Abstract
Purinergic P2 receptors, activated by endogenous ATP, are prominently expressed on neuronal and non-neuronal cells during development of the auditory periphery and central auditory neurons. In the mature cochlea, extracellular ATP contributes to ion homeostasis, and has a protective function against noise exposure. Here, we focus on the modulation of activity by extracellular ATP during early postnatal development of the lower auditory pathway. In mammals, spontaneous patterned activity is conveyed along afferent auditory pathways before the onset of acoustically evoked signal processing. During this critical developmental period, inner hair cells fire bursts of action potentials that are believed to provide a developmental code for synaptic maturation and refinement of auditory circuits, thereby establishing a precise tonotopic organization. Endogenous ATP-release triggers such patterned activity by raising the extracellular K+ concentration and contributes to firing by increasing the excitability of auditory nerve fibers, spiral ganglion neurons, and specific neuron types within the auditory brainstem, through the activation of diverse P2 receptors. We review recent studies that provide new models on the contribution of purinergic signaling to early development of the afferent auditory pathway. Further, we discuss potential future directions of purinergic research in the auditory system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the early 1970s ATP was recognized as a neurotransmitter molecule [1], opening a whole new research field of purinergic transmission and cellular signaling. Numerous studies revealed the importance of purinergic signaling in glial and neuronal communication, under both physiological and pathological conditions [2]. It was postulated that ATP is stored at different concentrations in probably every neuronal synaptic vesicle [3]. Vesicular release of ATP, and its co-release with other neurotransmitters, occur from both neurons and astrocytes [4,5,6,7,8,9,10,11]. Alternatively, ATP can be delivered to the extracellular space via membrane channels, such as connexin hemichannels [12,13,14], pannexin 1 [15,16,17], Ca2+ homeostasis modulator 1–3 [18,19,20], volume-regulated anion channels [21, 22], maxi-anion channels [23], and the P2X7 receptor [24]. Once released, extracellular ATP and its breakdown product adenosine exert a wide range of cellular effects by activating plasma membrane-localized receptors.
Purinoreceptors were first described in 1976 [25], and thereafter classified into two receptor families: P1 and P2, based on their affinity for adenosine and ATP/ADP [26]. Over the following years, an expanded nomenclature has been established: P2 receptors were further divided in two families, P2X and P2Y, each containing several subtypes, while P1 receptors were denominated as adenosine receptors [27]. P2X receptors are trimers or hexamers formed from individual subunits encoded by seven distinct genes and designated P2X1 to P2X7 [28]. They assemble to form ATP-gated ion channels, permeable to Na+, K+, and Ca2+ [29, 30]. All P2Y receptors are coupled to G-proteins, typically composed of seven transmembrane domains. In mammals, there are eight P2Y receptors divided into two subgroups based on the G-protein engagement: P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors are coupled to Gq and activate the phospholipase C-inositol 1,4,5-triphosphate pathway, while the receptors of the second subgroup, composed of P2Y12, P2Y13, and P2Y14, couple to Gi/o and inhibit adenylyl cyclase [31]. Four adenosine receptors have been cloned and characterized so far: A1, A2A, A2B, and A3. A1 and A3 are coupled to the Gi/o that reduces cAMP levels via inhibition of adenylyl cyclase. On the other hand, A2A and A2B activate Gs proteins and stimulate cAMP production [32].
Purinergic signaling is an essential component of sensory transduction and information coding in the visual, auditory, olfactory, and gustatory systems [33,34,35]. Along the ascending auditory pathway, purinergic receptors are expressed in many cell types (cochlea and spiral ganglion neurons (SGNs) [35]; cochlear nucleus (CN) [36]; medial nucleus of the trapezoid body (MNTB) [37]; and lateral and medial superior olive (LSO and MSO) [38]). In past years, we have gained new insights into the role and mechanisms of purinergic signaling in the developing auditory system, including propagation of Ca2+ waves across the inner ear [39, 40], modulation of Ca2+ spikes in inner hair cells (IHCs) [41,42,43], refinement and stimulation of outer hair cell (OHC) afferents [44, 45], and modulation of transmitter release and action potential (AP) discharges at specific brainstem synapses [46,47,48].
The extraction of sound frequencies from complex sound stimuli is accomplished in the inner ear, through the excitation of hair cells. Based on their position and the specific physical properties of that particular portion of the basilar membrane, the hair cells are excited only by a narrow band of sound frequencies [49]. This so-called tonotopic organization gives rise to precise maps of frequencies in all nuclei of the ascending auditory pathway up to the auditory cortex [50]. Similar to topographically arranged sensory maps in the visual and somatosensory systems [51,52,53], the establishment of tonotopic maps depends on two successive processes: (1) the genetically programmed distribution of molecular cues guiding the appropriate axonal projections to their synaptic partners, and (2) the activity-dependent refinement of such established connections [54,55,56]. In the auditory system, rearrangement of primary synaptic contacts begins before hearing onset [57, 58], guided by the spontaneous patterned activity generated in the inner ear and conveyed from there along the afferent auditory pathway [41, 59,60,61,62]. To what extent and through which mechanisms purinergic signaling contributes to the bursting activity of hair cells and, subsequently, central auditory neurons, is still a matter of debate. This review focuses on recent findings on the role of ATP in the cochlea and auditory brainstem during the sensitive developmental phase of the auditory system before and shortly after hearing onset.
Purinergic Signaling in the Developing Cochlea
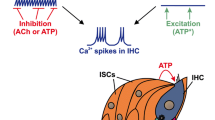
The cochlea is a spiral-shaped cavity within the bony labyrinth of the inner ear containing the organ of Corti, the sensory epithelium made up of the two types of sensory hair cells, embedded in several types of supporting cells (Fig. 1). The IHCs serve as transducers of a sound-evoked mechanical stimulus into a graded receptor potential, while the OHCs contribute to the increase in frequency selectivity and hearing sensitivity as part of an active nonlinear amplifier [63, 64]. The adult-like cytoarchitecture and function of the organ of Corti is fully established shortly after the onset of acoustically evoked signal processing (subsequently termed ‘hearing onset’ for the sake of brevity) [65, 66], which in altricial rodents is around postnatal day twelve (P12) (mouse [61, 67,68,69]; gerbil [70, 71]; rat [72]). Several physiological and anatomical features characterize an immature cochlea: (1) spontaneous Ca2+ waves which spread throughout the organ of Corti, at that time organized into the greater epithelial ridge containing Kölliker’s organ, IHCs, inner phalangeal cells, and border cells, and the lesser epithelial ridge, comprising OHCs and lateral non-sensory supporting cells [73, 74]; (2) Ca2+-dependent regenerative APs generated by IHCs and OHCs [41, 75]; and (3) reorganization of the afferent fibers of the SGNs which initially innervated both IHCs and OHCs, and subsequently establish the morphologically distinct IHC-type I SGN-fiber synapses and OHC-type II SGN-fiber synapses [76, 77].
Schematic of the organ of Corti as in prehearing mice, shown in cross section. Two types of sensory cells - inner hair cells (IHCs) and outer hair cells (OHCs) are tightly packed between the immature tectorial membrane (TM) and several types of supporting cells that control the homeostasis of extracellular fluids. P2 receptors are differentially expressed in sensory and supporting cells. The inset depicts the position of the organ of Corti within the temporal bone relative to other middle and inner ear structures. KO, Kölliker’s organ; ISC, inner supporting cell; OSC, outer supporting cell; Type I and II AF, spiral ganglion neuron afferent fibers; PC, pillar cell; BM, basilar membrane; DC, Deiters’ cell.
Intercellular Ca2+ waves, spontaneous Ca2+ APs, and refinement of hair cell-SGN synapses are essential for the development of the Organ of Corti, as well as for the structural reorganization of neuronal circuitries in the central auditory system [44, 58, 75, 76, 78,79,80,81,82,83,84,85]. There is ample evidence that purinergic signaling plays an important role in these early developmental processes. The current hypotheses are discussed below.
Inner Hair Cells
In adult animals, processing of acoustic information starts with transduction of mechanical pressure oscillations (sound wave) into graded receptor potentials of IHCs and electrical AP firing of SGNs. Before hearing onset, IHCs are irresponsive to sound, in part because of the closed external auditory meatus. Still, IHCs exhibit spontaneous Ca2+-dependent spikes, evoking bursts of APs in type I afferent fibers of SGNs, as shown by numerous studies in recent years crucially contributing to our understanding of the functional development of the cochlea.
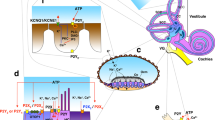
IHCs in the immature cochlea generate Ca2+ APs, whereas mature IHCs encode sound amplitude by graded receptor potentials [80, 86,87,88]. This transition, occurring around hearing onset, is mediated by the developmental change in the pattern of K+ channel expression in the IHC [89, 90]. Beforehand, i.e. during the first two postnatal weeks, ATP-mediated currents occur spontaneously in Kölliker’s organ [42]. This transient structure located medial to IHCs (Fig. 1) consists of tall columnar inner supporting cells (ISCs) which slowly degrade after hearing onset [73, 91]. Via ATP, the ISCs elicit the spreading intercellular Ca2+ waves that reach IHCs and affect their activity [41, 92]. The exact mechanism of initiation and expansion of these Ca2+ signals is still unclear, but they bear similarities with Ca2+ waves found in glia, neurons, epithelial cells, and many other cell types [93]. In the organ of Corti, intercellular Ca2+ waves seem to be initiated by the release of ATP via connexin Cx26 and Cx30 hemichannels [39, 94, 95], followed by the autocrine and paracrine activation of P2Y1, P2Y2, and P2Y4 receptors, and successive triggering of the PLC-IP3 pathway (Fig. 2) [96, 97]. Hemichannels are also able to release IP3 into the extracellular space, where it could bind to IP3 receptors expressed on the surface of supporting cells and potentiate the spread of Ca2+ waves [98, 99]. Additionally, connexin Cx26 and Cx30 build homo- and heteromeric gap junction proteins that allow the passage of IP3 and Ca2+ between cells (Fig. 2), thus enabling further propagation of intercellular Ca2+ waves [39, 92, 100]. Intracellular Ca2+ transients open Ca2+-activated Cl– channels TMEM16A, thus allowing the efflux of Cl– ions, followed by charge-balancing K+ efflux and water osmosis, resulting in crenation, i.e. shrinking of ISCs [101]. When Ca2+ waves reach the vicinity of IHCs, elevated levels of extracellular K+ initiate the following sequence of events: depolarization of the surrounding IHCs, generation of Ca2+ spikes, and glutamate release from IHCs, which finally triggers AP bursts in SGNs [101]. The accompanying crenation of ISCs enhances K+ clearance, thereby limiting the exposure of IHC to elevated [K+]e and preventing tonic IHC firing. This mechanism is thought to sculpt and coordinate firing activity in small groups of neighboring IHCs and SGNs [96, 101, 102]. To what extent ATP-induced Ca2+ waves influence the firing of IHCs, and subsequently SGN afferent fibers, is still a matter of debate. Yet, it is important to consider the different properties of immature IHCs: they fire spontaneous Ca2+ APs during the first postnatal week due to their depolarized resting membrane potential [43, 103], whereas in the second week, an external trigger in the form of ATP or an ATP-induced increase in [K+]e is required to elicit firing [42, 101]. This is in agreement with the finding that P4-6 IHCs generate single, or several successive, fast Ca2+ transients, independent of Ca2+ waves originating in Kölliker’s organ [40]. Still, Ca2+ waves in an ISC trigger additional bursts of Ca2+ transients in several neighboring IHCs, thereby enhancing and synchronizing their activity. Consistent with intrinsic AP firing in early postnatal IHCs, their activity is normal in Cx26 and Cx30 KO mice, which are devoid of Ca2+ waves in ISCs [84]. However, these IHCs fail to develop adult-like properties, suggesting a role of Ca2+ waves in shaping bursting activity during the prehearing period and thereby promoting maturation [83, 104].
Schematic of the proposed mechanism controlling the inner hair cell membrane potential. ATP, spontaneously released through connexin Cx 26 and Cx 30 hemichannels (1), binds to the auto- and heteroreceptors P2Y1, P2Y2, and P2Y4 (2) on inner supporting cells (ISCs). G-protein coupled P2YRs activate the PLC-IP3 signaling pathway that elevates the intracellular Ca2+ concentration. Connexin gap junctions (3) allow diffusion of IP3 and Ca2+ to neighboring ISCs, thus building an intercellular Ca2+ wave that reaches the vicinity of IHCs. Elevated [Ca2+]i activates the TMEM16A channel (4), leading to Cl– efflux that is balanced by K+ efflux through the leak K+ channels (5). The rise in extracellular K+ concentration depolarizes the IHC, resulting in a series of Ca2+-dependent APs shaped mainly by the KV delayed rectifier K+ channel (6) and the CaV1.3 Ca2+ channel (7). Further sculpting of the IHC firing pattern is achieved via SK2 K+ channel (8) that is activated by the Ca2+ influx through the surrounding α9α10 nicotinic ACh receptors (9) and P2X3 receptors (10). Additional Ca2+ entry and IHC depolarization might be achieved through P2X2 receptors (11) located in the proximity of stereocilia. AF, spiral ganglion neuron afferent fiber; EF, medial olivocochlear efferent fiber.
Another intriguing question is whether ATP can directly instigate/modulate the activity of immature IHCs. Several lines of evidence imply that ATP can affect IHCs through P2XRs: (1) IHCs express P2X2, P2X3, and P2X7 receptors [105,106,107,108]; (2) rat IHCs show ATP-induced inward currents starting as early as P1, with the largest amplitudes shortly before hearing onset [42]; and (3) focal application of 10 µmol/L–100 µmol/L ATP on neonatal IHCs elicits trains of APs in postsynaptic SGNs [41, 62]. Furthermore, superfusion of the cochlea with 100 µmol/L ATP also depolarizes IHCs in mouse and gerbil [43, 103]. However, application of the P2XR antagonist TNP-ATP causes IHC depolarization as well, while superfusion of low concentrations of ATP (3 nmol/L–10 nmol/L) hyperpolarizes IHCs [43]. These seemingly paradoxical results are explained by the putative involvement of different P2Rs (Fig. 2): (1) P2X2Rs in the proximity of stereocilia, activated by higher ATP concentrations might cause the IHC depolarization, and (2) P2X3Rs, coupled to SK2 channels at the IHC base and activated by nanomolar ATP concentrations might, on the contrary, lead to the hyperpolarization (Fig. 2) [43]. It is worth noting that SK2 channels are also indirectly coupled to heteromeric α9α10 nicotinic acetylcholine (ACh) receptors (Fig. 2). Their activation by efferent olivocochlear fibers causes Ca2+ influx, which in turn leads to opening of SK2 channels, hyperpolarization of IHCs through K+-efflux, and subsequent modulation of the AP discharge pattern [43, 103, 109,110,111,112]. Hence, ATP might act synergistically with ACh by modulating SK2 currents [43]. The studies showing that ATP application on IHCs elicits inward currents and depolarization of IHCs and AP firing in SGNs [41,42,43, 62, 103] were published before the mechanism of ISC-[K+]e-elicited IHC-activation had been revealed. Therefore, a potential indirect activation of IHCs via ISCs cannot be excluded, because the experimental setting in these earlier studies included the latter cells. On the other hand, in a recent study of the neonatal mouse cochlea, IHCs were probed by applying 1 µmol/L ATP from the pillar side to avoid an indirect effect through the ISCs [40]. Here, ATP application evoked bursts of Ca2+ transients in IHCs that preceded Ca2+ elevations in ISCs, consistent with direct stimulation of P2Rs on IHCs. However, the ATP concentration around active ISCs sufficient for further spreading of Ca2+ waves is estimated to be < 1 µmol/L [113], while the amount of endogenous ATP around immature IHCs, and whether it is sufficient for a functional effect, remains to be elucidated.
In summary, ATP instigates intercellular Ca2+ waves spreading through the developing cochlea, causing a K+-efflux from ISCs, and eventually depolarizing IHCs. In the first postnatal week, extracellular K+ synchronizes the intrinsic spiking of neighboring IHCs. Following the development of a hyperpolarized resting membrane potential during the second postnatal week, large Ca2+ waves arise as a necessary trigger of IHC bursting.
Outer Hair Cells
In mammals, locus-specific oscillation of OHCs along the basilar membrane amplifies the mechanical stimulus at corresponding IHCs in a tonotopic manner, increasing the sensitivity, frequency selectivity, and dynamic range of cochlear mechano-electrical stimulus transduction (“cochlear active process”) [63, 114,115,116]. The electromotility of mature OHCs can be reduced by ATP, thus affecting hearing sensitivity [12, 117]. In juvenile mice, purinergic signaling has been linked to the function of type II SGN fibers [45]. OHCs are contacted by the efferent fibers of medial olivocochlear neurons and by afferent fibers originating in type II SGNs. The former innervation conveys a suppression of OHC activity, serving to aid the detection of signals in noise and protect the cochlea from high-level sounds [118]. The role of afferent type II fibers is, on the other hand, still inconclusive [119]. One possible function is to drive the medial olivocochlear reflex, thus creating a negative feedback control of OHCs [120]. Another possibility is a nociceptive function through their concomitant activation via glutamate and ATP at trauma-inducing sound levels [121, 122].
During the first three postnatal weeks, type II fibers exhibit small and slow glutamatergic excitatory postsynaptic currents (EPSCs) elicited by sporadic vesicular glutamate release from OHCs [45]. It has been proposed that only concerted activation of groups of OHCs by very loud stimuli elicits AP firing in type II fibers [123, 124]. Type II fibers might also be depolarized by ATP released from supporting cells, possibly in response to damage of surrounding OHCs [45, 122]. It is possible that ATP evokes two distinct responses in type II afferents: high concentrations most likely stimulate P2X2Rs [106, 122], while low, submicromolar ATP preferentially activates G-protein-coupled P2Y receptors, apparently P2Y2Rs, resulting in closure of voltage-dependent KCNQ channels and higher excitability of SGN fibers [122, 125].
The OHC–type II afferent fiber synapse is established early in development (mouse: between E18 and P0 [76]), undergoing a substantial refinement in the first postnatal week, when OHCs exhibit Ca2+-dependent spikes similar to IHCs [75, 126]. The Ca2+ waves originating from Kölliker’s organ spread to the lesser epithelial ridge and activate P2YRs on Deiters’ cells, the non-sensory supporting cells underneath OHCs [44, 127]. Through the connexin hemichannels, Deiters’ cells release ATP, activating autologous P2YRs, but also the P2X3Rs of OHCs. The latter synchronize the otherwise spontaneous and random OHC Ca2+ signals and increase their firing rate [44]. This process is necessary for the proper refinement of the afferent projections onto OHCs, and for the maturation of OHCs, especially those in the apical cochlear region [75]. Additionally, type II fibers can be directly modulated by ATP, even shortly after hearing onset, which may contribute to synaptic maturation [45]. It still remains to be investigated whether the spontaneous OHC activity contributes to the bursting pattern of type II SGNs. Nevertheless, it can be concluded that purinergic signaling plays an important role in the maturation of OHC synapses and in the activation of type II SGN fibers.
Spiral Ganglion Neurons
SGNs are a heterogeneous group of bipolar neurons sending their peripheral afferents to the organ of Corti, while their central axons compose the auditory nerve (AN) projecting to the CN [128,129,130]. Type I SGNs account for 90%–95% of the primary afferent fibers, innervating a single IHC in groups of 5–30 [131]. Myelinated axons of type I SGNs are perfectly suited for fast transmission of APs from the cochlea, representing the main input to the central auditory system. The remaining 5%–10% are extensively branched afferents of type II SGNs, synapsing on multiple OHCs. The thin unmyelinated axons of type II SGNs project to the granule-cell region of the CN, probably relaying nociceptive information about trauma-inducing sounds [121, 129]. At birth, IHCs and OHCs are innervated by both types of SGN afferents, after which extensive pruning of the fibers in the first postnatal week leads to the adult-like wiring of hair cells [76, 77]. During this period, distinct P2Rs are expressed on type I and II SGNs, playing an important role in the synaptic refinement.
Both types of SGNs exhibit comparable ATP-gated somatic currents [132]. Several P2XRs reach maximal expression in SGNs during the first (P2X1 [133]; P2X3 [107, 134]; P2X7 [108]) and the second postnatal week (P2X2 [135]). In addition, five types of P2YRs are expressed in SGNs before the onset of hearing (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y12) [125]. A developmental role has been assigned to the P2X2/3 heteromeric receptor for promoting reorganization of promiscuous SGN innervation of hair cells [136]. This was shown by exposing P4 spiral ganglion explants to ATP analogues. Activation of P2X2/3 receptors inhibited BDNF-mediated neurite growth and branching, indicating an effect on pruning and the retraction of promiscuous SGN neurites from hair cells.
In addition to the role in refinement of hair cell-SGN synapses, purinergic signaling modulates the AP activity of SGNs. APs in type I SGNs are generally triggered by glutamate released from IHCs [62, 137, 138]. Extracellular ATP was shown to raise intracellular Ca2+ in the fiber and soma of type I SGNs [139], and puff application of ATP onto isolated type I SGNs evoked inward somatic currents [140]. Still, at SGN dendrites (IHC-SGN type I synapse), exogenous ATP induced only a small subthreshold voltage change in the presence of glutamate receptor blockers, failing to elicit APs [41]. Consistent with a rather modulatory role, the somatic P2YRs have been suggested to account for facilitation of AP propagation along type I nerve fibers [140, 141]. In contrast, the activation of type II SGN fibers seems to be mediated by both P2X and P2Y receptors, which seem to be potent enough to trigger APs [45, 122].
In conclusion, current data suggest direct modulation of both IHCs and OHCs and the respective type I and type II SGNs through yet not fully revealed combinations of P2Rs. Also, IHCs and type I SGNs can be indirectly activated, through the instigation of Ca2+-waves in Kölliker’s organ, subsequently causing a rise in the extracellular K+ concentration. Apparently, both mechanisms play crucial roles in the postnatal maturation of hair cells and SGNs, and in the refinement of their synaptic connections. Furthermore, by modulating spontaneous bursting activity of SGNs that is conveyed to the central nervous system, purinergic signaling may also contribute to the development of upstream brainstem nuclei.
Purinergic Signaling in the Auditory Brainstem
Sound features are encoded into series of APs, generated by SGNs (N. VIII pars cochlearis) and conveyed by their axons to second-order auditory brainstem neurons in the CN [142]. They in turn project to the third-order neurons, such as nuclei of the superior olivary complex (Fig. 3), nuclei of the lateral lemniscus, and to the inferior colliculus in the midbrain [143]. Distinct neuron types within the auditory brainstem express P2 receptors, and several studies have shown the effects of purinergic signaling on neuronal activity during early development.
Mammalian auditory brainstem with schematic wiring. Acoustic information is transduced into graded receptor potential by the inner hair cells (IHCs). This analog information is encoded into action potentials, conveyed by auditory nerve (AN) fibers. In the cochlear nucleus, the obligatory first central station along the afferent pathway, the information is segregated into streams processing different sound features. In the anteroventral part of the cochlear nucleus (AVCN), the major targets of AN fibers are stellate cells (multipolar cells, SCs), spherical bushy cells (SBCs), and globular bushy cells (GBCs). The medial superior olive (MSO) is the first stage of processing of interaural time differences through the computation of bilateral excitatory inputs from the SBC plus an inhibitory projection from the medial nucleus of the trapezoid body (MNTB). Note that MNTB neurons are activated by the contralateral GBCs. Interaural level differences are first calculated in the lateral superior olive (LSO), where excitatory input from the ipsilateral SBC and inhibitory input from the ipsilateral MNTB converge. Excitatory neurons are shown in green and yellow, inhibitory neurons in red.
Cochlear Nucleus
In the CN, each AN fiber bifurcates into an ascending branch projecting to the anteroventral cochlear nucleus (AVCN), and a descending branch projecting to the posteroventral (PVCN) and dorsal cochlear nuclei [142]. Thus, each AN fiber projects to all three tonotopically organized CN partitions [144]. There, different types of second-order neurons form the starting points of distinct ascending auditory processing pathways. Sound source localization relies on the integration of input signals generated at the two ears and conveyed from the ventral CN bushy cells (BCs) directly to the MSO and LSO, or indirectly via the MNTB (Fig. 3) [143, 145]. Large spherical BCs (see Fig. 3 SBC), densely packed in the anterior portion of the ventral CN, receive one to three large axosomatic terminals, i.e. the endbulbs of Held [146]. Small globular BCs (see Fig. 3 GBC) of the caudal AVCN and PVCN receive ~17 modified endbulbs [147]. Convergence of multiple excitatory inputs is thought to enhance the temporal accuracy of the BC firing with respect to presynaptic AN fibers [148]. Still, BCs do not function as a simple relay, since additional excitatory (cholinergic) and inhibitory (GABAergic/glycinergic) inputs shape their acoustically evoked firing [149,150,151,152,153,154,155,156,157,158]. In addition, recent in vitro and in vivo electrophysiological studies of BCs in gerbils provided evidence that ATP evokes membrane depolarization, and modulates the duration and frequency of APs during the early postnatal period [36, 46, 159]. Previous investigations had documented the expression of P2X2 receptor in the adult rat CN [37, 160, 161], but a possible contribution of purinergic signaling to nuclear development remained unknown. Using a wide range of electrophysiological, pharmacological, and histological methods, it was shown that BC somata express heteromeric P2X2/3 and P2Y1 receptors [36, 46, 159]. The Ca2+ entry through P2X2/3 activates PKC and thereby increases AP duration and frequency of firing. These effects persist for seconds [159], and this ongoing modulation of the firing activity is most likely mediated through a Ca2+–PKC-induced attenuation of the Kv conductance. Such modulation allows a larger Ca2+ influx [162] which might account for various forms of synaptic plasticity [163,164,165], and potentially also for the functional maturation of the endbulb of Held–BC synapse. Possible direct modulation of glutamate receptors through P2X2/3Rs can be excluded [159]. The activation of P2Y1Rs, on the other hand, is not sufficient to increase the AP firing per se, despite the induction of prominent Ca2+ release from internal stores [36].
The strongest ATP effects on BC activity occur before hearing onset, slowly waning until P23 [36, 46, 159]. This time-course matches the maturation profile of AMPA currents [159], and might be a compensatory mechanism to facilitate spiking during the period of pronounced AMPAR desensitization in immature BCs [166,167,168]. Direct application of ATP in vivo can evoke BC spiking under the pharmacological inhibition of glutamate receptors [46]. However endogenously released ATP does not seem to evoke APs independent of an AN drive, but rather facilitates the generation of glutamate-mediated APs by shortening the excitatory postsynaptic potential–AP delay [159]. In the first postnatal week, BCs are sensitive to ATP throughout the ventral CN. In the further course of development, the responsiveness to ATP becomes topographically restricted to low-frequency BCs in the rostral AVCN [159]. Stellate cells (SCs), the other principal neuronal type in the CN, are devoid of P2Rs at both neonatal and adult stages [36, 46, 159]. Considering the common innervation pattern, but different developmental time-courses of BCs and SCs [68], it is conceivable that purinergic signaling might contribute to the faster maturation of auditory processing in BCs.
The origin of ATP in the CN remains elusive. The possibility of a co-release of ATP with glutamate from excitatory endbulb terminals, or with GABA or glycine from inhibitory inputs can be excluded by stimulation of the respective inputs in acute slice preparations [46]. Therefore, ATP might originate from astrocytes, with glutamate from the endbulb triggering its release as part of a complex tripartite synapse [169]. Astrocyte-derived ATP has been shown to promote the postsynaptic insertion of AMPA receptors, the phosphorylation-dependent inhibition of postsynaptic and extrasynaptic GABA receptors, and the Ca2+-dependent inactivation of postsynaptic NMDA receptors, thus affecting the neuronal excitability and/or bidirectional modulation of synaptic strength [7, 170,171,172,173].
Taken together, endogenously released ATP specifically modulates the activity of BCs in a tonotopic manner during the critical period of synaptic refinement within the CN. Postsynaptic P2X2/3Rs increase the efficacy of the immature endbulb of Held–BC synapse, likely promoting synaptic strengthening during development.
Superior Olivary Complex
Several studies have addressed the potential role of purinergic signaling in the nuclei of the superior olivary complex (SOC). In the MNTB, large principal neurons (Fig. 3) are intermingled with fibers of the trapezoid body [174]. Each neuron receives excitatory synaptic input from a single globular BC of the contralateral CN through a giant axosomatic terminal, the calyx of Held [175], as well as through small conventional glutamatergic inputs from yet unidentified sources [176, 177]. In addition, the principal neurons receive inhibitory inputs that arise in the ventral nucleus of the trapezoid body and the MNTB itself [178, 179], but the respective impact of these projections is still a matter of debate [180,181,182]. The MNTB neurons also express purinergic receptors [37, 38, 160], which seem to be involved in the early postnatal development of excitatory and inhibitory signal transmission as shown both by in vitro and in vivo experiments [46, 48, 183, 184]. In the MNTB of prehearing gerbils, ATPγS evokes depolarization and P2XR-mediated currents in ~50% of neurons [46]. The ATPγS-evoked currents are sensitive to TNP-ATP, a specific blocker of P2X1, P2X3, and P2X2/3 receptors. Furthermore, in 11% of the MNTB neurons recorded just after hearing onset, ATPγS significantly increases both the spontaneous and acoustically evoked firing rates in vivo. In addition, ATPγS application increases the frequency of spontaneous excitatory and/or inhibitory postsynaptic currents in MNTB neurons of the rat recorded around hearing onset [48]. These results suggest an ATP-mediated facilitation of transmitter release at non-calyceal inputs. Detailed physiological examination revealed the presence of P2X3 receptor subunits (likely as a component of the P2X2/3R heteromer) on cell bodies and/or axons of the excitatory non-calyceal inputs [48]. Additional P2X1 subunits (possibly as part of the P2X1/2R) have been postulated to exist on presynaptic inhibitory terminals, whereas P2YR-mediated effects at the calyx-MNTB neuron synapse can be excluded [48].
There are also reports of a functional role of P1Rs at the calyx terminal [183, 184]. During high-frequency activity, endogenously released adenosine binds to presynaptic A1Rs, which in turn leads to the inhibition of presynaptic Ca2+ currents, eventually resulting in modest synaptic depression [183, 184]. These effects peak during the first postnatal week and become weaker with postnatal development [183].
The MNTB principal cells, which receive their excitatory input through the calyx of Held synapse, function as fast, sign-inverting relays, involved in the processing of sound source information [185] by providing reliable and well-timed inhibitory inputs to the LSO and MSO (Fig. 3). In addition to the inhibitory projection from the ipsilateral MNTB, the LSO neurons receive direct ipsilateral excitatory inputs from BCs, and the integration of both is the basis for the binaural processing of interaural level differences [186, 187]. Interaural time differences are encoded by bipolar MSO neurons, which receive direct excitatory projections from BCs of both sides [188]. Both the MSO and LSO neurons of adult rats express somatic P2X4 and P2X6 receptor subunits [38]. In the gerbil MSO, however, no purinergic signaling was found at the beginning of the second postnatal week by means of whole cell recording [46]. Yet, under the same conditions, around half of the LSO cells showed apparent P2X currents. A study in P1–P6 mice and rats reported pre- and postsynaptic P2Rs on the BC-LSO synapse [47]. Here, presynaptically bound ATP can increase the miniature EPSC frequency or decrease the miniature EPSC amplitude. Whether these effects are mediated by two distinct subgroups of P2 receptors, perhaps also affecting different types of LSO neurons, remains to be determined. Presynaptic P2XRs, boosting spontaneous synaptic events, have been shown in the dorsal horn of the spinal cord [189], the solitary tract nucleus [190], and the hippocampus [191]. On the contrary, in neurons of the medial habenula and hippocampus, presynaptic P2YR activation has been shown to inhibit spontaneous glutamate release [191, 192]. It is conceivable that different subsets of LSO neurons have different expression patterns of P2X and P2Y receptors, but the physiological importance of this distinction is not yet clear. Postsynaptic P2Rs appear to be P2XRs located either extrasynaptically, or on synapses from non-CN inputs [47], such as inputs from the LNTB or VNTB [193]. The functional importance of these projections is not fully understood, and considering the subtle effects seen in slice experiments, purinergic signaling might play only a minor modulatory role in LSO activity. Yet, it cannot be excluded that we still lack comprehensive knowledge about the role of ATP in the developing superior olivary complex circuit.
Physiological Relevance
Since the seminal work of Hubel and Wiesel in the visual system [194,195,196] it is well established that specific neuronal activity during critical developmental periods is required to produce high precision and strength of synaptic contacts in adult neuronal networks [197, 198]. Spontaneous neuronal activity can be observed in many systems before the onset of sensory processing, thereafter becoming stimulus-evoked, and thus representing early sensory experience. Temporally correlated spontaneous activity, originating in neighboring receptor cells, seems crucial for the refinement of initially promiscuous synaptic contacts and the generation of precise topographic maps along the afferent sensory pathways [78]. Orderly topographic connections from the HC sensors, via hierarchically organized CNS centers, to specific cortical areas represent the fundamental principle of the tonotopic organization in the auditory system [199]. Similar to the somatosensory and visual systems [200], patterned spontaneous activity in the afferent auditory pathway is important for the development of an orderly central nuclear organization, here tonotopy [82]. In the pre-hearing cochlea, groups of 5–10 neighboring (i.e. tonotopically similar) IHCs correlate the firing of SGN neurons projecting to CNS [41, 201]. This synchronous IHC activity contributes to the development of central synapses, but it is also important for maturation of the IHC itself. Extracellular ATP seems to play a central role in driving the Ca2+ waves that coordinate the activity of IHCs [96]. In addition, purinergic signaling drives the refinement of OHC–SGN type II synapses by increasing and synchronizing OHC activity [44]. Impairment of ATP-induced Ca2+ waves reduces the number of ribbon synapses and afferent fibers on OHCs [44]. This may render type II SGNs silent, since they require concerted input of many OHCs for their activation. Prehearing bursting activity is important for axonal distribution, pruning, and synapse formation between AN fibers and CN neurons [202]. Similar to activity modulation in the cochlea, endogenous ATP release in the CN increases the fidelity of immature endbulb of Held–BC synapses. This effect, mediated by P2X2/3Rs may contribute to synaptic strengthening through Hebbian-like plasticity [203]. P2XRs and NMDARs are similar in terms of relative Ca2+ permeability, and both receptor types have been shown to be involved in Ca2+-driven plasticity processes [204]. However, activation of P2XRs can mediate a substantial Ca2+ influx at resting membrane potential [205], as opposed to NMDARs. The intracellular Ca2+ can be further increased through the P2Y1R-induced PLC-IP3-Ca2+ pathway [36]. Generally, both synaptic potentiation and depression depend on cytoplasmic Ca2+ signals [163, 206], which can cause bidirectional changes in synaptic strength [207, 208] . Hence, it is conceivable that synaptic pruning or strengthening of immature endbulb–BC synapses depends on ATP-mediated signaling.
Perspectives
Although recent electrophysiological and immunohistological studies in the developing auditory system have provided new mechanistic insights into the functional roles of ATP signaling, there are several aspects which remain to be elucidated. Significant progress has been made in understanding the effects of ATP on the physiology of developing hair cells. The current hypothesis proposes an indirect effect of ATP through the Ca2+-dependent activation of TMEM16A channels expressed on ISCs and the subsequent increase in [K+]e [96, 101]. Local rapid changes of [K+]e promote the coordinated activity of neighboring IHCs and SGNs, causing synchronous bursting of central auditory neurons within respective isofrequency zones, thus potentially providing cues for the refinement and maturation of central circuits [59, 96, 101, 102]. However, what causes the initial spontaneous release of ATP from ISCs, and what is the potential role of P2Rs expressed on IHCs, remains elusive. Furthermore, the recent finding that Ca2+ waves, initiated and boosted by ATP in the greater epithelial ridge, travel to the lesser epithelial ridge, where they affect the activity of OHCs [44], raises the question of the functional importance of spontaneous AP firing in OHCs. Does it drive the refinement of OHC afferent innervation, or is it required for the maturation of SGNs and their central projections? Given that early in development both SGN type I and type II send afferent projections to OHCs, it remains intriguing to what extent the OHC activity contributes to the early bursting of the AN. Surprisingly, bursting activity persists in VGlut3−/− mice that show no glutamate release from IHCs [59]. Apparently, SGNs deprived of synaptic excitation become hyperexcitable, and fire bursts of APs in response to the K+ efflux from the ISCs [59]. Whether P2Rs, expressed on both IHC-SGN synapses and SGN somata, might contribute to the hyperexcitability of SGNs is an open question.
Among the central auditory neurons, purinergic enhancement of synaptic efficacy at the immature endbulb of Held–BC synapse in the CN has been well characterized [46, 159]. Still, the potential source and the mechanism of ATP release remain unknown. In addition, a better understanding of the development of auditory function would be gained from an animal model lacking purinergic modulation. Furthermore, the functional contribution of purinergic signaling in the SOC circuits is still inconclusive. Current studies indicate rather mild modulatory effects on transmitter release [46,47,48, 183, 184], yet the functional impact on sound signal processing is still unknown. Future studies need to reveal whether purinergic signaling in third-order neurons plays such a prominent role as in the developing cochlea and CN neurons. Finally, higher auditory structures, such as midbrain (inferior colliculus), diencephalon (medial geniculate body), and auditory cortex have not yet been investigated regarding the potential contribution of ATP signaling to the development or function of the mature system.
References
Burnstock G. Purinergic nerves. Pharmacol Rev 1972, 24: 509–581.
Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol 2020, 1202: 1–12.
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends in Neurosciences 2009, 32: 19–29.
Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 2007, 129: 485–491.
Ho T, Jobling AI, Greferath U, Chuang T, Ramesh A, Fletcher EL, Vessey KA. Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front Cell Neurosci 2015, 9: 389.
Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci 2002, 22: 4794–4804.
Lalo U, Palygin O, Verkhratsky A, Grant SGN, Pankratov Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci Rep 2016, 6: 33609.
Richardson PJ, Brown SJ. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem 1987, 48: 622–630.
Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, et al. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 2018, 9: 370.
Sperlágh B, Sershen H, Lajtha A, Vizi ES. Co-release of endogenous ATP and [3H]noradrenaline from rat hypothalamic slices: origin and modulation by α2-adrenoceptors. Neuroscience 1997, 82: 511–520.
Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M. CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci Signal 2019, 12: eaaw5450. doi:10.1126/scisignal.aaw5450.
Zhao H-B, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A 2005, 102: 18724–18729.
Yi C, Mei X, Ezan P, Mato S, Matias I, Giaume C, Koulakoff A. Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ 2016, 23: 1691–1701.
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 2008, 28: 4702–4711.
Chen J, Zhu Y, Liang C, Chen J, Zhao H-B. Pannexin1 channels dominate ATP release in the cochlea ensuring endocochlear potential and auditory receptor potential generation and hearing. Sci Rep 2015, 5: 10762.
Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A 2006, 103: 7655–7659.
Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, et al. Mechanosensitive release of adenosine 5’-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 2014, 62: 1486–1501.
Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron 2018, 98: 547–561.e10.
Ma J, Qi X, Yang C, Pan R, Wang S, Wu J, et al. Calhm2 governs astrocytic ATP releasing in the development of depression-like behaviors. Mol Psychiatry 2018, 23: 883–891.
Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013, 495: 223–226.
Gaitán-Peñas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernández-Dueñas V, Barrallo-Gimeno A, et al. Investigation of LRRC8-mediated volume-regulated anion currents in xenopus oocytes. Biophys J 2016, 111: 1429–1443.
Dunn PJ, Salm EJ, Tomita S. ABC transporters control ATP release through cholesterol-dependent volume-regulated anion channel activity. J Biol Chem 2020. doi:10.1074/jbc.RA119.010699.
Zhao B, Gu L, Liu K, Zhang M, Liu H. Maxi-anion channels play a key role in glutamate-induced ATP release from mouse astrocytes in primary culture. Neuroreport 2017, 28: 380–385.
Xiong Y, Teng S, Zheng L, Sun S, Li J, Guo N, et al. Stretch-induced Ca2+ independent ATP release in hippocampal astrocytes. J Physiol (Lond) 2018, 596: 1931–1947.
Burnstock G. Purinergic receptors. J Theor Biol 1976, 62: 491–503.
Burnstock G. A basis for distinguishing two types of purinergic receptors. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven Press, 1978: 107–118.
Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv 2018, 2: 2398212818817494.
North RA. Molecular physiology of P2X receptors. Physiol Rev 2002, 82: 1013–1067.
Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 2011, 63: 641–683.
Samways DSK, Li Z, Egan TM. Principles and properties of ion flow in P2X receptors. Front Cell Neurosci 2014, 8: 6.
Kügelgen I von. Pharmacology of P2Y receptors. Brain Res Bull 2019, 151: 12–24.
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: The state of the art. Physiol Rev 2018, 98: 1591–1625.
Rotermund N, Schulz K, Hirnet D, Lohr C. Purinergic signaling in the vertebrate olfactory system. Front Cell Neurosci 2019, 13: 112.
Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 2013, 7: 264.
Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci 2009, 32: 128–141.
Milenkovic I, Rinke I, Witte M, Dietz B, Rübsamen R. P2 receptor-mediated signaling in spherical bushy cells of the mammalian cochlear nucleus. J Neurophysiol 2009, 102: 1821–1833.
Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X(1)-P2X(6) receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups. J Comp Neurol 2000, 427: 485–507.
Koehl A, Schmidt N, Rieger A, Pilgram SM, Letunic I, Bork P, et al. Gene expression profiling of the rat superior olivary complex using serial analysis of gene expression. Eur J Neurosci 2004, 20: 3244–3258.
Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A 2008, 105: 18770–18775.
Eckrich T, Blum K, Milenkovic I, Engel J. Fast Ca2+ transients of inner hair cells arise coupled and uncoupled to Ca2+ waves of inner supporting cells in the developing mouse cochlea. Front Mol Neurosci 2018, 11: 264.
Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature 2007, 450: 50–55.
Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci 2010, 30: 1539–1550.
Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, et al. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci 2011, 14: 711–717.
Ceriani F, Hendry A, Jeng J-Y, Johnson SL, Stephani F, Olt J, et al. Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. EMBO J 2019, 38: e99839. doi:10.15252/embj.201899839.
Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature 2009, 461: 1126–1129.
Dietz B, Jovanovic S, Wielsch B, Nerlich J, Rubsamen R, Milenkovic I. Purinergic modulation of neuronal activity in developing auditory brainstem. J Neurosci 2012, 32: 10699–10712.
Kreinest M, Müller B, Winkelhoff J, Friauf E, Löhrke S. Miniature EPSCs in the lateral superior olive before hearing onset: regional and cell-type-specific differences and heterogeneous neuromodulatory effects of ATP. Brain Res 2009, 1295: 21–36.
Watano T, Calvert JA, Vial C, Forsythe ID, Evans RJ. P2X receptor subtype-specific modulation of excitatory and inhibitory synaptic inputs in the rat brainstem. J Physiol (Lond) 2004, 558: 745–757.
Bekesy G von. Experiments in Hearing. New York: McGraw-Hill Book Company, 1960.
Tsukano H, Horie M, Ohga S, Takahashi K, Kubota Y, Hishida R, et al. Reconsidering tonotopic maps in the auditory cortex and lemniscal auditory thalamus in mice. Front Neural Circuits 2017, 11: 14.
Harding-Forrester S, Feldman DE. Somatosensory maps. Handb Clin Neurol 2018, 151: 73–102.
Kremkow J, Jin J, Wang Y, Alonso JM. Principles underlying sensory map topography in primary visual cortex. Nature 2016, 533: 52–57.
Weigand M, Sartori F, Cuntz H. Universal transition from unstructured to structured neural maps. Proc Natl Acad Sci U S A 2017, 114: E4057–E4064.
Karmakar K, Narita Y, Fadok J, Ducret S, Loche A, Kitazawa T, et al. Hox2 genes are required for tonotopic map precision and sound discrimination in the mouse auditory brainstem. Cell Rep 2017, 18: 185–197.
Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res 1999, 297: 187–195.
Macova I, Pysanenko K, Chumak T, Dvorakova M, Bohuslavova R, Syka J, et al. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J Neurosci 2019, 39: 984–1004.
Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci 2003, 6: 282–290.
Clause A, Kim G, Sonntag M, Weisz CJC, Vetter DE, Rűbsamen R, Kandler K. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron 2014, 82: 822–835.
Babola TA, Li S, Gribizis A, Lee BJ, Issa JB, Wang HC, et al. Homeostatic control of spontaneous activity in the developing auditory system. Neuron 2018, 99: 511–524.e5.
Rübsamen R, Schäfer M. Ontogenesis of auditory fovea representation in the inferior colliculus of the Sri Lankan rufous horseshoe bat, Rhinolophus rouxi. J Comp Physiol A 1990, 167: 757–769.
Sonntag M, Englitz B, Kopp-Scheinpflug C, Rübsamen R. Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: an in vivo study in mice. J Neurosci 2009, 29: 9510–9520.
Tritsch NX, Rodriguez-Contreras A, Crins TTH, Wang HC, Borst JGG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci 2010, 13: 1050–1052.
Hudspeth AJ. Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci 2014, 15: 600–614.
Ekdale EG. Form and function of the mammalian inner ear. J Anat 2016, 228: 324–337.
Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl 1985, 422: 1–69.
Johnson SL, Franz C, Knipper M, Marcotti W. Functional maturation of the exocytotic machinery at gerbil hair cell ribbon synapses. J Physiol (Lond) 2009, 587: 1715–1726.
Alford BR, Ruben RJ. Physiological, behavioral and anatomical correlates of the development of hearing in the mouse. Ann Otol Rhinol Laryngol 1963, 72: 237–247.
Müller MK, Jovanovic S, Keine C, Radulovic T, Rübsamen R, Milenkovic I. Functional development of principal neurons in the anteroventral cochlear nucleus extends beyond hearing onset. Front Cell Neurosci 2019, 13: 119.
Song L, McGee J, Walsh EJ. Frequency- and level-dependent changes in auditory brainstem responses (ABRS) in developing mice. J Acoust Soc Am 2006, 119: 2242–2257.
McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus). Hear Res 1996, 100: 68–79.
Woolf NK, Ryan AF. The development of auditory function in the cochlea of the mongolian gerbil. Hear Res 1984, 13: 277–283.
Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res 1987, 429: 75–84.
Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol 2007, 51: 571–583.
Mammano F, Bortolozzi M. Ca2+ signaling, apoptosis and autophagy in the developing cochlea: Milestones to hearing acquisition. Cell Calcium 2018, 70: 117–126.
Jeng J-Y, Ceriani F, Hendry A, Johnson SL, Yen P, Simmons DD, et al. Hair cell maturation is differentially regulated along the tonotopic axis of the mammalian cochlea. J Physiol (Lond) 2020, 598: 151–170.
Huang L-C, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 2007, 134: 2925–2933.
Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci U S A 1992, 89: 6324–6327.
Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 2010, 11: 18–29.
Clause A, Lauer AM, Kandler K. Mice lacking the alpha9 subunit of the nicotinic acetylcholine receptor exhibit deficits in frequency difference limens and sound localization. Front Cell Neurosci 2017, 11: 167.
Corns LF, Johnson SL, Roberts T, Ranatunga KM, Hendry A, Ceriani F, et al. Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nat Commun 2018, 9: 4015.
Dayaratne MWN, Vlajkovic SM, Lipski J, Thorne PR. Kölliker’s organ and the development of spontaneous activity in the auditory system: implications for hearing dysfunction. Biomed Res Int 2014, 2014: 367939.
Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci 2009, 12: 711–717.
Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, et al. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc Natl Acad Sci U S A 2013, 110: 8720–8725.
Johnson SL, Ceriani F, Houston O, Polishchuk R, Polishchuk E, Crispino G, et al. Connexin-mediated signaling in nonsensory cells is crucial for the development of sensory inner hair cells in the mouse cochlea. J Neurosci 2017, 37: 258–268.
Yu WM, Goodrich LV. Morphological and physiological development of auditory synapses. Hear Res 2014, 311: 3–16.
Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 1998, 394: 281–284.
Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol (Lond) 2003, 548: 383–400.
Wong AB, Jing Z, Rutherford MA, Frank T, Strenzke N, Moser T. Concurrent maturation of inner hair cell synaptic Ca2+ influx and auditory nerve spontaneous activity around hearing onset in mice. J Neurosci 2013, 33: 10661–10666.
Kros CJ. How to build an inner hair cell: challenges for regeneration. Hear Res 2007, 227: 3–10.
Marcotti W. Functional assembly of mammalian cochlear hair cells. Exp Physiol 2012, 97: 438–451.
Hinojosa R. A note on development of Corti’s organ. Acta Otolaryngol 1977, 84: 238–251.
Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, et al. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal 2010, 6: 167–187.
Leybaert L, Sanderson MJ. Intercellular Ca(2+) waves: mechanisms and function. Physiol Rev 2012, 92: 1359–1392.
Mazzarda F, D’Elia A, Massari R, Ninno A de, Bertani FR, Businaro L, et al. Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca2+ signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab Chip 2020. doi:10.1039/d0lc00427h.
Schütz M, Scimemi P, Majumder P, Siati RD de, Crispino G, Rodriguez L, et al. The human deafness-associated connexin 30 T5M mutation causes mild hearing loss and reduces biochemical coupling among cochlear non-sensory cells in knock-in mice. Hum Mol Genet 2010, 19: 4759–4773.
Babola TA, Kersbergen CJ, Wang HC, Bergles DE. Purinergic signaling in cochlear supporting cells reduces hair cell excitability by increasing the extracellular space. Elife 2020. doi:10.7554/eLife.52160.
Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium 2007, 41: 77–86.
Gossman DG, Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes 2008, 15: 305-315.
Liu WJ, Yang J. Developmental expression of inositol 1, 4, 5-trisphosphate receptor in the post-natal rat cochlea. Eur J Histochem 2015, 59: 2486.
Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun 2003, 307: 362–368.
Wang HC, Lin C-C, Cheung R, Zhang-Hooks Y, Agarwal A, Ellis-Davies G, et al. Spontaneous activity of cochlear hair cells triggered by fluid secretion mechanism in adjacent support cells. Cell 2015, 163: 1348–1359.
Harrus A-G, Ceccato J-C, Sendin G, Bourien J, Puel J-L, Nouvian R. Spiking pattern of the mouse developing inner hair cells is mostly invariant along the tonotopic axis. Front Cell Neurosci 2018, 12: 407.
Sendin G, Bourien J, Rassendren F, Puel J-L, Nouvian R. Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea. Proc Natl Acad Sci U S A 2014, 111: 1999–2004.
Johnson SL, Wedemeyer C, Vetter DE, Adachi R, Holley MC, Elgoyhen AB, Marcotti W. Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses. Open Biol 2013, 3: 130163.
Brändle U, Zenner H-P, Ruppersberg JP. Gene expression of P2X-receptors in the developing inner ear of the rat. Neurosci Lett 1999, 273: 105–108.
Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Järlebark L, et al. Expression of the P2X 2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci 1999, 19: 8377–8388.
Huang L-C, Ryan AF, Cockayne DA, Housley GD. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem Cell Biol 2006, 125: 681–692.
Nikolic P, Housley GD, Thorne PR. Expression of the P2X7 receptor subunit of the adenosine 5’-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neurootol 2003, 8: 28–37.
Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol (Lond) 2004, 560: 691–708.
Moglie MJ, Fuchs PA, Elgoyhen AB, Goutman JD. Compartmentalization of antagonistic Ca2+ signals in developing cochlear hair cells. Proc Natl Acad Sci U S A 2018, 115: E2095–E2104.
Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 2000, 288: 2366–2368.
Zachary S, Nowak N, Vyas P, Bonanni L, Fuchs PA. Voltage-gated calcium influx modifies cholinergic inhibition of inner hair cells in the immature rat cochlea. J Neurosci 2018, 38: 5677–5687.
Rodriguez L, Simeonato E, Scimemi P, Anselmi F, Calì B, Crispino G, et al. Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc Natl Acad Sci U S A 2012, 109: 14013–14018.
He DZZ, Lovas S, Ai Y, Li Y, Beisel KW. Prestin at year 14: progress and prospect. Hear Res 2014, 311: 25–35.
Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 2008, 18: 370–376.
Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev 2001, 81: 1305–1352.
Yu N, Zhao H-B. ATP activates P2x receptors and requires extracellular Ca(++) participation to modify outer hair cell nonlinear capacitance. Pflugers Arch 2008, 457: 453–461.
Smith DW, Keil A. The biological role of the medial olivocochlear efferents in hearing: separating evolved function from exaptation. Front Syst Neurosci 2015, 9: 12.
Zhang KD, Coate TM. Recent advances in the development and function of type II spiral ganglion neurons in the mammalian inner ear. Semin Cell Dev Biol 2017, 65: 80–87.
Froud KE, Wong ACY, Cederholm JME, Klugmann M, Sandow SL, Julien J-P, et al. Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun 2015, 6: 7115.
Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, et al. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 2015, 25: 606–612.
Liu C, Glowatzki E, Fuchs PA. Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci U S A 2015, 112: 14723–14727.
Weisz CJC, Lehar M, Hiel H, Glowatzki E, Fuchs PA. Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. J Neurosci 2012, 32: 9528–9536.
Weisz CJC, Glowatzki E, Fuchs PA. Excitability of type II cochlear afferents. J Neurosci 2014, 34: 2365–2373.
Huang L-C, Thorne PR, Vlajkovic SM, Housley GD. Differential expression of P2Y receptors in the rat cochlea during development. Purinergic Signal 2010, 6: 231–248.
Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci 2008, 28: 1798–1803.
Berekméri E, Fekete Á, Köles L, Zelles T. Postnatal development of the subcellular structures and purinergic signaling of deiters’ cells along the tonotopic axis of the cochlea. Cells 2019. doi:10.3390/cells8101266.
Liberman MC. Single-neuron labeling in the cat auditory nerve. Science 1982, 216: 1239–1241.
Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol 1988, 278: 581–590.
Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol 1987, 255: 560–70.
Meyer AC, Moser T. Structure and function of cochlear afferent innervation. Curr Opin Otolaryngol Head Neck Surg 2010, 18: 441–6.
Jagger DJ, Housley GD. Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J Physiol (Lond) 2003, 552: 525–33.
Nikolic P, Housley GD, Luo L, Ryan AF, Thorne PR. Transient expression of P2X1 receptor subunits of ATP-gated ion channels in the developing rat cochlea. Brain Res Dev Brain Res 2001, 126: 173–182.
Huang L-C, Greenwood D, Thorne PR, Housley GD. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J Comp Neurol 2005, 484: 133–143.
Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5’-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol 1998, 393: 403–414.
Greenwood D, Jagger DJ, Huang L-C, Hoya N, Thorne PR, Wildman SS, et al. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development 2007, 134: 1407–1417.
Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 2002, 5: 147–154.
Kim KX, Payne S, Yang-Hood A, Li S-Z, Davis B, Carlquist J, et al. Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. J Neurosci 2019, 39: 4434–4447.
Cho H, Harada N, Yamashita T. Extracellular ATP-induced Ca2+ mobilization of type I spiral ganglion cells from the guinea pig cochlea. Acta Otolaryngol 1997, 117: 545–552.
Ito K, Dulon D. Nonselective cation conductance activated by muscarinic and purinergic receptors in rat spiral ganglion neurons. Am J Physiol, Cell Physiol 2002, 282: C1121–C1135.
Ito K, Dulon D. Purinergic signaling in cochleovestibular hair cells and afferent neurons. Purinergic Signal 2010, 6: 201–209.
Osen KK. Course and termination of the primary afferents in the cochlear nuclei of the cat. An experimental anatomical study. Arch Ital Biol 1970, 108: 21–51.
Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 2003, 60: 457–474.
Sando I. The anatomical interrelationships of the cochlear nerve fibers. Acta Otolaryngol 1965, 59: 417–436.
Middlebrooks JC. Sound localization. Handb Clin Neurol 2015, 129: 99–116.
Cao X-J, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength, and short-term plasticity. J Neurophysiol 2010, 104: 2308–2320.
Spirou GA, Brownell WE, Zidanic M. Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J Neurophysiol 1990, 63: 1169–1190.
Joris PX, Carney LH, Smith PH, Yin TC. Enhancement of neural synchronization in the anteroventral cochlear nucleus. I. Responses to tones at the characteristic frequency. J Neurophysiol 1994, 71: 1022–1036.
Dehmel S, Kopp-Scheinpflug C, Weick M, Dorrscheidt GJ, Rubsamen R. Transmission of phase-coupling accuracy from the auditory nerve to spherical bushy cells in the Mongolian gerbil. Hear Res 2010, 268: 234–249.
Englitz B, Tolnai S, Typlt M, Jost J, Rubsamen R. Reliability of synaptic transmission at the synapses of Held in vivo under acoustic stimulation. PLoS One 2009, 4: e7014.
Goyer D, Kurth S, Gillet C, Keine C, Rubsamen R, Kuenzel T. Slow cholinergic modulation of spike probability in ultra-fast time-coding sensory neurons. eNeuro 2016. doi:10.1523/ENEURO.0186-16.2016.
Keine C, Rubsamen R. Inhibition shapes acoustic responsiveness in spherical bushy cells. J Neurosci 2015, 35: 8579–8592.
Keine C, Rubsamen R, Englitz B. Inhibition in the auditory brainstem enhances signal representation and regulates gain in complex acoustic environments. Elife 2016. doi:10.7554/eLife.19295.
Keine C, Rübsamen R, Englitz B. Signal integration at spherical bushy cells enhances representation of temporal structure but limits its range. Elife 2017. doi:10.7554/eLife.29639.
Kuenzel T, Borst JGG, van der Heijden M. Factors controlling the input-output relationship of spherical bushy cells in the gerbil cochlear nucleus. J Neurosci 2011, 31: 4260–4273.
Kuenzel T, Nerlich J, Wagner H, Rubsamen R, Milenkovic I. Inhibitory properties underlying non-monotonic input-output relationship in low-frequency spherical bushy neurons of the gerbil. Front Neural Circuits 2015, 9: 14.
Nerlich J, Keine C, Rubsamen R, Burger RM, Milenkovic I. Activity-dependent modulation of inhibitory synaptic kinetics in the cochlear nucleus. Front Neural Circuits 2014, 8: 145.
Nerlich J, Kuenzel T, Keine C, Korenic A, Rubsamen R, Milenkovic I. Dynamic fidelity control to the central auditory system: synergistic glycine/GABAergic inhibition in the cochlear nucleus. J Neurosci 2014, 34: 11604–11620.
Jovanovic S, Radulovic T, Coddou C, Dietz B, Nerlich J, Stojilkovic SS, et al. Tonotopic action potential tuning of maturing auditory neurons through endogenous ATP. J Physiol (Lond) 2017, 595: 1315–1337.
Xiang Z, Bo X, Burnstock G. P2X receptor immunoreactivity in the rat cochlea, vestibular ganglion and cochlear nucleus. Hear Res 1999, 128: 190–196.
Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, et al. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 1999, 407: 11–32.
McCobb DP, Beam KG. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage activated calcium channels. Neuron 1991, 7: 119–27.
Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 1999, 9: 305–313.
Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic Ca 2+i elevation. J Neurophysiol 1999, 81: 781–787.
Mahajan G, Nadkarni S. Intracellular calcium stores mediate metaplasticity at hippocampal dendritic spines. J Physiol (Lond) 2019, 597: 3473–502.
Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. J Physiol 1998, 511 (Pt 3): 861–869.
Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci 2001, 21: 9487–9498.
Chanda S, Xu-Friedman MA. A low-affinity antagonist reveals saturation and desensitization in mature synapses in the auditory brain stem. J Neurophysiol 2010, 103: 1915–1926.
Illes P, Burnstock G, Tang Y. Astroglia-derived ATP modulates CNS neuronal circuits. Trends Neurosci 2019, 42: 885–898.
Gordon GRJ, Baimoukhametova DV, Hewitt SA, Rajapaksha WRAKJS, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nature neuroscience 2005, 8: 1078–1086.
Gordon GRJ, Iremonger KJ, Kantevari S, Ellis-Davies GCR, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 2009, 64: 391–403.
Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci 2006, 26: 9006–9009.
Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 2014, 12: e1001747.
Morest DK. The collateral system of the medial nucleus of the trapezoid body of the cat, its neuronal architecture and relation to the olivo-cochlear bundle. Brain Res 1968, 9: 288–311.
Morest DK. The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z Anat Entwicklungsgesch 1968, 127: 201–220.
Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol 1998, 79: 3127–3142.
Hamann M, Billups B, Forsythe ID. Non-calyceal excitatory inputs mediate low fidelity synaptic transmission in rat auditory brainstem slices. Eur J Neurosci 2003, 18: 2899–2902.
Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol 1991, 314: 684–706.
Albrecht O, Dondzillo A, Mayer F, Thompson JA, Klug A. Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse. Front Neural Circuits 2014, 8: 83.
Mc Laughlin M, van der Heijden M, Joris PX. How secure is in vivo synaptic transmission at the calyx of Held? J Neurosci 2008, 28: 10206–10219.
Kopp-Scheinpflug C, Dehmel S, Tolnai S, Dietz B, Milenkovic I, Rübsamen R. Glycine-mediated changes of onset reliability at a mammalian central synapse. Neuroscience 2008, 157: 432–445.
Lorteije JAM, Rusu SI, Kushmerick C, Borst JGG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci 2009, 29: 13770–13784.
Kimura M, Saitoh N, Takahashi T. Adenosine A(1) receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol (Lond) 2003, 553: 415–426.
Wong AYC, Billups B, Johnston J, Evans RJ, Forsythe ID. Endogenous activation of adenosine A1 receptors, but not P2X receptors, during high-frequency synaptic transmission at the calyx of Held. J Neurophysiol 2006, 95: 3336–3342.
Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 2010, 90: 983–1012.
Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol 1986, 247: 457–476.
Spangler KM, Warr WB, Henkel CK. The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J Comp Neurol 1985, 238: 249–262.
Warr WB. Fiber degeneration following lesions in the anterior ventral cochlear nucleus of the cat. Exp Neurol 1966, 14: 453–474.
Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 1997, 389: 749–753.
Jin Y-H, Bailey TW, Li B-Y, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 2004, 24: 4709–4717.
Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 2005, 25: 6286–6295.
Price GD, Robertson SJ, Edwards FA. Long-term potentiation of glutamatergic synaptic transmission induced by activation of presynaptic P2Y receptors in the rat medial habenula nucleus. Eur J Neurosci 2003, 17: 844–850.
Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech 2000, 51: 330–354.
Hubel DH, Wiesel TN. Integrative action in the cat’s lateral geniculate body. J Physiol (Lond) 1961, 155: 385–398.
Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol (Lond) 1970, 206: 419–436.
Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol 1963, 26: 978–993.
Mudd DB, Balmer TS, Kim SY, Machhour N, Pallas SL. TrkB activation during a critical period mimics the protective effects of early visual experience on perception and the stability of receptive fields in adult superior colliculus. J Neurosci 2019, 39: 4475–4488.
Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci U S A 1996, 93: 602–608.
Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci 2010, 4: 79.
Hanganu-Opatz IL. Between molecules and experience: role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Res Rev 2010, 64: 160–176.
Zhang-Hooks Y, Agarwal A, Mishina M, Bergles DE. NMDA receptors enhance spontaneous activity and promote neuronal survival in the developing cochlea. Neuron 2016, 89: 337–350.
Baker CA, Montey KL, Pongstaporn T, Ryugo DK. Postnatal development of the endbulb of held in congenitally deaf cats. Front Neuroanat 2010, 4: 19.
Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, et al. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol (Lond) 2006, 571: 563–578.
Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience 2009, 158: 137–148.
Egan TM, Samways DSK, Li Z. Biophysics of P2X receptors. Pflugers Arch 2006, 452: 501–512.
Mateos-Aparicio P, Rodríguez-Moreno A. Calcium dynamics and synaptic plasticity. Adv Exp Med Biol 2020, 1131: 965–84.
Jędrzejewska-Szmek J, Damodaran S, Dorman DB, Blackwell KT. Calcium dynamics predict direction of synaptic plasticity in striatal spiny projection neurons. Eur J Neurosci 2017, 45: 1044–1056.
Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science 1999, 285: 1870–1874.
Acknowledgements
IM and SJ were supported by the Deutsche Forschungsgemeinschaft (DFG Grant 954/3-1) as a part of the priority program 1608 “Ultrafast and temporally precise information processing: normal and dysfunctional hearing.” We thank Rudolf Rübsamen for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jovanovic, S., Milenkovic, I. Purinergic Modulation of Activity in the Developing Auditory Pathway. Neurosci. Bull. 36, 1285–1298 (2020). https://doi.org/10.1007/s12264-020-00586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00586-4