Abstract
Gastrostomy is performed when the patient needs to be fed through the gastrointestinal tract but cannot eat orally. Currently, gastrostomy is mostly performed endoscopically, but this is not possible in every case. To nourish esophageal cancer patients who need neo-adjuvant chemoradiotherapy and the use of the stomach to replace the esophagus when performing esophagectomy after chemoradiotherapy treatment, we performed laparoscopic gastrostomy from the anterior wall of the stomach. This is a case series analysis of patients with esophageal cancer who were indicated for neoadjuvant chemoradiotherapy and had large tumors that could not undergo endoscopic gastrostomy. During the period from April 2020 to January 2022, we performed laparoscopic gastrostomy for six patients with stage cT3N0M0 esophageal cancer. These patients were characterized by the inability to eat or drink by mouth because a large tumor obstructed the lumen of the esophagus. The surgery results were successful in all patients, with operative times ranging from 20 to 45 min. There were no cases of accidents or complications. The patient was nourished again by the gastrointestinal tract within 24 h and was transferred to the chemoradiotherapy department after 3 to 4 days for neo-adjuvant chemoradiotherapy treatment. Laparoscopic gastrostomy from the anterior wall of the stomach is a safe technique with a short operation time and a quick postoperative recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A gastrostomy is a surgical procedure that is performed on patients who need enteral nutrition but are unable to obtain food through the mouth. The placement of a gastrostomy feeding tube was first described by Stamm in 1894, in which an open surgical technique was carried out to place a gastrostomy tube into the stomach. This technique existed for a long time until the early 1980s, when it was gradually replaced by percutaneous endoscopic gastrostomy (PEG) [1]. More than 40 years after PEG was presented at the annual meeting of the American Society for Gastrointestinal Endoscopy in 1980, this technique has been widely applied and has shown many advantages compared to open gastrostomy [2]. However, unfortunately, PEG is not always feasible for certain cases in which a gastrostomy tube cannot reach the stomach due to upper gastrointestinal tract obstruction. Additionally, some authors also propose the solution of gastrostomy through computed tomography (CT)-guided gastrostomy tube placement. However, this method has a high rate of accidents and complications and a low success rate, so clinicians rarely prescribe it [3]. Therefore, surgical gastrostomy still plays an important and indispensable role in clinical practice.
There have been many reports applying laparoscopic surgery to gastrostomy with different techniques, from laparoscopic-assisted surgery to complete laparoscopic surgery. These reports demonstrate the difficulties faced by surgeons when it takes a long time to create the Witzel tunnel through total laparoscopic surgery. Therefore, most authors only opt for laparoscopic surgery in the form of assisted laparoscopic surgery or a combination of laparoscopic surgery with endoscopy for gastrostomy tube placement [1, 4,5,6]. Among the reports on laparoscopic gastrostomy, laparoscopic tubularized continent gastrostomy introduced by Marco Lotti et al. in 2020 is worth mentioning. The author and his collaborators presented a technique that partially removed the greater omentum in the greater curvature and created a gastric flap by a beveled cut at this position. The gastric flap was then brought to the outside [7]. This technique showed many advantages and has been highly appreciated by other authors in cases where minimally invasive surgery was not feasible or unsuccessful [8]. However, this method caused failure of the nutritional integrity of the greater curvature. Therefore, gastrostomy in patients with esophageal cancer indicated for neo-adjuvant chemoradiation followed by esophagectomy is not feasible because the use of the stomach to replace the esophagus is not safe since the main nutrient supply vessel for the upper parts of the stomach has been damaged.
Starting from the actual demands for the treatment of patients with esophageal cancer requiring neo-adjuvant chemoradiotherapy and considering esophagectomy, we present our technique for laparoscopic gastrostomy creation from the anterior of the body-fundus of the stomach to meet the above requirements without any references to other authors.
Patients and Methods
Patient Selection
This study consisted of a series of six patients with advanced esophageal cancer who were unable to consume food orally, and endoscopy was unsuccessful in passing through their stomachs to perform PEG. As a result, these patients were advised to undergo surgical placement of a gastrostomy feeding tube prior to receiving neo-adjuvant chemoradiotherapy.
Written informed consent was obtained from all patients in our study, approved by the Hanoi Medical University Institutional Ethical Review Board, Vietnam (Decision no. 4889/QĐ-ĐHYHN, on Oct 21, 2022). The Director of Hanoi Medical University signed on Oct 21, 2022.
Surgical Procedures
-
Step 1: After general anesthesia, the patient was placed in the supine position, hands close to the body and legs closed. A laparoscopic surgery system was placed on the left-hand side of the patient's head. The surgeons stand on the right side of the patient.
-
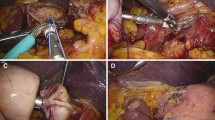
Step 2: A 10-mm trocar was placed to the lower border of the umbilicus by employing the open Hasson and gas insufflation technique. Then, a 5-mm trocar was inserted at the intersection of the right midclavicular line and the horizontal umbilical line. A 12-mm trocar was positioned at the intersection of the left midclavicular line and the horizontal umbilical line. An additional 10-mm trocar was placed to the left of the epigastric midline, which was the output position of the gastrostomy tube (Fig. 1).
-
Step 3: A 60-mm endoscopic stapler was used to make a beveled cut at the anterior wall of the upper body-fundus of the stomach so that an approximately 60 mm x 10 mm (length x diameter) gastrostomy tube could be placed with one end free and the other connected with the stomach (Fig. 2).
-
Step 4: The free end of the tube was brought out via the 10-mm trocar hole at the left of the epigastrium, the tube was cut off, hemostasis was performed, and the edge of the tube was attached to the abdominal wall using Vicryl 2.0 thread. A 16F Foley catheter was inserted into the stomach via the newly created tube, and the ball was pumped to fix the sonde (Fig. 3).
-
Step 5: We examined the cut line. Hemostasis was carried out if necessary by using a clip. Then, the air was cleared, and the trocar holes were closed.
Results
During the period from April 2020 to January 2022, we performed laparoscopic gastrostomy following the technique described above on six male patients. The clinical characteristics and surgical results are shown in Table 1. All six patients were hospitalized because of lower thoracic esophageal cancer with a large tumor causing esophageal obstruction, and the fiberscope could not be passed through. These patients were indicated for gastrostomy feeding prior to neo-adjuvant chemoradiotherapy. All six patients underwent laparoscopic gastrostomy without complications. The first day after surgery, mild pain occurred, and the patients were administered intravenous paracetamol 1 g. Re-examination after 1 month showed that all six patients had good incisions, with neither fluid leakage nor inflammation around the catheter, and the Foley drainage tubes were replaced easily and safely.
Two patient had a good response after neo-adjuvant chemoradiotherapy and underwent thoracoscopic esophagectomy using the stomach to replace the esophagus. One patient responded well after preoperative chemoradiotherapy. However, this patient had a history of cirrhosis, and after the preoperative chemoradiotherapy, the cirrhosis worsened and did not meet the requirements for general anesthesia to remove the esophagus. The patient had the gastric tube removed after 3 months at the request of the patient and family. A follow-up examination 1 month after the catheter was removed showed that the position of the tube base was completely sealed. One year later, the patient died due to the recurrence and progression of cancer. The remaining three patients did not respond to preoperative chemoradiotherapy, and they received continued palliative care treatment. All three patients later died of cancer progression at 4, 6, and 7 months after surgery.
Discussion
A gastrostomy is one of the various methods performed in the gastrointestinal tract to nutritionally support patients who are unable to tolerate oral feeding in certain disease conditions. However, to perform surgery on these patients, safety must be a top priority. Moreover, the surgery should minimize complications, enhance a quick recovery, and be affordable. Furthermore, patients with esophageal cancer indicated for chemoradiotherapy require a faster recovery from gastrostomy surgery to help them receive chemoradiotherapy for treatment of the cancer.
The open surgery technique for gastrostomy was introduced in the late 18th century and has significantly improved over time [1]. Fortunately, the development of science and technology, including the development of the interventional gastroenterology specialty, has created great innovations in minimally invasive gastrostomy techniques. PEG has shown significant advantages over laparotomy. These benefits include less pain, fewer complications, and faster healing. Therefore, it is considered a first option for gastrostomy [2, 9]. However, not all patients can undergo PEG when fiberscope cannot access the stomach because of an obstruction of the gastrointestinal tract. Another minimally invasive technique recommended by interventional radiologists is CT scan-guided gastrostomy. Unfortunately, this technique has shown a high risk of complications, some of which are severe and require another surgery [3].
The invention and continuous development of laparoscopy since the first introduction of laparoscopic cholecystectomy by Phillip Mouret at the French surgical conference held in Lion in 1986 have enabled laparoscopy to be widely applied to various surgeries in general and to gastrostomy specifically. To date, numerous modified techniques have been reported, such as laparoscopi-assisted or totally laparoscopic Witzel gastrostomy (LWG), and laparoscopically assisted percutaneous endoscopic gastrostomy (LAPEG) [4,5,6]. These reports have shown positive results and offered great applications in practice. Among the publications on the application of laparoscopy in gastrostomy surgery, Marco Lotti et al., in May 2020, presented a technique for laparoscopic creation of a tubularized continent gastrostomy by partial resection of the greater curvature and catheterization with a large gastric curvature, which was considered safe and innovative by colleagues [8]. However, this technique requires resection of the greater curvature, which is time-consuming and requires good hemostasis. Moreover, the biggest disadvantage of this technique is that for patients with esophageal cancer indicated for neo-adjuvant chemoradiotherapy followed by esophagectomy, the use of gastric replacement is then not safe because part of the vascular arc above greater curvature, which is the main blood supply to the fundus stomach, has been damaged and is no longer intact [7].
Studies have shown that anastomotic leakage is one of the most serious and common complications of esophagectomy. The incidence of anastomotic leakage when using gastric replacement for the resected esophagus varies from 11.4% to 21.2%, and 2.5% to 20% of anastomotic leakages are ischemic. The reported mortality rate after esophagectomy related to anastomotic leakage is 7.2% to 35% [10]. Recently, Japanese authors demonstrated that gastric integrity during esophageal reconstructive surgery reduced the incidence of esophageal fistula to less than 1% [11]. Therefore, preserving the integrity of the stomach when using it to replace the esophagus in esophagectomy for esophageal cancer is a very important issue.
Based on the actual demands during the treatment of patients with esophageal cancer who need neo-adjuvant chemoradiotherapy and esophagectomy in the immediate future, we developed a technique to apply laparoscopy to create a gastrostomy orifice from the anterior of the body-fundus of the stomach to maximally preserve the integrity of the stomach. We used a 60-mm endoscopic stapler to vertically cut the anterior of the body-fundus of the stomach to create a gastrostomy tube of approximately 60 mm x 10 mm (LxD). The free end of the tube was brought to the outside before fixing it into the abdominal wall, and the other end was continuously connected with the stomach to ensure a nourishing blood supply to the created coil. This method is simple, safe, and fast. The duration of the first surgery was 45 min; however, the following surgeries were faster, and the fifth surgery took only 20 min. Although some surgeons may be concerned about the nourishing blood supply of this tube, this is not a problem since the rich network of blood vessels in the gastric wall will ensure a sufficient blood supply to the catheter. The results of all five cases in our study showed a very good supply. In addition, some surgeons may worry about the leakage of gastric juice or food through root of the catheter. This did not occur in any of the six cases in our study, because we take the gastrostomy tube through the left rectus abdominis muscle and root of the gastrostomy tube is located toward the fundus, which is the upper part of the stomach. We re-examined and monitored the patients’ condition one month after surgery and found that the patients in the study showed good results with no complications.
Creation of gastrostomy from the anterior of the stomach was performed by fashioning a solid tunnel from the stomach to the outside; therefore, the replacement of the Foley drainage catheter was conducted simply, safely, and quickly. Two of our six patients underwent esophagectomy after neo-adjuvant chemoradiotherapy, and a reconstructed stomach was used to replace the removed esophagus. We found that the stomach after gastrostomy preserved its anatomical integrity and nourishment function, complying with the requirements for its use to reconstruct and replace the resected esophagus. In these two cases, the result of esophagectomy for radical treatment of esophageal cancer was good, with no complications occurring after the surgery. Another patient responded to chemoradiotherapy well, and the patient could eat and drink; however, this patient was not allowed esophagectomy due to having certain internal disease conditions. This patient asked for removal of the tube 3 months after laparoscopic gastrostomy. There was no leakage of fluid or food out of the tube, and the catheter foot had healed well.
From the results of this study, we believe that laparoscopic gastrostomy creation from the anterior of the body-fundus of the stomach that we have introduced can be extended to all patients requiring gastrostomy for which endoscopic techniques cannot be performed.
Conclusion
Laparoscopic gastrostomy to create a gastrostomy orifice in the anterior of the body-fundus of the stomach is safe and effective and meets the criteria for anatomical integrity as well as gastric nutrition when the stomach is required to replace the esophagus in esophagectomy.
References
Mizrahi I, Garg M, Divino CM, Nguyen S (2014) Comparison of laparoscopic versus open approach to gastrostomy tubes. J Soc Laparoendosc Surg 18(1):28–33. https://doi.org/10.4293/108680813X13693422520927
Ponsky JL (2021) Percutaneous endoscopic gastrostomy: after 40 years. Gastrointest Endosc 93(5):1086–1087. https://doi.org/10.1016/j.gie.2020.09.036
Yasin JT, Schuchardt PA, Atkins N et al (2020) CT-guided gastrostomy tube placement-a single center case series. Diagn Interv Radiol 26(5):464–469. https://doi.org/10.5152/dir.2020.19471
Sayadi Shahraki M, Berjis N, Bighamian A, Mahmoudieh M, Shahabi Shahmiri S, Sheikhbahaei E (2020) Minimally invasive technique for gastrostomy tube insertion: a novel laparoscopic approach. Asian J Endosc Surg 13(4):610–613. https://doi.org/10.1111/ases.12780
Hsieh JS, Wu CF, Chen FM, Wang JY, Huang TJ (2007) Laparoscopic Witzel gastrostomy--a reappraised technique. Surg Endosc 21(5):793–797. https://doi.org/10.1007/s00464-006-9018-6
Tanaka T, Ueda T, Yokoyama T et al (2019) Laparoscopic percutaneous endoscopic gastrostomy is useful for elderly. J Soc Laparoendosc Surg 23(2). https://doi.org/10.4293/JSLS.2019.00011
Lotti M, Carrara G, Lovece A, Giulii CM (2020) Laparoscopic tubularized continent gastrostomy: an alternative to tube gastrostomies. Updat Surg 72(3):901–905. https://doi.org/10.1007/s13304-020-00795-6
Tebala GD, Bond-Smith G (2021) Laparoscopic tubularized gastrostomy: a valid alternative to percutaneous endoscopic gastrostomy. Updat Surg 73(2):779–780. https://doi.org/10.1007/s13304-020-00849-9
Mahawongkajit P, Techagumpuch A, Limpavitayaporn P et al (2020) Comparison of introducer percutaneous endoscopic gastrostomy with open gastrostomy in advanced esophageal cancer patients. Dysphagia 35(1):117–120. https://doi.org/10.1007/s00455-019-10017-w
Davies J, Pernar L, Eble D et al (2020) Use of a laparoscopic Witzel gastrostomy without gastropexy in bariatric and general surgery. Obes Surg 30(11):4631–4635. https://doi.org/10.1007/s11695-020-04871-z
Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS (2021) Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 34(1). https://doi.org/10.1093/dote/doaa039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, Q.A. Laparoscopic Gastrostomy from the Anterior Wall of the Stomach: a Simple Gastrostomy Technique with Maximal Preservation of Stomach Integrity. Indian J Surg (2024). https://doi.org/10.1007/s12262-023-04010-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-023-04010-9