Abstract
Laparoscopic bariatric surgery has become the gold standard for the treatment of morbidly obese patients. This experimental study aimed to develop a new, anatomically relevant, high-fidelity simulator to train advanced laparoscopic Roux-en-Y gastric bypass (LRYGB). An anatomical model similar to human abdominal organs was established using ex vivo porcine organs as materials. A three-dimensional (3D) printer was applied to print the laparoscopic simulation training box, including the placement of the model base, diaphragm fixators, and training box–height adjustment device. A total of six experts simulated LRYGB surgery and evaluated the model’s face, content validity, and realism of surgical technique through a questionnaire. The simulator was highly realistic in appearance, anatomy, touch, instrumentation, and core surgical procedures. All participants recommended the application of this model in bariatric surgery training. Compared with the actual operation, this model has enough fidelity and is easy to reproduce, which provides effective support for future surgical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is an emerging epidemic. Laparoscopic Roux-en-Y gastric bypass (LRYGB) has become one of the most commonly used bariatric surgery methods [1, 2]. LRYGB is a technically complex and challenging procedure. With the increasing popularity of bariatric surgery and the growing number and demand of patients, more and more surgeons perform advanced laparoscopic bariatric surgery without specific training, resulting in short-term and long-term complications for patients [3,4,5]. Literature has reported that the learning curve of LRYGB needs to be experienced between 50 and 100 cases, during which the incidence of complications may increase [6]. Although residents are fewer direct surgeons in the LRYGB, the American Society of Metabolic and Bariatric Surgeons and the American Society of Gastrointestinal and Endoscopic Surgeons have adopted the guidelines for bariatric surgery programs and established the Center for Weight Loss Surgical training [7,8,9,10]. In China, residents undergoing standardized training rarely contact LRYGB or receive LRYGB training except for basic laparoscopic examination (i.e., diagnosis, cholecystectomy, and appendectomy), which is detrimental to patients receiving or starting such procedures in the future.
Current models based on surgical simulation include box trainers, virtual reality simulators, cadaver models, and live animals [11, 12]. The box trainer provides a simple, convenient, and inexpensive model that enables the trainer to acquire basic laparoscopic skills, including laparoscopic suture, knotting, and coordination training, but cannot simulate surgical steps or specialized surgeries [13]. Virtual reality simulators lack tactile feedback when using surgical instruments, and the costs associated with purchasing and maintaining simulators are very high [14]. The supply of cadaver models is limited due to reusability, availability, price, and related ethical issues [15]. Experiments on living animals require specialized anesthesia and laboratories, which are expensive and ethically problematic [16, 17]. This has created a need for low-cost, realistic, widely available models to train residents for advanced LRYGB training.
So far, there is little development of LRYGB in vitro training models. Currently, known models can only simulate enteroenterostomy, but cannot completely simulate the entire operation of LRYGB [18].
The goal of our simulation lab is to design a high-fidelity surgical model for LRYGB training that can provide high-quality training without the drawbacks of current modeling tools. At the same time, weight-loss experts evaluated the model with questionnaires.
Materials and Methods
Model Establishment
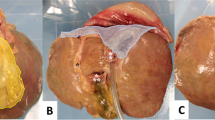
The raw materials for the model were obtained from the designated slaughterhouse, including the continuous lower esophagus, diaphragm, stomach, upper jejunum, liver, bile, spleen, pancreas, and all the mesangium (omentum majus and lesser omentum), lymph nodes, blood vessels, and other soft tissues connected between the organs mentioned above (Fig. 1A). The digestive tract was rinsed with antibacterial preservatives and special solvents to keep its elasticity as much as possible. The lot was put into a particular organ preservation bag and mold, frozen to − 12 °C, and rewarmed with a special solvent at normal temperature for 4–8 h until the organ model became utterly soft. The diaphragm and abdominal organs could be fixed according to the layout of the simulated box to restore a scene similar to that of living animals (Fig. 1B).
Demonstration of LRYGB Simulator. A Ex vivo porcine organ configuration. B The diaphragm is suspended by three plastic tapes. C The self-designed surgical platform. D Abdominal wall skin fitted with trocar ports for insertion of instruments. E Flexible metal snake bone fix camera. F Participants practice on simulated laparoscopic surgery platform
A self-printed surgical operating platform (Fig. 1C) was used in the experiment, including a monitor, a camera, a laparoscopic simulation training box, an abdominal wall skin, a model base, a light source, and a porcine diaphragm suspension device. The silicone thickness of the abdominal wall skin allowed the placement of a laparoscopic trocar (Fig. 1D). The height of the operating platform could be adjusted up and down to simulate the pneumoperitoneum. The endoscope holder was a flexible metal conduit that could support a 30° camera (Fig. 1E), which allowed the surgeon to operate independently.
Study Design
This study was conducted in the innovation laboratory of our hospital and was approved by our hospital ethics committee. A total of six surgical weight-loss specialists were recruited for the surgery to test the model. First, we introduced the model’s composition, application method, and surgical procedure to the experts. Then, the experts simulated LRYGB on the simulator, including creating a small gastric pouch, creating a biliopancreatic limb, making a Roux limb, side-side intestinal anastomosis, and gastrojejunostomy. After the operation, the face validity, content validity, and surgical technical realism of the model were assessed by filling in the 5-grade Likert scale questionnaire. All participants answered the questions anonymously.
Simulated Surgical Procedure
The lesser omentum was separated at about 5 cm below the cardia with an ultrasonic knife, the retrogastric tunnel was cut by ultrasonic dissection and extension toward the angle of His (Fig. 2A), and the stapler was inserted to cut and anastomose the stomach (Fig. 2B). The small gastric pouch tunnel was continuously dissected and extended toward the posterior space of His angle. The stomach was cut and anastomosed toward His angle (Fig. 2C). The stapler was 1–1.5 cm away from the gastroesophageal junction. A small gastric pouch was made through the stapler (Fig. 2D). The small intestine was measured from the initiation of the Treitz ligament to 50 cm distal, an ultrasonic knife separated the mesentery, and the small intestine was cut and anastomosed with a stapler. The small intestine of 150 cm was measured in the distal end direction. The one # silk thread mark, Roux limb mark, and biliopancreatic limb were respectively made into small incisions with an ultrasonic knife (Fig. 2E). The stapler was inserted to cut. Anastomosis and the common hole were closed with a 2–0 absorbable suture (Fig. 2F). The distal stump of the intestine (Roux limb) was protruded forward into the small gastric pouch. A small incision was made on the anterior wall of the small gastric pouch and the small intestine with an ultrasonic knife, and a stapler was inserted to cut and anastomose. A 2–0 absorbable suture was exploited to close the common hole of the gastrojejunostomy. The anastomotic diameter was approx.—1.2 ~ 1.5 cm (Fig. 2G , H). Methylene blue dye was injected through the nasogastric tube to detect the leakage of gastrojejunostomy (Fig. 2I).
Ex vivo LRYGB procedure. A Dissect along the lesser curvature of the stomach. B Stapler cut and staple stomach. C Establish a posterior gastric tunnel. D Creation of the gastric pouch. E Creation of biliopancreatic limp and Roux limb. F Common hole of the intestinal anastomosis. G Small incision at the anterior wall of the gastric pouch. H Suture close the common anastomosis. I Methylene blue dye detect leakage
Results
Six surgeons successfully completed LRYGB on the simulator and conducted an anonymous survey. All participants agreed that the tactile response of the model was very realistic during suturing and operative space. It was also reasonably realistic regarding anatomical authenticity, tissue texture, and organ size and shape. Five surgeons rated the tactile response to the energy instrument as very realistic, while another surgeon rated it as fairly realistic (Table 1).
All surgeons strongly agreed that this model was easy to handle and should replace the living animal models, which could shorten the learning curve, improve the trainees’ surgical skills and confidence, and reduce patient risk. Therefore, it is recommended to use the model in LRYGB training. In addition, most participants considered the surgical operation of the model realistic (Table 1). All surgeons agreed that it was very realistic to establish a small gastric pouch, biliopancreatic limb, Roux limb, side-side intestinal anastomosis, and gastrojejunostomy. In addition, most participants believed that the model could simulate the dissociation of lesser omentum and the identification of the Treitz ligament by ultrasonic scalpel (Table 2).
Discussion
We developed a novel ex vivo porcine organ training model for LRYGB surgery. A total of six surgical specialists strongly recommended the use of the newly developed simulations in LRYGB training. Firstly, it could shorten the learning curve and improve the surgical skills of the trainees. Secondly, it was also simpler and more convenient than the living animal models. Thirdly, the organs were easy to preserve, the training could be repeated, and the cost was low. Therefore, it could replace the use of living animals.
Due to the short working hours of the residents, the demand for specific surgical skills, the increase of trainees, and the increasing emphasis on the efficiency of the operating room, the traditional “apprenticeship” training opportunities for residents, are significantly reduced [19, 20]. In addition, improving patients’ safety awareness and increasing medical dispute cases have reduced the opportunities for surgeons to train residents. In this case, simulation training has developed into an essential part of advanced laparoscopic surgical technique training. The LRYGB model was designed and evaluated by bariatric surgery specialists. The model was easy to operate, which could improve surgeons’ surgical skills and confidence to reduce the risk of patients.
Currently, the animal training model involved in LRYGB training can only train the operator’s surgical techniques, but cannot simulate the core surgical steps of LRYGB, so it cannot be widely used [21]. A study recruited eight surgeons who performed side-to-side sutures on pig cadaver models and then LRYGB on actual patients. This result found that the surgeon’s performance in the model was related to his performance in the operating room. Still, the experiment did not simulate other core procedures of LRYGB surgery [22]. A recent study analyzed human cadaver models preserved using the Thiel method, whose organs were similar to actual patients in terms of color and elasticity. Laparoscopic bariatric cadavers were the best training model. However, the limitations of this model include complex and expensive preservative processes, reusability, cost, and ethical issues [23]. The model we designed can simulate the core surgical procedure required for LRYGB surgery, and the operation is realistic. The model has surgical anatomy similar to that of humans. Surgeons can adjust the height of the training box according to their individual differences and organ size, simulate pneumoperitoneum, and puncture to the skin of the abdominal wall of the training box. In addition, the model can also be used for the application of advanced instruments such as ultrasonic knife and staplers and the operation of LRYGB core surgical steps. Previously, the operation of advanced instruments required special operating rooms. These manipulation techniques are realistic, and the model is portable and easy to replicate, making it suitable for any surgical training center. In addition, in our model, trainees can perform other advanced procedures, such as laparoscopic sleeve gastrectomy and fundoplication. All surgeons agreed that the model was realistic in terms of operative space, organ size and shape, sutures, and tactile response when using energy instruments. Our model could also evaluate surgical outcomes, such as anastomotic leakage. Six weight-loss experts have made small gastric pouches without leakage. Compared with synthetic or virtual simulators, we believe this model has obvious advantages in evaluating surgical outcomes.
Traditionally, the advanced laparoscopic skills of LRYGB can only be mastered by senior surgeons. Residents proficient in advanced laparoscopic skills in the early stages of training may have more opportunities to participate in laparoscopic bariatric surgery. Our model allows residents to learn advanced laparoscopic skills and helps them get through the early stages of the learning curve. Once entering the operating room, these residents can calmly use the skills acquired in the simulation environment to practice and shorten the learning curve, which is also the focus of our follow-up experimental research.
Our study also has several limitations: First, due to the tremendous operative difficulty, technical complexity, and many complications of LRYGB, we only recruited six bariatric surgeons for the test, and more experienced evaluators still need to confirm the high fidelity of the simulator. Second, it is uncertain whether the skills acquired in the simulator will be directly transferred to the operating environment, and validation studies are needed in the future. Third, this study cannot simulate tissue bleeding. Similar studies have injected the red liquid into the porcine abdominal aorta in the past, but this is not realistic [24]. However, our experiment simulated the core surgical steps of LRYGB. Currently, we are investigating the use of 3D printing materials to simulate blood vessels and tissue bleeding.
In conclusion, all experts agreed that the model is a good training tool for surgeons. The successful development of this advanced surgical model may lead to the development of effective surgical evaluation tools for other specialties.
References
Salminen P, Helmiö M, Ovaska J et al (2018) Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2017.20313
Buchwald H, Oien DM (2013) Metabolic/bariatric surgery worldwide 2011. Obes Surg 23(4):427–436. https://doi.org/10.1007/s11695-012-0864-0
Yuce TK, Holmstrom A, Soper NJ et al (2021) Complications and readmissions associated with first assistant training level following elective bariatric surgery. J Gastrointest Surg 25(8):1948–1954. https://doi.org/10.1007/s11605-020-04787-0
Lefere S, Onghena L, Vanlander A et al (2021) Bariatric surgery and the liver-mechanisms, benefits, and risks. Obes Rev 22(9):e13294. https://doi.org/10.1111/obr.13294
Gribsholt SB, Svensson E, Richelsen B et al (2018) Rate of acute hospital admissions before and after Roux-en-Y gastric bypass surgery: a population-based cohort study. Ann Surg 267(2):319–325. https://doi.org/10.1097/SLA.0000000000002113
Breaux JA, Kennedy CI, Richardson WS (2007) Advanced laparoscopic skills decrease the learning curve for laparoscopic Roux-en-Y gastric bypass. Surg Endosc 21(6):985–988. https://doi.org/10.1007/s00464-007-9203-2
Telem DA, Jones DB, Schauer PR et al (2018) Updated panel report: best practices for the surgical treatment of obesity. Surg Endosc 32(10):4158–4164. https://doi.org/10.1007/s00464-018-6160-x
McBride CL, Rosenthal RJ, Brethauer S et al (2017) Constructing a competency-based bariatric surgery fellowship training curriculum. Surg Obes Related Dis 13(3):437–441. https://doi.org/10.1016/j.soard.2016.10.013
Morton JM, Garg T, Nguyen N (2014) Does hospital accreditation impact bariatric surgery safety? Ann Surg 260(3):504–8. https://doi.org/10.1097/SLA.0000000000000891
Schirmer B (2011) Guidelines for institutions granting bariatric privileges for using laparoscopic techniques. Surg Endosc 25(3):669–670. https://doi.org/10.1007/s00464-010-1375-5
Schlottmann F, Herbella FAM, Patti MG (2021) Simulation for foregut and bariatric surgery: current status and future directions. J Laparoendosc Adv Surg Tech A 31(5):546–550. https://doi.org/10.1089/lap.2021.0080
Wang X, Zhang K, Hu W et al (2021) A new platform for laparoscopic training: initial evaluation of the ex-vivo live multivisceral training device. Surg Endosc 35(1):374–382. https://doi.org/10.1007/s00464-020-07411-z
Li MM, George J (2017) A systematic review of low-cost laparoscopic simulators. Surg Endosc 31(1):38–48. https://doi.org/10.1007/s00464-016-4953-3
Reznick RK, MacRae H (2006) Teaching surgical skills–changes in the wind. N Engl J Med 355(25):2664–2669. https://doi.org/10.1056/NEJMra054785
Ahmed K, Jawad M, Abboudi M et al (2011) Effectiveness of procedural simulation in urology: a systematic review. J Urol 186(1):26–34. https://doi.org/10.1016/j.juro.2011.02.2684
Brook NR, Dell’Oglio P, Barod R et al (2019) Comprehensive training in robotic surgery. Curr Opin Urol 29(1):1–9. https://doi.org/10.1097/MOU.0000000000000566
Schmitt B, Wacker C, Ikemoto L et al (2014) A transparent oversight policy for human anatomical specimen management: the University of California, Davis experience. Acad Med 89(3):410–414. https://doi.org/10.1097/ACM.0000000000000135
Zevin B, Dedy NJ, Bonrath EM et al (2017) Comprehensive simulation-enhanced training curriculum for an advanced minimally invasive procedure: a randomized controlled trial. Surg Obes Relat Dis 13(5):815–824. https://doi.org/10.1016/j.soard.2016.11.019
Wohlrab K, Jelovsek JE, Myers D (2017) Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol 217(5):522–526. https://doi.org/10.1016/j.ajog.2017.05.017
Nashaat A, Sidhu HS, Yatham S et al (2019) Simulation training for lobectomy: a review of current literature and future directions†. Eur J Cardio-Thoracic Surg 55(3):386–394. https://doi.org/10.1093/ejcts/ezy276
Zevin B, Aggarwal R, Grantcharov TP (2012) Simulation-based training and learning curves in laparoscopic Roux-en-Y gastric bypass. Br J Surg 99(7):887–895. https://doi.org/10.1002/bjs.8748
Boza C, Varas J, Buckel E et al (2013) A cadaveric porcine model for assessment in laparoscopic bariatric surgery–a validation study. Obes Surg 23(5):589–593. https://doi.org/10.1007/s11695-012-0807-9
Ruiz-Tovar J, Prieto-Nieto I, García-Olmo D et al (2019) Training courses in laparoscopic bariatric surgery on cadaver thiel: results of a satisfaction survey on students and professors. Obes Surg 29(11):3465–3470. https://doi.org/10.1007/s11695-019-04003-2
Schlottmann F, Murty NS, Patti MG (2017) Simulation model for laparoscopic foregut surgery: the University of North Carolina foregut model. J Laparoendosc Adv Surg Tech A 27(7):661–665. https://doi.org/10.1089/lap.2017.0138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, J., Mao, J., Chen, H. et al. Development and Validity of a Novel Ex Vivo Porcine Organs Laparoscopic Roux-en-Y Gastric Bypass Training Model. Indian J Surg (2022). https://doi.org/10.1007/s12262-022-03639-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-022-03639-2