of SCS was examined in animals with severe and moderate mechanical hypersensitivity: the 50% PWT of rats with moderate mechanical hypersensitivity was significantly increased from 3.42 ± 0.72 g to 9.79 ± 3.56 g (P < 0.01) during 15-min SCS (Fig. 3C1). The 50% PWT almost returned to pre-surgical levels. Meanwhile, the 50% PWT of rats with severe mechanical hypersensitivity only increased to 1.89 ± 1.10 g from a Pre-SCS level of 0.55 ± 0.10 g (P >0.05) (Fig. 3C1). To compare the analgesic effect of SCS between animals with severe and moderate mechanical hypersensitivity, the 50% PWT during SCS (Dur-SCS) was measured; this represented the mean value of 50% PWT at 15 min (15 min-SCS) and 30 min (30 min-SCS) after SCS was started. The data showed that the Dur-SCS 50% PWT in rats with moderate mechanical hypersensitivity (8.85 ± 2.77 g) was significantly increased from the Pre-SCS level (3.42 ± 0.72 g) (P < 0.01, Fig. 3C2), suggesting an increase in the pain threshold. The Dur-SCS in rats with moderate mechanical hypersensitivity was also significantly greater than that in rats with severe mechanical hypersensitivity (1.20 ± 0.53 g, P < 0.01, Fig. 3C2), suggesting that a better analgesic effect is produced by SCS in rats with moderate mechanical hypersensitivity.
Stimulation Intensity–Analgesic Response Relationship
The relationship between stimulation intensity and analgesic effect was studied using various levels of stimulation intensity from 20% MT to 80% MT. The results showed an intensity–response relationship except for the 40% MT (Fig. 4A1, A2, and Table S1).
The analgesic effect of SCS at different intensities and frequencies in short-term SCS and the effect of continuous prolonged SCS at 4 frequencies. A1, A2 Time course of the analgesic effect at different MTs of SCS at 50 Hz. There is an intensity-dependent and reversible elevation in the 50% PWT in rats with moderate mechanical hypersensitivity (also see Table S1). It appears that SCS at 60% MT or above is required to have a reliable analgesic effect. B1, B2 Time course of the analgesic effect of SCS at different frequencies at 80% MT. All frequencies (2, 15, 50, 100 Hz, 10 kHz, and 2/100 Hz alternating frequencies) of SCS showed similar degrees of analgesic effects in rats with moderate mechanical hypersensitivity. For short-term SCS, Pre-SCS represents the 50% PWT at 0 min in A1 and B1; Dur-SCS, mean 50% PWT at 15 and 30 min in B1; Post-SCS, 50% PWT at 60 min in A1 and B1. C1, C2 Time course for changes in 50% PWT during and after prolonged SCS in 80% MT at four frequencies. Prolonged SCS up to 24 h at 2, 50, 100 Hz, and 10 kHz had a similar persistent analgesic effect. The analgesic effect disappeared 1 h after termination of the SCS except in the 50 Hz group. Pre-SCS, 50% PWT at time 0 in C1; Dur-SCS, mean 50% PWT every 2 h from 2 to 24 h in C1; Post-SCS, 50% PWT at 25 h in C1. Repeated ANOVA with Dunnett's multiple comparisons test; **P < 0.01, ***P < 0.001 vs Pre-SCS; #P < 0.05, ##P < 0.01, ###P < 0.001 vs Dur-SCS in the No-SCS group.
No Stimulation Frequency–Analgesic Response Relationship
Our previous work demonstrated that the analgesic effect of EA is frequency dependent [15, 25, 26]. Therefore, we examined the analgesic effect of SCS at 6 different frequencies (2-, 15-, 50-, 100-Hz SCS, 10-kHz SCS, and 2/100-Hz alternating SCS). Compared with its own Pre-SCS value or Dur-SCS in the No-SCS group, the 50% PWT in each frequency group was similarly increased after SCS (Fig. 4B1 and B2). There was no significant difference in the analgesic effect among these frequency groups (Table S2).
Sustained Analgesia Induced by Prolonged SCS
To imitate prolonged treatment with SCS in clinical situations, the analgesic effect of SCS at four selected frequencies (2, 50, 100 Hz, and 10 kHz) was evaluated during continuous 24-h stimulation. The 50% PWT data were collected every other hour throughout the 24-h period of treatment. The SCS at all four frequencies had similar prolonged analgesic effects during the 24 h (Fig. 4C1, C2; 2-Hz Pre-SCS: 3.78 ± 0.75 g, Dur–SCS: 8.21 ± 0.96 g; 50-Hz Pre-SCS: 3.63 ± 0.31 g, Dur-SCS: 9.21 ± 0.79 g; 100-Hz Pre-SCS: 3.21 ± 0.47 g, Dur–SCS: 8.56 ± 0.85 g; 10-kHz Pre-SCS: 3.02 ± 0.48 g, Dur-SCS: 9.10 ± 0.60 g). No significant difference was found between any frequency groups (Table S3). The analgesic effect was persistent without showing any sign of tolerance during 24 h of continuous stimulation. The after-effect of 24 h-SCS was determined. The values of 50% PWT in all frequency groups returned to the pre-stimulation baseline level 60 min after termination of the SCS except in the 50-Hz group (Fig. 4C1, C2, and Table S3). This is in sharp contrast with EA analgesia, in which tolerance occurs after prolonged stimulation >6 h, suggesting a major difference in the underlying analgesic mechanism between the two interventions.
Involvement of Opioid Receptors in the Analgesic Effect of SCS
The increase in 50% PWT following 2-Hz SCS was blocked by the MOR-specific antagonist CTOP and the KOR-specific antagonist Nor-BNI but not by the DOR-specific antagonist NTI (Fig. 5, Tables S4 and S5), suggesting that MOR and KOR are involved in mediating the analgesic effect of 2-Hz SCS.
Effects of opioid receptor antagonists on the analgesic effect of 2-Hz SCS. A1, A2 CTOP (3 and 10 μg), an MOR-specific antagonist, significantly attenuates the analgesic effect. B1, B2 NTI (3 and 10 mg/kg), a DOR-specific antagonist, does not affect the analgesic effect. C1, C2 Nor–BNI (5.74 and 22.96 μg), a KOR-specific antagonist, significantly reduces the analgesic effect. Pre-SCS, 50% PWT at 0 min in A1, B1, and C1; Dur-SCS, mean 50% PWT at 15 and 30 min in A1, B1, and C1; Post-SCS, 50% PWT at 60 min in A1, B1, and C1. Repeated ANOVA with Dunnett's multiple comparisons test; *P < 0.05, **P <0.01, ***P < 0.001 vs Pre-SCS; #P < 0.05, ##P < 0.01, ###P < 0.001 vs Dur-SCS in the SCS + Veh group. CTOP, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; NTI, naltrindole; Nor-BNI, nor-binaltorphimine.
The increase in 50% PWT at 100-Hz SCS was blocked by the KOR-specific antagonist Nor-BNI, but not by CTOP or NTI (Fig. 6, Tables S6 and S7). These results suggest that KOR is involved in mediating the analgesic effect of SCS at 100 Hz.
Effects of opioid receptor antagonists on the analgesic effect of 100-Hz SCS. A1, A2 CTOP (10 and 30 μg), an MOR-specific antagonist, does not affect the analgesic effect. B1, B2 NTI (3 and 10 mg/kg), a DOR-specific antagonist, does not affect the analgesic effect. C1, C2 Nor-BNI (5.74 and 22.96 μg), a KOR-specific antagonist, significantly reduces the analgesic effect. Pre-SCS, 50% PWT at 0 min in A1, B1, and C1; Dur-SCS, mean 50% PWT at 15 and 30 min in A1, B1, and C1; Post-SCS, 50% PWT at 60 min in A1, B1, and C1. Repeated ANOVA with Dunnett's multiple comparisons test; **P < 0.01, ***P < 0.001 vs Pre-SCS; #P < 0.05, ###P < 0.001 vs Dur-SCS in the SCS + Veh group.
The analgesic effects of 10-kHz SCS were blocked by any of the three receptor antagonists tested, Nor-BNI, CTOP, and NTI (Fig. 7, Tables S8 and S9), suggesting that MOR, DOR, and KOR all participate in mediating the analgesic effect of 10-kHz SCS.
Effects of opioid receptor antagonists on the analgesic effect of 10-kHz SCS. A1, A2 CTOP (10 and 30 μg), an MOR-specific antagonist, significantly reduces the analgesic effect. B1, B2 NTI (3 and 10 mg/kg), a DOR-specific antagonist, significantly counteracts the hypersensitivity suppressive effect. C1, C2 Nor-BNI (5.74 and 22.96 μg), a KOR-specific antagonist, significantly reduces the analgesic effect. Pre-SCS, 50% PWT at 0 min in A1, B1, and C1; Dur-SCS, mean 50% PWT at 15 and 30 min in A1, B1, and C1; Post-SCS, 50% PWT at 60 min in A1, B1, and C1. Repeated ANOVA with Dunnett's multiple comparisons test; **P < 0.01, ***P < 0.001 vs Pre-SCS; #P < 0.05, ##P < 0.01, ###P < 0.001 vs Dur-SCS in the SCS + Veh group.
Met-enk Content in CSF
In addition to the pharmacological evidence that opioid peptides and opioid receptors are involved, changes in the content of Met-enk in CSF were determined following SCS. The results clearly showed that the concentration of Met-enk in CSF significantly increased from 9.68 ± 1.96 pg/mL in the No-SCS group to 18.70 ± 1.30 pg/mL after 2-Hz SCS. There was a trend of increased Met-enk content (14.43 ± 4.41 pg/mL) after 10-kHz SCS, but this did not reach a statistically significant level compared with the No-SCS group. The content of Met-enk remained unchanged after 100-Hz SCS (7.95 ± 1.89 pg/mL, Fig. 8).
Discussion
As an important technique in neuromodulation, SCS has been used more widely in recent years for chronic pain management. The underlying mechanisms of the analgesic effect are not fully understood, and efforts are being made to improve the analgesic efficacy. Using the SNI rat model with moderate mechanical hypersensitivity, we characterized the antinociceptive effect of SCS and studied the mechanism of action. In particular, we were interested in whether endorphins and opioid receptors are involved and whether the selection of pulse frequency is critical for the therapeutic effect.
Analgesic Effect and Stimulation Parameters of SCS
The analgesic effect of SCS may be affected by many factors such as the segments of the spinal cord stimulated, the severity of neuropathic pain, and stimulation parameters such as waveform, pulse width, intensity, and frequency.
It has been reported that the analgesic effect of SCS was more efficacious when applied to the spinal segments containing injured spinal nerves or above [23, 24], which is consistent with the hypotheses that the analgesic effect of SCS is based on gate control theory. We chose the T13–L1 vertebral segments corresponding to L4, L5, and L6 of the spinal cord as the stimulation sites since the SNI rats had injuries to the spinal nerves derived from L4, L5, and L6. The gate control hypothesis for SCS-induced analgesia is also supported by the fact that no tolerance developed after continuous SCS stimulation for 24 h. A significant reduction in the analgesic effect has been reported in rats receiving EA continuously for 6 h [17]. It is believed that central endorphins play an important role in mediating the analgesic effect of EA [15].
The analgesic effect of SCS was compared in the SNI model at different severities of mechanical hypersensitivity (moderate vs severe). Our data demonstrated that the analgesia of SCS was more effective in rats with moderate mechanical hypersensitivity. This is in agreement with previously reported results [23, 24].
We investigated the analgesic effect of SCS at different intensities (corresponding to 20%, 40%, 60%, and 80% MT). It can be predicted that the stimulation intensity is positively correlated with the analgesic effect of the SCS within the motor threshold (Fig. 4), consistent with previous reports [27,28,29]. The stimulation intensity corresponding to 80% MT was chosen to study the mechanism of SCS and dramatic antinociception was produced in the rat model of neuropathic pain.
The frequency of the SCS may serve as an independent signal transmitting neuromodulation information to the CNS. It has been demonstrated that stimulation of peripheral nerves by EA or TEAS produces pain relief in a frequency-dependent manner [15,16,17]. For example, 2-Hz TEAS is more effective than 100 Hz in reducing neuropathic pain [30]. In contrast, 100-Hz TEAS is more efficacious in muscle spasm-induced pain resulting from upper neuron injuries [31]. We hypothesized that SCS-induced analgesia would also have frequency specificity since the pulses used in peripheral nerve stimulation and SCS are almost identical except for a difference in the stimulation intensity used in these two modalities. In addition, primary afferent nerves are critically involved in mediating EA analgesia. These nerves can also be activated by SCS with electrodes placed near the dorsal root and dorsal horn of the spinal cord. However, similar to some previous findings [28, 31], our data showed that SCS at different frequencies (2, 15, 50, 100, 2/100 Hz and 10 kHz) had a similar degree of analgesia. This finding does not agree with some of the clinical reports in which 10-kHz SCS is more effective than traditional SCS [32]. One possibility is that patients may not be able to tolerate the high stimulation intensity (80% MT) used in animal studies. An uncomfortable feeling is associated with SCS at lower frequencies and higher intensity. In contrast, 10-kHz stimulation delivers much more energy per unit time but does not produce an uncomfortable paresthesia feeling. Therefore, it is more tolerable at higher intensities by patients. A new stimulation pattern, namely, differential target multiplexed programming SCS, has been reported to be more effective than traditional SCS [33,34,35,36]. Unfortunately, the neurochemical mechanism of these stimulation patterns is not fully understood and was not included in the present study.
The results of the present work suggest that SCS at various frequencies has similar antinociceptive efficacy and similar sustained effects following prolonged stimulation. It should be noted that only one SNI model of neuropathic pain was used in the present study. Other models of various kinds of pain should also be studied before any conclusion can be drawn regarding the frequency specificity of SCS in pain relief.
Opioid Receptors and SCS
Analgesic Mechanisms: SCS vs EA
Our previous work showed that endogenous opioid peptides and their receptors play a key role in EA analgesia [15,16,17]. EA at 2 Hz increases the release of enkephalin in the central nervous system, binding to the MOR and the DOR to induce analgesic effects. On the other hand, EA at 100 Hz increases the release of dynorphin and activates the KOR [15,16,17].
In the present study, SCS at 2 Hz increased the concentration of Met-enk in rat CSF, and the analgesic effect was blocked by an MOR-specific antagonist. These data suggest that, similar to EA, the analgesic effect of SCS at 2 Hz is mediated by Met-enk and MOR.
The analgesic effect of 2-Hz SCS was also significantly attenuated by a KOR-selective antagonist, indicating that KOR might also be involved in mediating the analgesia induced by 2-Hz SCS.
The analgesic effect of 100-Hz SCS was completely prevented by a KOR antagonist. Furthermore, no change in Met-enk concentration was found in the CSF, and the analgesic effect was not blocked by MOR or DOR antagonists, suggesting that 100-Hz SCS is mediated by KOR. It is likely that 100-Hz SCS has a neurochemical mechanism similar to that of 100-Hz EA: it selectively stimulates dynorphin release from the spinal cord, activating KOR to produce analgesia. Notably, that the variance in Met-enk concentration in CSF was large in this study, and more animals in future studies will be able to increase the power of statistical analysis.
Sato and colleagues found that the analgesic effect of 60-Hz SCS is mediated by DOR [13]. However, in our study, the analgesic effect of 100-Hz SCS was slightly lower in the presence of a DOR antagonist but did not achieve statistical significance. The difference may be because continuous administration by osmotic minipumps was used in their study, but a bolus i.p. injection was used in ours.
The results suggest that the analgesic mechanisms of SCS and EA have similarities and differences. The main reason for the differences might be that the EA signal is mediated only by primary affect nerve fibers, while SCS not only activates sensory neurons but also interneurons in the spinal cord. The latter may have much diversified responses.
It should be noted that our results are consistent with those reported previously: low frequency (2 or 4 Hz) SCS activates the MOR, and high frequency (50, 60, or 100 Hz) SCS activates the KOR [13], our results demonstrated that low-frequency SCS also activates the KOR.
10-kHz SCS
The analgesic effect of SCS at 10 kHz was blocked by antagonists against all three types of opioid receptor (MOR, DOR, and KOR). Our recent work using a single-unit recording technique in rats demonstrated that the signals of EA-like peripheral electric stimulation are transmitted into the CNS by Aβ and Aδ primary afferent sensory fibers. The highest pulse frequency recorded in the primary sensory neurons is ~250 Hz even though the stimulation frequency is up to 1000 Hz. This is presumably due to the refractory period of neurons [37]. Therefore, the frequency of 10-kHz SCS was too high to be transmitted to CNS neurons. Data from the present study showed that SCS at 100 Hz and 10 kHz induced similar reductions in the nociceptive response. It is reasonable to hypothesize that the actual or effective stimulation frequency of SCS at 10 kHz may not be >250 Hz in spinal neurons. Another possibility is that the 10-kHz SCS may be able to block the transmission of nociceptive signals in unmyelinated C fibers or other neurons by keeping them refractory.
Unlike the development of tolerance seen after prolonged stimulation with EA, no sign of decreased analgesic effect was found during 24 h of continuous SCS. This suggests that mechanisms other than endorphins play an important role in SCS analgesia.
The gate control theory may also be used to explain the analgesic effect of SCS. Dynorphinergic neurons are inhibitory interneurons that play a key role in the gate control mechanism. They receive signals from primary afferent Aβ fibers and shut the gate. As a result, the pain signal is prevented from entering the CNS [38]. The analgesic effects of 2-, 100-Hz, 10-kHz SCS, and 100-Hz EA were all blocked by the KOR antagonist, indicating that SCS at these frequencies activates inhibitory dynorphinergic interneurons to produce analgesia.
In summary, SCS had an efficacious antinociceptive effect with minimal after-effects in the SNI model of moderate neuropathic pain. The antinociceptive effect of SCS was intensity-dependent and persistent without developing tolerance under our experimental conditions. Many endorphins and their receptors may be involved in mediating the effect. There are similarities and differences in the mechanisms underlying SCS and EA.
References
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg 1967, 46: 489–491.
Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: Clinical efficacy and potential mechanisms. Pain Pract 2018, 18: 1048–1067.
Melzack R, Wall PD. Pain mechanisms: A new theory. Pain Forum 1996, 5: 3–11.
Guan Y. Spinal cord stimulation: Neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep 2012, 16: 217–225.
Tazawa T, Kamiya Y, Kobayashi A, Saeki K, Takiguchi M, Nakahashi Y. Spinal cord stimulation modulates supraspinal centers of the descending antinociceptive system in rats with unilateral spinal nerve injury. Mol Pain 2015, 11: 36.
Saadé NE, Barchini J, Tchachaghian S, Chamaa F, Jabbur SJ, Song Z, et al. The role of the dorsolateral funiculi in the pain relieving effect of spinal cord stimulation: A study in a rat model of neuropathic pain. Exp Brain Res 2015, 233: 1041–1052.
Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience 2013, 247: 134–144.
Ultenius C, Song ZY, Lin PY, Meyerson BA, Linderoth B. Spinal GABAergic mechanisms in the effects of spinal cord stimulation in a rodent model of neuropathic pain: Is GABA synthesis involved? Neuromodulation 2013, 16: 114–120.
Song ZY, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 2011, 152: 1666–1673.
Yang F, Xu Q, Shu B, Tiwari V, He SQ, Vera-Portocarrero LP, et al. Activation of cannabinoid CB1 receptor contributes to suppression of spinal nociceptive transmission and inhibition of mechanical hypersensitivity by Aβ-fiber stimulation. Pain 2016, 157: 2582–2593.
Sun L, Tai L, Qiu Q, Mitchell R, Fleetwood-Walker S, Joosten EA, et al. Endocannabinoid activation of CB1 receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur J Pain 2017, 21: 804–814.
Ding XH, Hua F, Sutherly K, Ardell JL, Williams CA. C2 spinal cord stimulation induces dynorphin release from rat T4 spinal cord: Potential modulation of myocardial ischemia-sensitive neurons. Am J Physiol Regul Integr Comp Physiol 2008, 295: R1519–R1528.
Sato KL, King EW, Johanek LM, Sluka KA. Spinal cord stimulation reduces hypersensitivity through activation of opioid receptors in a frequency-dependent manner. Eur J Pain 2013, 17: 551–561.
Tonelli L, Setti T, Falasca A, Martignoni E, Torcia E, Calcaterra FM, et al. Investigation on cerebrospinal fluid opioids and neurotransmitters related to spinal cord stimulation. Appl Neurophysiol 1988, 51: 324–332.
Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003, 26: 17–22.
Ulett GA, Han S, Han JS. Electroacupuncture: Mechanisms and clinical application. Biol Psychiatry 1998, 44: 129–138.
Han JS, Terenius L. Neurochemical basis of acupuncture analgesia. Annu Rev Pharmacol Toxicol 1982, 22: 193–220.
Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87: 149–158.
Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980, 20: 441–462.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw (Von Frey hairs). Journal of Neuroscience Methods 1994, 53: 55–63.
Ju J, Shin DJ, Na YC, Yoon MH. Role of spinal opioid receptor on the antiallodynic effect of intrathecal nociceptin in neuropathic rat. Neurosci Lett 2013, 542: 118–122.
Chen XH, Han JS. All three types of opioid receptors in the spinal cord are important for 2/15 Hz electroacupuncture analgesia. Eur J Pharmacol 1992, 211: 203–210.
Smits H, Ultenius C, Deumens R, Koopmans GC, Honig WMM, van Kleef M, et al. Effect of spinal cord stimulation in an animal model of neuropathic pain relates to degree of tactile “allodynia.” Neuroscience 2006, 143: 541–546.
Smits H, van Kleef M, Joosten EA. Spinal cord stimulation of dorsal columns in a rat model of neuropathic pain: Evidence for a segmental spinal mechanism of pain relief. Pain 2012, 153: 177–183.
Xing GG, Liu FY, Qu XX, Han JS, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol 2007, 208: 323–332.
Jiang YX, Wang Y, Liu HX. Comparison between therapeutic effects of transcutaneous electrical nerve stimulation with the frequency of 2 Hz and 100 Hz on Chronic inflammatory pain in rats. ZhongGuo Zhong Xi Yi Jie He Za Zhi 2001, 21: 923–925.
Shechter R, Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, et al. Conventional and kilohertz-frequency spinal cord stimulation produces intensity- and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 2013, 119: 422–432.
Sato KL, Johanek LM, Sanada LS, Sluka KA. Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg 2014, 118: 464–472.
Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional-SCS vs burst-SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: Effect of amplitude. Neuromodulation 2018, 21: 19–30.
Han JS. Acupuncture analgesia: Areas of consensus and controversy. Pain 2011, 152: S41–S48.
Zhao WL, Wang C, Li ZZ, Chen L, Li JB, Cui WD, et al. Efficacy and safety of transcutaneous electrical acupoint stimulation to treat muscle spasticity following brain injury: A double-blinded, multicenter, randomized controlled trial. PLoS One 2015, 10: e0116976.
Song ZY, Viisanen H, Meyerson BA, Pertovaara A, Linderoth B. Efficacy of kilohertz-frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation Technol Neural Interface 2014, 17: 226–235.
Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016, 79: 667–677.
Vallejo R, Kelley CA, Gupta A, Smith WJ, Vallejo A, Cedeño DL. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain 2020, 16: 1744806920918057.
Fishman M, Cordner H, Justiz R, Provenzano D, Merrell C, Shah B, et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract 2021, 21: 912–923.
Gong WY, Johanek LM, Sluka KA. A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physical activity in an animal model of neuropathic pain. Anesth Analg 2016, 122: 1178–1185.
Huo R, Han SP, Liu FY, Shou XJ, Liu LY, Song TJ, et al. Responses of primary afferent fibers to acupuncture-like peripheral stimulation at different frequencies: Characterization by single-unit recording in rats. Neurosci Bull 2020, 36: 907–918.
Duan B, Cheng LZ, Ma QF. Spinal circuits transmitting mechanical pain and itch. Neurosci Bull 2018, 34: 186–193.
Acknowledgement
We thank Beijing PINS Medical Co., Ltd., China for technical assistance. Special thanks go to Fu Xu (Beijing PINS Medical Co., Ltd.) and colleagues for modifying the SCS equipment for animal studies. This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology, China (2016YFC0105501, 2019YFD1712000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has conflict of interest regarding the contents of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhai, FJ., Han, SP., Song, TJ. et al. Involvement of Opioid Peptides in the Analgesic Effect of Spinal Cord Stimulation in a Rat Model of Neuropathic Pain. Neurosci. Bull. 38, 403–416 (2022). https://doi.org/10.1007/s12264-022-00844-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00844-7