Abstract
Neuroligins (NLs) are postsynaptic cell-adhesion proteins that play important roles in synapse formation and the excitatory-inhibitory balance. They have been associated with autism in both human genetic and animal model studies, and affect synaptic connections and synaptic plasticity in several brain regions. Yet current research mainly focuses on pyramidal neurons, while the function of NLs in interneurons remains to be understood. To explore the functional difference among NLs in the subtype-specific synapse formation of both pyramidal neurons and interneurons, we performed viral-mediated shRNA knockdown of NLs in cultured rat cortical neurons and examined the synapses in the two major types of neurons. Our results showed that in both types of neurons, NL1 and NL3 were involved in excitatory synapse formation, and NL2 in GABAergic synapse formation. Interestingly, NL1 affected GABAergic synapse formation more specifically than NL3, and NL2 affected excitatory synapse density preferentially in pyramidal neurons. In summary, our results demonstrated that different NLs play distinct roles in regulating the development and balance of excitatory and inhibitory synapses in pyramidal neurons and interneurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synaptic adhesion proteins have been shown to regulate the formation, maturation, and maintenance of synapses and synaptic transmission. They are therefore considered to be critical in the development of neuronal connectivity in the central nervous system. Neuroligins (NLs) belong to a family of cell-adhesion proteins which are postsynaptically localized and mediate the differentiation of postsynaptic organization. NL1 and NL2 proteins are mainly expressed in post-excitatory and post-inhibitory synapses respectively [1,2,3], and NL3 is expressed in both [4]. NL4 is localized to glycinergic post-synapses [5]. With such a specific expression pattern of each NL, they have been found to differentially regulate the development and balance of excitatory and inhibitory synapses [6,7,8,9,10,11].
In microscopic studies, overexpression of NL1/2/3 increases the density of vesicular glutamate transporter 1 (vGlut1) and vesicular gamma-aminobutyric acid (GABA) transporter (vGAT) clusters in cultured hippocampal neurons, indicating common upregulation of both excitatory and inhibitory synapses [8,9,10], among which NL2 shows a preference for inhibitory synapse formation and reduces the excitatory/inhibitory (E/I) ratio [8]. Electrophysiological results have shown that overexpression of NL2 selectively affects the inhibitory but not the excitatory transmission in cultured hippocampal neurons [11], while overexpression of NL1 increases the frequency of both miniature inhibitory synaptic currents (mIPSCs) and miniature excitatory synaptic currents (mEPSCs) [9]. However, controversial results from Sudhof’s group have shown that the EPSC amplitude, specifically the N-methyl-D-aspartate receptor (NMDAR) current, changes in a similar overexpression system, but not the properties of mIPSCs or mEPSCs [12]. The disparities between the microscopic and electrophysiological studies or among the electrophysiological studies might have resulted from the different sensitivities of the assays applied or the culture densities of neurons. Nevertheless, the studies suggest that NL1/2/3 can all affect the formation and transmission of excitatory and inhibitory synapses, NL1 having a preference for excitatory synapses and NL2 for inhibitory synapses.
Overexpression may cause protein mislocation and thereby introduce more artefactual problems, nevertheless, the NL knockdown or knockout studies have led to similar functional annotations of NLs. Knockdown of NL1/2/3 with specific shRNAs effectively reduces the vGlut1 density in cultured hippocampal neurons. In addition, knockdown of NL1/2 but not NL3 reduces spine and vGAT density [8]. Knockout of NL1 reduces EPSC but not IPSC amplitude in hippocampus and somatosensory cortex [11, 13, 14]. Knockout of NL2 mainly reduces vGAT puncta in the hippocampus as well as defects in inhibitory neurotransmission in both the hippocampus and somatosensory cortex [2, 13, 15]. Conditional knockout of NL3 in Purkinje cells produces a small but significant reduction in mEPSC amplitude [16]. Although neither the synaptic density nor synaptic size is altered in NL1/2/3 single- or triple-knockout mice [15, 17, 18], sparse knock-down of NL1 reduces synapse number and spine density in cortical layer 2/3 pyramidal neurons [19], indicating a potential chronic compensation for the synapse loss caused by NL deficiency.
While there are abundant studies of the function of NL in pyramidal neurons, their functions in interneurons are barely known. It has been shown that NMDAR-mediated synaptic transmission is significantly reduced in the cerebellar stellate and hippocampal parvalbumin (PV) interneurons of NL1 and NL3 PV conditional-knockout mice, respectively [20, 21]. The unitary IPSC amplitude evoked in fast spiking interneurons is 40%–50% lower in the somatosensory cortex of NL2-knockout mice [2]. However, it remains unclear whether such alterations in PV neurotransmission are due to an intracellular deficit or to modulation of neighboring cells. Moreover, it is difficult to determine the synaptogenic role of NLs in interneurons from brain slice studies as it is difficult to identify synaptic subtypes on a single neuron.
To determine the differential capacities of NLs to regulate the formation of subtypes of synapse and to clarify their differential contribution to E/I balance in pyramidal neurons and interneurons, we specifically knocked down NL expression using a virus-mediated shRNA approach and examined the synapse formation in different neurons.
Materials and Methods
Animals
Embryonic day 18 (E18) Sprague-Dawley rats were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. All animal procedures were in accordance with the guidelines of the Animal Advisory Committee at Zhejiang University following the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Animal Committee of Zhejiang University.
Antibodies
Antibodies were from the following companies or self-made: mouse monoclonal CaMKII (1:500, Abcam, ab22609), mouse monoclonal GAD67 (1:500, Chemicon, MAB5406), rabbit polyclonal PSD-95 (1:500, self-made, generated against the amino-acids 77–299 fragment of rat PSD-95 and purified on a Affi-gel affinity column), rabbit anti-GABA A Receptor alpha 6 (1:500, Abcam, ab117100), rabbit anti-GAPDH (1:1000, Cell Signaling, 2118S), rabbit anti-GFP (1:1000, self-made), rabbit anti-NL1 (1:1000, self-made, generated against the amino-acids 718–844 fragment of NL1), rabbit anti-NL2 (1:1000, self-made, generated against the amino-acids 699–837 fragment of NL2), and rabbit anti-NL3 (1:1000, self-made, generated against the amino-acids 706–825 fragment of NL3). The rabbit anti-GFP antibody cross-reacted with YFP and Venus and was raised against GST-GFP fusion proteins and purified with His-GFP fusion proteins coupled with Affi-gel beads (Bio-Rad, Catalog 153-6099) as previously described [22]. The NL antigen was purified on a His-tag affinity column. The NL antiserum derived from rabbit was purified on an Affi-gel affinity column.
Secondary antibodies were from Invitrogen (Cy3-mouse, A10521; Cy3-rabbit, A10520; 488-rabbit, A-11008; 488-mouse, A-11029; 647-mouse, A-21235; and 647-rabbit, A-21443) and Thermo Scientific (goat anti-mouse-680, 35518; goat anti-rabbit-680, 35568; goat anti-mouse-800, 35521; and goat anti-rabbit-800, 35571).
Constructs
The YFP-tagged mouse NL1 and NL2 were gifts from Dr. A.M. Craig (Washington University). The YFP-tagged NL3 was PCR-amplified from a pBC SK+ vector (Kazusa, mKIAA 1480) and inserted into a pEYFP-N1 vector (from Dr. D. Chang, Hong Kong University of Science and Technology) by SalI/BamHI for signal peptide (amino-acids 1–34) before the YFP sequence and by BsrGI/XbaI for the rest of the sequence (amino-acids 35–825) after the YFP sequence.
The shRNA plasmids were cloned by inserting shRNA double-strand DNAs into the pSuper vector (a gift from Dr. N. Ip, Hong Kong University of Science and Technology) by BglII/XhoI. The pH1-shRNA fragments in the pSuper constructs were then swapped with the pH1 fragment in the modified pFUGW vector by EcoRI/XhoI to make the shRNA lentiviral constructs. The shRNA double-strand DNA sequences were made by annealing the primer pairs shown in Table 1. The HIV-1 packing vector Δ8.9 and the VSVg envelope glycoprotein plasmid were generous gifts from Dr. C. Lois (Massachusetts Institute of Technology).
Lentivirus Production and Infection
Lentiviruses were produced from transfected HEK293T cells. shNL constructs in pFUGW vector or the control vector, VSVg envelope glycoprotein plasmid, and HIV-1 packing vector Δ8.9 were mixed at a ratio of 2:1:1.5 in μg and transfected into HEK293T cells using Lipofectamine 2000 (Gibco) according to the manufacturer’s instructions. The transfection medium was replaced by fresh medium every 6–8 h. Lentiviruses were harvested 48 h after transfection by collecting the medium and centrifugation at 1000 rpm at 4°C for 5 min to remove cell debris. The medium was then filtered through a 0.45-µm filter, aliquoted, and stored at −80°C. Fifteen microliters of harvested medium was used to infect neurons cultured in 12-well plates, followed by biochemical analyses or immunocytochemistry 6–7 days after infection.
Neuron Culture and Immunocytochemistry
Dissociated rat primary cortical neurons were cultured as previously described [23], except that the brain region was cortex instead of hippocampus, and 3–5 μL of DNase was added during digestion. Neurons were fixed in 4% paraformaldehyde (Sinopharm, Beijing, China) and 4% sucrose in 1× PBS for 20 min and then incubated with 0.2% Triton X-100 in 1× PBS for 10 min at room temperature. The neurons were then blocked with 10% normal donkey serum in 1× PBS for 2 h at room temperature and incubated with primary antibodies for 1 h at room temperature or at 4°C overnight. After washing 3 times with 1× PBS, the cells were incubated with secondary antibodies for 2 h at room temperature. After three washes with 1× PBS for 10 min each, neurons were mounted in Mowiol 4-88-glycerol mounting solution containing 6 g glycerol (Sango Biotech, Shanghai, China), 2.4 g Mowiol 4-88 (Calbiochem, Darmstadt, Germany), 6 mL ddH2O, 12 mL 0.2 mol/L Tris (pH 8.5, HX Bio, Hangzhou, China), and 2.5% DABCO (1,4-diazabicyclo[2.2.2]octane, Sigma Aldrich, Darmstadt, Germany).
Immunoblotting
Crude lysates were collected in 1× Laemmli sample buffer and boiled at 100°C for 5 min. Protein extracts were separated by SDS-PAGE and then transferred to a nitrocellulose membrane (Pall, New York, USA). Membranes were incubated with the corresponding antibodies for 1 h at room temperature or overnight at 4°C after blocking with 1% skimmed milk in 1× TBS (6.057 g Tris, 8.5 g NaCl, 3.4 mL HCl, ddH2O added to 1 L, pH 7.6). The membranes were then incubated with corresponding secondary antibodies and imaged on a LI-COR (Lincoln, NE) Odyssey CLx Imaging System. GAPDH was used as an internal control.
Microscopy, Image Processing, and Analysis
Slides were visualized under a Nikon (Tokyo, Japan) A1R confocal scanning microscope in the core facilities of Zhejiang University School of Medicine. Images were captured with the same parameters and analyzed blindly. The control and experimental groups were processed in parallel.
Statistics
Statistics were carried out using Graphpad 6 prism software (San Diego, CA). Unless otherwise specified, comparisons between two groups were made with the unpaired two-sample Student’s t-test. Multiple comparisons were carried out using the one-way ANOVA Newman-Keuls test. At least 45 neurons from at least 3 individual experiments were analyzed.
Results
Pyramidal Neurons and Interneurons Differ in Synapse Formation
To examine the regulatory role of NLs in specific synapse formation, we knocked down the expression of endogenous NLs and analyzed the synapse density on both pyramidal neurons and interneurons. We first tested in the primary cultured cortical neurons the reliability of commercial antibodies as specific neuronal or synaptic markers using an immunocytochemical approach. We found that the ratio between neuronal populations marked by CaMKII and GAD67 was ~7:3 (Fig. S1), which was similar as the ratio between pyramidal neuron and interneuron in rat cortex. The PSD-95 and GABA(A)Rα1 antibodies showed distinguishable puncta along dendrites (Fig. S2). We then set out to use CaMKII as the marker of pyramidal neurons, GAD67 as the interneuron marker, and PSD-95 as the excitatory synapse marker. Because the inhibitory synapses where NL2/3 are localized are mainly GABAergic [3, 4, 11], and from our results, the GABA(A)Rα1 antibody worked much better than the gephyrin antibody for quantification (data not shown), we decided to use GABA(A)Rα1 as the GABAergic synapse marker.
We then calculated the growing puncta densities of excitatory and GABAergic inhibitory synaptic markers separately in pyramidal neurons and interneurons. Primary cortical neurons from rats on embryonic day 18 (E18) were cultured and stained with the above antibodies in combination from days in vitro (DIV) 2 to DIV 16 (Figs. 1 and S3–4). We found that PSD-95 had a higher density and exerted earlier growth in pyramidal neurons than in interneurons (Fig. 1B–C). The density of GABA(A)Rα1 was higher in interneurons throughout the culture period than in pyramidal neurons. It showed a steady and continuous growth in pyramidal neurons while in interneurons it increased slowly from DIV 6 to DIV 12 and more rapidly afterwards (Fig. 1D–E). This result indicates that the excitatory and GABAergic synapses have differential growth in pyramidal neurons and interneurons.
Development of excitatory and GABAergic synapses in cultured cortical neurons. A PSD-95 and GABA(A)Rα1 puncta formation from DIV 2 to DIV 16 in cultured cortical pyramidal neurons (PN) and interneurons (IN). Scale bar, 10 μm. B–E Quantitation of PSD-95 and GABA(A)Rα1 puncta in pyramidal neurons and interneurons (mean ± SEM; n = 3 independent experiments).
Specific Knockdown of Neuroligin Causes Indistinctive Changes in the Localization and Expression of the Other Ligins
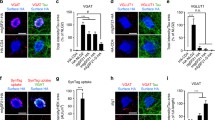
We used the RNAi strategy to elucidate the function of NLs in subtyped synapse formation. We designed NL1/2/3-specific shRNAs and first verified their knockdown efficiency and specificity in overexpressing HEK293T cells (Fig. 2). shRNAs in lentiviral backbone plasmids were transfected together with venus-tagged NL1/2/3 plasmids into HEK293T cells. The results of immunoblotting showed that shNL1, shNL2, and shNL3 effectively and specifically knocked down the expression of NL1, NL2, and NL3, respectively.
Validation of shRNA efficiency and specificity in neuroligin knockdown in HEK293T cells. A–C Immunoblots of NLs after shRNA knockdown in HEK293T cells. A shNL1 specifically and efficiently knocked down NL1 expression (0.082 ± 0.033 for shNL1, 1.436 ± 0.204 for shNL1-scr, 0.705 ± 0.171 for shNL1-2, 1.013 ± 0.070 for shNL2, and 1.019 ± 0.059 for shNL3, normalized to control). B shNL2 specifically and efficiently knocked down NL2 expression (1.594 ± 0.235 for shNL1, 1.337 ± 0.169 for shNL1-scr, 1.135 ± 0.049 for shNL1-2, 0.110 ± 0.033 for shNL2, and 1.430 ± 0.068 for shNL3, normalized to control). C shNL3 specifically and efficiently knocked down NL3 expression (0.783 ± 0.323 for shNL1, 1.293 ± 0.063 for shNL1-scr, 1.153 ± 0.215 for shNL1-2, 0.897 ± 0.085 for shNL2, and 0.166 ± 0.078 for shNL3, normalized to control; one-way AVONA, *P <0.05, **P <0.01, ***P <0.001; mean ± SEM; n = 3 independent experiments).
Therefore, shNL1, shNL2, and shNL3 were selected to generate Lentiviruses for specific NL knockdown, and the knockdown effects on endogenous NLs were further examined in cultured cortical neurons. Neurons were infected at DIV 6 by viruses expressing different shRNAs and the expression levels of the different NLs were assessed by immunoblotting at DIV 13. We found a very high infection efficiency in neurons (Fig. S5). Immunoblotting results showed that shNL1, shNL2, and shNL3 effectively and specifically knocked down the expression of endogenous NL1, NL2, and NL3 correspondingly in cultured cortical neurons (Fig. 3A, D, G). To confirm that the deficit of one NL affected the expression pattern of other NLs in neurons, we quantified the NL puncta density on the dendrites of both pyramidal neurons and interneurons. We found that the shNL1 lentivirus effectively reduced the NL1 puncta density to 70.5% of the control level in pyramidal neurons and to 51.4% in interneurons (Figs. 3B–C and S6). shNL2 reduced the NL2 puncta density to 31.7% of the control level in pyramidal neurons and to 43.1% in interneurons (Figs. 3E–F and S7), and for shNL3, to 57.4% and 54.9%, respectively (Figs. 3H–I and S8). In addition, none of the shNLs caused non-specific alterations in the expression patterns of other NLs (Fig. 3B, C, E, F, H, I). This result indicated that NL-specific shRNAs specifically and effectively knock down the expression of the target proteins, without causing significant changes of the others.
Validation of shRNA efficiency and specificity in neuroligin knockdown in cultured cortical neurons. A Immunoblots and quantification of NL1 expression after shRNA knockdown (0.216 ± 0.078 for shNL1, 1.02 ± 0.079 for shNL2, and 0.748 ± 0.013 for shNL3, normalized to control). B Representative images of NL1 puncta after shRNA knockdown in pyramidal neurons (PN) and interneurons (IN) at DIV 16. Scale bars, 10 μm. C Quantification of images as in B (0.229 ± 0.022 for shNL1, 0.516 ± 0.023 for shNL2, and 0.414 ± 0.047 for shNL3 in PNs; and 0.247 ± 0.015, 0.654 ± 0.055, and 0.630 ± 0.051, respectively in INs, normalized to control in PN or IN). D Immunoblots and quantification of NL2 expression after shRNA knockdown (1.050 ± 0.216 for shNL1, 0.266 ± 0.043 for shNL2, and 0.955 ± 0.061 for shNL3, normalized to control). E Representative images of NL2 puncta after shRNA knockdown at DIV 16. Scale bars, 10 μm. F Quantification of images as in E (0.371 ± 0.036 for shNL1, 0.218 ± 0.018 for shNL2, and 0.312 ± 0.036 for shNL3 in PNs; and 0.286 ± 0.031, 0.246 ± 0.016, and 0.394 ± 0.043, respectively, in INs, normalized to control in PNs or INs). G Immunoblots and quantification of NL3 expression after shRNA knockdown (1.033 ± 0.137 for shNL1, 0.749 ± 0.048 for shNL2, and 0.261 ± 0.126 for shNL3, normalized to control). H Representative images of NL3 puncta after shRNA knockdown at DIV 16. Scale bars, 10 μm. I Quantification of images as in H (0.529 ± 0.055 for shNL1, 0.583 ± 0.044 for shNL2, and 0.240 ± 0.029 for shNL3 in PNs; and 0.672 ± 0.044, 0.594 ± 0.035, and 0.239 ± 0.033, respectively, in INs, normalized to control in PNs or INs; one-way ANOVA test, *P < 0.05, **P < 0.01, ***P < 0.001; mean ± SEM. n = 3 independent experiments).
Neuroligin Isoforms Contribute Differentially to Excitatory and GABAergic Synapse Formation
The knockdown specificity of shNLs largely excluded the compensatory effects from other NLs as in knockout studies. We therefore infected the cultured cortical neurons with shRNA-expressing viruses and examined the two types of synapses separately in pyramidal neurons and interneurons. Using the synapse development assay described above (Fig. 1), we infected neurons at DIV 6 and quantified the PSD-95 density at DIV 13, and infected neurons at DIV 10 and quantified GABA(A)Rα1 density at DIV 16. In order to minimize toxic effects of the virus, we infected neurons with very small amounts of virus sufficient for quantification and changed the medium every 4–6 h after infection.
We found that knockdown of NL1/2/3 all caused significant reductions in the density of PSD-95 puncta in pyramidal neurons, shNL1 and shNL2 being more effective than shNL3 (Fig. 4A, C). Meanwhile, in interneurons, both shNL1 and shNL3 effectively reduced the PSD-95 puncta density, shNL3 showing higher potency, while shNL2 barely had any effect (Fig. 4B, D). This result indicates that NL1 and NL3 are able to regulate the formation of excitatory synapses in both pyramidal neurons and interneurons, while NL2 influences excitatory synapses on pyramidal neurons only. NL1 had a similar effect on regulating excitatory synapse formation in both types of neurons, while NL3 had a stronger effect on interneurons.
PSD-95 puncta in cultured cortical neurons after shNL lentiviral infection. A, B Representative images of PSD-95 puncta in cultured cortical pyramidal neurons (A) and interneurons (B). Green, GFP; red, PSD-95; blue, CaMKII/GAD67. Scale bars, 50 μm for whole-cell images and 10 μm for enlarged images. C–D Quantification of PSD-95 density in pyramidal neurons (C) and interneurons (D) (0.796 ± 0.048 for shNL1, 0.820 ± 0.006 for shNL2, and 0.892 ± 0.036 for shNL3 in pyramidal neurons; 0.898 ± 0.028, 0.964 ± 0.007, and 0.835 ± 0.023, respectively, in interneurons, normalized to control; one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001; mean ± SEM; n = 3 independent experiments).
From the GABA(A)Rα1 staining, we found that only shNL2 significantly reduced the puncta density in pyramidal neurons (Fig. 5A, C). Surprisingly, shNL1 was more potent in reducing GABAergic synapse density in interneurons than shNL2 (Fig. 5B, D). Taken together, these results indicated that NL2 regulates the formation and maintenance of GABAergic synapses in both pyramidal neurons and interneurons, NL1 regulates GABAergic synapse formation in interneurons only, while NL3 has no influence on GABAergic synapses in either type of neuron.
GABA(A)Rα1 puncta in cultured cortical neurons after shNL lentiviral infection. A, B Representative images of GABA(A)Rα1 puncta in cultured cortical pyramidal neurons (A) and interneurons (B). Green, GFP; red, GABA(A)Rα1; blue, CaMKII/GAD67. Scale bars, 50 μm for whole-cell images and 10 μm for enlarged images. C, D Quantification of GABA(A)Rα1 density in pyramidal neurons (C) and interneurons (D) (0.627 ± 0.169 for shNL1, 0.443 ± 0.098 for shNL2, and 0.715 ± 0.049 for shNL3 in pyramidal neurons; 0.522 ± 0.073, 0.665 ± 0.069, and 0.942 ± 0.115 in interneurons, respectively, normalized to control; one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001; mean ± SEM; n = 4 independent experiments).
Discussion
Autism spectrum disorder is increasingly being considered a disease of the synapse [24] that involves many synapse adhesion proteins [25]. Neurexins and NLs are membrane proteins located on opposing synaptic sites and interact with each other to generate new synapses. Recent electrophysiological studies have revealed that NLs have different functions in synaptic transmission in pyramidal neurons and interneurons [2, 18, 26], and that this may be due to their specific subcellular expression patterns in neurons. Enormous efforts have been devoted to delineating the detailed functions of NLs in the brain as well as the underlying mechanisms. Yet, different approaches have led to different results and interpretations due to technical limitations and side-effects. For example, overexpressed protein may be mislocalized and introduce a gain-of-function effect or side-effect, such as overexpressed and mislocalized NL2 which has been reported to reduce both GABAergic and glutamatergic dendritic postsynaptic protein clusters [27]. Therefore, carefully monitoring the protein expression level is important in reproducing the endogenous protein function. Knockout or transgenic mice reveal chronic alterations in neuronal morphology and activity, which sometimes cause compensatory effects. Here, we chose the shRNA knockdown strategy to examine the acute changes in synapse development in different neurons. As NL4 immunoreactivity has been shown to be faint or diffuse in many forebrain areas, especially the cortex [5], we did not knock down NL4 in cultured cortical neurons. We designed the NL isoform-specific shRNAs carefully and examined their knockdown efficiency and specificity in both exogenous and endogenous systems. It is worth noting that the shRNA knockdown efficiency was underestimated as the viral infection efficiency in neurons was 70%–80% for immunoblotting assays. We also confirmed that acute knockdown of one NL neither affected the expression levels nor the expression patterns of the other NL family members. In addition, as most of the NL-knockout mice failed to show any change in spine density [13,14,15, 18, 19], which was different from what was found in cultured neurons, we reasoned that the delayed synapse formation during the early stage may not be revealed in adult knockout mice or there might be compensatory effects in the brain to bypass the synapse loss caused by NL deficiency. Therefore, examining the synapse development in cultured neurons within the proper time window could be an effective way to understand the functions of NLs in synapse formation.
In summary, our result showed detailed and differential roles of NL isoforms in subtype synapse formation in pyramidal neurons and interneurons. NL1 not only functions in the formation of both types of synapse in pyramidal neurons, as reported, but also functions in GABAergic synapse formation in interneurons. NL2 plays a preferential role in GABAergic synapse formation in both pyramidal neurons and interneurons. NL3 preferentially regulates excitatory synapse formation in interneurons and plays a modest role in pyramidal neurons. Therefore, we conclude that NL1/2/3 has a distinct capability to regulate the E/I balance in pyramidal neurons and interneurons.
E/I balance is critical for the normal function of circuits, and is believed to be crucial in the pathology of autism. Research has also shown that the excitatory/inhibitory synapse ratio and subunit composition of neurotransmitter receptors are altered in mice with the autistic NL mutation [8,9,10, 28,29,30,31], and E/I rebalance by elevating inhibitory neuron activity rescues the defects in social behavior [32]. Interestingly, PV-cell deficits have also been found in NL3 R451C-knockin mice [18, 26] and NL2-knockout mice [2]. However, there was no clear conclusion if such PV-cell deficits were direct consequences of the intracellular changes of NL protein function or the compensatory effects of loss of function of NL during development. Our results further provide evidence that a deficit of NL in interneurons would cause a synapse-formation defect in this type of neuron and therefore may lead to an E/I imbalance. Dissociated neuronal cultures from mice with an NL autistic mutation could provide a more direct validation of this study. Moreover, electrophysiological approaches will be needed to further examine the roles of NLs in regulating synaptic transmission.
References
Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A 1999, 96: 1100–1105.
Gibson JR, Huber KM, Sudhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci 2009, 29: 13883–13897.
Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 2004, 83: 449–456.
Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci 2007, 26: 1738–1748.
Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A 2011, 108: 3053–3058.
Hoon M, Bauer G, Fritschy JM, Moser T, Falkenburger BH, Varoqueaux F. Neuroligin 2 controls the maturation of GABAergic synapses and information processing in the retina. J Neurosci 2009, 29: 8039–8050.
Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 2009, 63: 628–642.
Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 2005, 307: 1324–1328.
Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, et al. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem 2005, 280: 17312–17319.
Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A 2004, 101: 13915–13920.
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007, 54: 919–931.
Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J 2009, 28: 3244–3255.
Chanda S, Hale WD, Zhang B, Wernig M, Sudhof TC. Unique versus redundant functions of neuroligin genes in shaping excitatory and inhibitory synapse properties. J Neurosci 2017, 37: 6816–6836.
Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci 2010, 30: 2115–2129.
Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, et al. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav 2009, 8: 114–126.
Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science 2012, 338: 128–132.
Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron 2006, 51: 741–754.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007, 318: 71–76.
Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, et al. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat Neurosci 2012, 15: 1667–1674.
Zhang B, Sudhof TC. Neuroligins are selectively essential for NMDAR signaling in cerebellar stellate interneurons. J Neurosci 2016, 36: 9070–9083.
Polepalli JS, Wu H, Goswami D, Halpern CH, Sudhof TC, Malenka RC. Modulation of excitation on parvalbumin interneurons by neuroligin-3 regulates the hippocampal network. Nat Neurosci 2017, 20: 219–229.
Cao M, Xu J, Shen C, Kam C, Huganir RL, Xia J. PICK1-ICA69 heteromeric BAR domain complex regulates synaptic targeting and surface expression of AMPA receptors. J Neurosci 2007, 27: 12945–12956.
Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci 2010, 13: 22–24.
Wang M, Li H, Takumi T, Qiu Z, Xu X, Yu X, et al. Distinct defects in spine formation or pruning in two gene duplication mouse models of autism. Neurosci Bull 2017, 33: 143–152.
Tian Y, Zhang ZC, Han J. Drosophila studies on autism spectrum disorders. Neurosci Bull 2017, 33: 737–746.
Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013, 78: 498–509.
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004, 119: 1013–1026.
Zeidan A, Ziv NE. Neuroligin-1 loss is associated with reduced tenacity of excitatory synapses. PLoS One 2012, 7: e42314.
Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 2005, 48: 229–236.
Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 2000, 101: 657–669.
Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol 2003, 90: 3950–3957.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477: 171–178.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31571049 and 81561168022), the National Basic Research Program of China (2015CB910801), Zhejiang Provincial Natural Science Foundation of China (LR19H090001 and LD19H090002), a joint grant from the National Natural Science Foundation of China and the Research Grants Council of Hong Kong, China (8151101104 and N_HKUST625/15) and Fundamental Research Funds for the Central Universities of China. We appreciate the Core Facilities of Zhejiang University School of Medicine for technical support, and Mrs. SS Liu, ZXN Lin and GF Xiao for their help with confocal microscopy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, QQ., Xu, J., Liao, TL. et al. Neuroligins Differentially Mediate Subtype-Specific Synapse Formation in Pyramidal Neurons and Interneurons. Neurosci. Bull. 35, 497–506 (2019). https://doi.org/10.1007/s12264-019-00347-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00347-y