Abstract
Mucinous breast carcinoma (MBC) is a particular type of breast cancer involving the presence of extracellular mucin. MBC accounts for approximately 4% of invasive breast cancers. MBC is divided into two as pure mucinous breast carcinoma (PMBC) and mixed mucinous breast carcinoma (MMBC) according to cell cellularity ratio. We aimed to investigate the difference between MBC subtypes in terms of clinical and survival. The data of 33 patients who were operated on for MBC between January 2010 and January 2021 in our clinic were analyzed retrospectively. Demographic data, hospital admission complaints, radiological diagnostic methods, surgical technique, histopathological and immunohistochemical examination, and survival time of the patients included in the study were examined. The patients were divided into two groups, PMBC and MMBC, and compared. During the study, MBC was detected in 33 (2.16%) of 1522 patients operated on with breast cancer diagnosis in our clinic. Of the MBC patients, 23 (69.7%) were PMBC and 10 (30.3%) were MMBC. PMBC and MMBC patients were compared in clinical, histopathological, and survival. In the PMBC group, statistically significant tumor size was larger, and survival time was longer in pathological and radiological terms. In addition, axillary dissection rate and N stage were more advanced in the MMBC group. It is essential to distinguish subgroups as MMBC and PMBC according to the amount of extracellular mucin of MBC. Although PMBC is detected in larger sizes at the time of diagnosis than MMBC, MMBC has more axillary metastases and worse survival because it contains invasive components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucinous breast carcinoma (MBC) constitutes approximately 4% (1–6%) of invasive breast cancers [1,2,3]. It is common in postmenopausal women [4]. On physical examination, MBC is a gelatinous lesion with regular borders and an easily recognizable elastic mass. Tumor size is between 1 and 20 cm (mean = 3 cm). MBC has better survival than other breast carcinoma types since its 10-year survival is 90%.
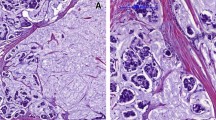
Histopathological examination reveals superficial solid tumor islets within mucin pools. MBC can be divided into two groups according to cell cellularity ratio as pure mucinous breast carcinoma (PMBC) and mixed mucinous breast carcinoma (MMBC) [5].
While PMBC consists of tumor tissue with extracellular mucin production in more than 90% of the tumor, MMBC infiltrates ductal epithelial components without mucin [2]. Although there are case series in the literature showing that PMBC has a better prognosis and survival than MMBC, the limits of MBC subtypes are still being investigated [2, 6].
This study aimed to compare the MBC subtypes PMBC and MMBC in terms of clinical, radiological, histopathological, and survival and investigate their differences.
Patients and Methods
The data of patients with histopathological diagnosis of mucinous breast carcinoma who underwent surgery for breast cancer in Mersin University Faculty of Medicine, Department of General Surgery between January 2010 and January 2021 were retrospectively analyzed. Thirty-three (33) patients (2.16%) were included in the study. Two patients diagnosed with mucinous breast carcinoma due to a biopsy from the breast mass but who did not undergo surgery in our clinic were excluded from the study.
Data Collection
Demographic characteristics of the patients (age, gender), menstrual period, complaints on admission, radiological imaging methods and findings, location of the mass in the breast (right, left), biopsy performed for the mass (core biopsy), the surgical technique (lumpectomy and mastectomy), histopathological data, follow-up, and survival time were recorded. In addition, the data of mammography, breast ultrasonography (USG), and magnetic resonance imaging (MRI) were recorded. In addition, the size of the mass (T stage), the total number of removed and metastatic lymph nodes (N stage), and immunohistochemical data were recorded from the histopathology reports. American Joint Committee on Cancer staging manual used MBC staging [7]. In the evaluation of the data, www.e-picos.com New York software and MedCalc statistical package program were used.
Pure/Mixed Mucinous Carcinoma
On histopathological examination of MBC, there are small cell islands and glandular structures composed of uniform cells floating in large extracellular mucin pools. If the mucin content is more than 90% of the mucin/colloid structure, it is grouped as pure mucinous, and if it is less than 90%, it is grouped as mixed mucinous carcinoma [8]. Therefore, the patients included in the study were divided into two groups as pure and mixed mucinous breast carcinoma according to the postoperative histopathological examination and were compared with each other.
Immunohistochemical Examination
During the histopathological examination, hormone receptors (HR), estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 were evaluated by immunohistochemistry (IHC). In the evaluation of ER and PR, those with ≥ 10 (fmol/mg cytosol protein) were grouped as positive and those with < 10 as negative [9]. When HER2 was evaluated with the IHC method, it was determined that if 3( +) HER2 was present in the tumor cell, 1( +) tumor cell did not have HER2, and if 2( +) was found, the presence of HER2 was evaluated by fluorescent in situ hybridization (FISH) method [10]. According to IHC and FISH results, patients were grouped as HER2 negative and positive. In our study, the cut-off limit for Ki67 grouping was 20%. Therefore, patients were divided into Ki67 < 20% low and ≥ 20% high group.
Results
During the study, MBC was detected in 33 (2.16%) patients operated on with breast cancer diagnosis in our clinic. Of the patients with mucinous breast carcinoma, 23 (69.7%) were PMBC and 10 (30.3%) were MMBC.
All of the patients were women. While the mean age was 41.7 (SD 11.1) in the PMBC group 47.3 (SD 11.9) in the MMBC group, there was no significant difference between the groups regarding mean age (p = 0.21). When the patients were evaluated according to the menopausal period, 15 (65.2%) of the PMBC patients were premenopausal, 8 (34.8%) were in the postmenopausal period, while 4 (40%) were premenopausal and 6 (60%) were postmenopausal in the MMBC group. There was no significant difference between the groups regarding the menopausal period (p = 0.18) (Table 1).
While 26 (78.8%) of the patients with MBC had complaints about a mass in the breast, the mass was detected incidentally in 7 (21.2%) of them. The most common complaint of symptomatic patients was a painless palpable mass (61.5%), followed by nipple retraction (15.4%), skin retraction (15.4%), and nipple discharge (7.7%). There was no significant difference between the groups in terms of the presence of the symptom of a breast mass (p = 0.16) (Table 2).
Mammography (61%), breast USG (100%), and MRI (48.5%) were used as radiological imaging modalities. According to the location of the mass, it was located in the right breast in 56.5% of the patients in the PMBC group, and in the left breast in 43.5% of the patients, while it was in the right breast in 60% and the left breast in 40% in the MMBC group. There was no difference between the groups according to the mass’s lateralization (right/left) (p = 0.85). None of the patients had bilateral breast masses.
All patients had a preoperative biopsy with a core biopsy. According to the PMBC group, in the biopsy results, 8 (34.8) patients were diagnosed with PMBC, while 15 (65.2) patients could not be differentiated into subgroups. According to the biopsy results in the MMBC group, 7 (70%) patients could be diagnosed with MMBC in the preoperative period. While lumpectomy was applied to 16 (69.6%) patients in the PMBC group and 5 (50%) patients in the MMBC group, mastectomy was applied to 7 (30.4%) patients in the PMBC group and 5 (50%) patients in the MMBC group. There was no significant difference between the groups regarding surgical technique preference (p = 0.28) (Table 1).
Sentinel lymph node biopsy (SLNB) was applied to all patients for axillary lymph node staging. Axillary dissection was performed in patients with carcinoma metastasis as a result of SLNB. Axillary lymph node dissection was performed on 3 (13%) patients in the PMBC group and 5 (50%) patients in the MMBC group. It was determined that axillary lymph node metastasis was more common in the MMBC group than in the PMBC group, and there was a significant difference (p = 0.02). The median value of the total number of lymph nodes removed by axillary dissection was 13 (10–14) in the PMBC group and 16 (11–21) in the MMBC group. The median value of the number of metastatic lymph nodes was 1 (1), while 3 (1–6) were in the MMBC group. There was no significant difference between the groups in terms of the total number of lymph nodes removed and the number of metastatic lymph nodes (p = 0.25, p = 0.33, respectively) (Table 1).
Tumor size was evaluated with radiological imaging methods in the preoperative period and determined after histopathological examination in the postoperative period. According to the results of radiological imaging methods, the mean tumor size was 3.16 (SD 0.99) cm in the PMBC group, while it was 2.37 (SD 0.97) cm in the MMBC group. According to the histopathological examination results, the mean tumor size was 3.11 (SD 1.08 cm) in the PMBC group, while it was 2.11 (SD 0.93) cm in the MMBC group. There was a significant difference between the groups regarding tumor size after radiological and histopathological examination (p = 0.04, p = 0.02, respectively). The mean tumor size was lower in the MMBC group than in the PMBC group (Table 1).
During the histopathological examination, tumor size, the number of total and metastatic lymph nodes removed, ER, PR, cErbB2, and Ki67 levels were determined and reported in all patients.When evaluated in terms of T stage, there were 6 (26.1%) patients at T1 stage, 16 (69.6%) patients at T2 stage, and 1 (4.3%) at the T3 stage in the PMBC group. There were 3 (30%) patients at the T1 stage and 7 (70%) patients at the T2 stage in the MMBC group. When evaluated in terms of the N stage, 20 (87%) of the patients in the PMBC group were at n0, and 3 (13%) were at n1. Of the patients in the MMBC group, 4 (40%) were at n0, 5 (50%) at n1, and 1 (10%) at n2. While there was no difference between the groups according to the T stage, there was a significant difference according to the N stage (p = 0.79, p = 0.02, respectively) (Table 3).
When the HR in mucinous carcinoma cells was evaluated, 82.6% of the patients in the PMBC group were positive for ER, 65% for PR, and 26% for HER2, whereas in the MMBC group, 70% of the patients were positive for ER, 60% for PR, and 20% for HER2. The Ki67 rate was 20% or higher in 56.5% of the patients in the PMBC group and 40% of the patients in the MMBC group. There was no significant difference between the groups in terms of ER, PR, and HER2 receptor distribution and Ki67 level (p = 0.65, p = 0.77, p = 0.71, p = 0.46) (Table 3).
The mean follow-up period of the patients in the postoperative period was 61 (13–109) months in the PMBC group and 69 (39–103) months in the MMBC group. None of the patients were lost during the follow-up period. There was no difference between the groups regarding follow-up time (p = 0.45) (Table 1). In the 109-month follow-up period, those in the PMBC group were found to have a 94% survival rate until the 41st month and 87% from the 53rd month. In the MMBC group, the probability of survival was 89% at the 43rd month and 74% at the 53rd month. A total of four patients died of breast cancer-related causes. Two died in the PMBC group at the 41st and 53rd months, and the other two died at the 43rd and 74th months in the MMBC group. As seen in the hazard ratio value, since the confidence interval includes 1, it was determined that the time to exitus (months) did not differ according to the pathology groups (Kaplan–Meier, log-rank p = 0.35) (Table 4; Graph 1).
Discussion
Mucinous carcinomas are a rare and specific malignant tumor that accounts for 1–6% of invasive breast cancers [1, 3]. It is often seen in advanced age. They are smooth, soft, and mobile masses on examination. MBC is divided into PMBC and MMBC subgroups according to the distribution of mucin-producing cells [8]. In our study, PMBC and MMBC groups were compared in clinical, histopathological, and survival outcomes.
It has been reported that MBC is more common in women in the postmenopausal period, and PMBC is seen at a younger age than MMBC without making a statistical difference [2]. The incidence of MBC in our study was 2.16% and was consistent with the literature. In our study, the PMBC group (41.7, SD 11.1) was younger than the MMBC group (47.3, SD 11.9), but there was no statistically significant difference between the groups.
Mucinous carcinoma usually grows slowly and can reach a large size at the time of diagnosis. Often the first symptom of patients with MBC is a palpable mass. Sometimes, it can be detected incidentally. Invasion is rarely seen because of its regular borders and growth by pushing the surrounding tissue. MBC subgroups do not have specific symptoms [11, 12]. The most common symptom in our study was a painless palpable mass. There was no difference between the groups in terms of symptoms.
Among the MBC radiological imaging methods, mammography, USG, and breast MRI are used. Radiological imaging methods can show the pure and mixed separation of MBC at different rates. Microlobule borders on mammography are associated with high mucin content, while irregular or pointed borders correspond histologically to lower mucin percentages and infiltrating margins [13]. On USG, MBC is isoechoic or hypoechoic. Homogeneity in USG is associated with PMBC type and thus a better prognosis. While most PMBCs are isoechogenic to subcutaneous fat echo, MMBC is hypoechogenic [13, 14]. On breast MRI, PMBC contains a limited mass with extremely high signal intensity on the T2 sequence and benign-appearing kinetics with persistent augmentation, whereas MMBC may have more questionable imaging features [15, 16].
When evaluated according to tumor size and TNM stage, there are differences between MBC subgroups. In the study by Chaudhry et al., tumor size was more significant in the PMBC group than in the MMBC group. It was stated that mammography, USG, and breast MRI evaluated tumor size [17]. In our study, the radiology-pathological correlation was found in the measurement of tumor size. In comparing tumor size between the groups, the tumor in the PMBC group was found to be statistically significantly more significant than the MMBC group, both radiologically and pathologically (p < 0.05).
When the groups were evaluated according to the TNM staging system, the T stage was more advanced in the PMBC group without creating a statistically significant difference, while the N stage was found to be more advanced in the MMBC group with a statistically significant difference. This was interpreted as the tumor size was primarily due to the high mucin content of PMBC, and MMBC caused more lymph node involvement because it contains more invasive tumor components.
While the biopsy is highly effective for the preoperative diagnosis of MBC, the effectiveness of the biopsy may be limited in the differentiation of subgroups. In the study of Cyrta et al., a preoperative diagnosis of MMBC with biopsy was detected at 41%, whereas a PMBC diagnosis could not be made. The cytopathological identification of biopsy PMBC patients could not be made adequately [18]. Shield et al. stated that biopsy is sufficient for diagnosing mucinous carcinoma, but it is not possible to differentiate the lesion as PMBC or MMBC [19]. In our study, all patients were diagnosed with MBC by preoperative biopsy. With a biopsy, subgroup discrimination could be made at 34.8% in the PMBC group and 70% in the MMBC group. Therefore, a biopsy was thought to be limited in subgroup discrimination in MBC.
Mucinous tumors usually have high HR positivity in IHC evaluation [20]. Although HR positivity is a better prognostic indicator, it is more common in elderly patients. Rande et al. reported that HR positivity was higher in MBC than in invasive breast cancer, but there was no difference in HR positivity between PMBC and MMBC [21]. In the study by Budzik et al., HR positivity was high, and tumor differentiation was low in the PMBC group [22]. Her2 positivity and high Ki67 level are independent predictive values for breast cancer. However, her2 positivity is mainly low, and Ki67 is low in mucinous carcinoma [23, 24]. There was no significant difference between the groups in our study in terms of ER, PR, and HER2 receptor distribution and Ki67 level [25, 26].
MBC axillary lymph node metastasis is predictive for survival and is seen at a lower rate than invasive ductal and lobular carcinomas of the breast [12]. In subgroups of MBC, axillary lymph node metastasis is more common in MMBC than in PMBC. In MMBC, more axillary lymph node metastases occur because of the characteristics of invasive tumor components [3]. Axillary lymph node metastasis is seen in 2–14% of PMBC and 46–64% of MMBC [13]. SLBN was applied to all patients for axillary lymph node metastasis in our study. Due to SLNB positivity, axillary lymph node dissection was performed at 13% in the PMBC group and 50% in the MMBC group. SLNB positivity and the number of metastatic lymph nodes were statistically higher in the MMBC group (p = 0.02).
In the literature, it has been found that PMBC has better 5- and 10-year survival rates than MMBC [26, 27]. When evaluated in terms of 5-year survival rate in their study, Zhang et al. found a higher survival rate for MBC than non-mucinous breast cancer and PMBC than MMBC [26]. Although the 5-year survival rate and survival time were longer in PMBC than in MMBC in our study, there was no statistically significant difference between the groups regarding 5-year survival and survival time (p = 0.56 and p = 0.35, respectively). In the study by Komenaka et al., PMBC tumor size was larger than MMBC, but it was stated that tumor size did not affect axillary lymph node metastasis and survival. As the reason for this, they stated that the tumor is filled with mucin in PMBC and does not adversely affect metastasis and survival [28].
Conclusion
Mucinous breast carcinoma has better survival than other breast cancers. In addition, pure mucinous breast carcinoma has a better prognosis than mixed mucinous breast carcinoma due to less lymph node involvement and more hormone receptor positivity. However, because mixed mucinous breast carcinoma has invasive components, it is diagnosed in smaller sizes, has a more aggressive course, and worsens survival. For these reasons, it is imperative to distinguish between pure and mixed mucinous breast carcinoma in mucinous breast carcinoma patients.
References
Anderson WF, Chu KC, Chang S, Sherman ME (2004) Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev 13(7):1128–1135
Di Saverio S, Gutierrez J, Avisar E (2008) A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat 111(3):541–547. https://doi.org/10.1007/s10549-007-9809-z
Park S, Koo J, Kim JH, Yang WI, Park BW, Lee KS (2010) Clinicopathological characteristics of mucinous carcinoma of the breast in Korea: comparison with invasive ductal carcinoma-not otherwise specified. J Korean Med Sci 25(3):361–8. https://doi.org/10.3346/jkms.2010.25.3.361
Li CI (2010) Risk of mortality by histologic type of breast cancer in the United States. Horm Cancer 1(3):156–165. https://doi.org/10.1007/s12672-010-0016-8
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH (2011) Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer 14(4):308–13. https://doi.org/10.4048/jbc.2011.14.4.308
Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M (2008) Mucinous breast carcinoma: a large contemporary series. Am J Surg 196(4):549–551. https://doi.org/10.1016/j.amjsurg.2008.06.013
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99
World Health Organization (1982) Histological typing of breast tumors. Tumori 68:181–98
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–84. https://doi.org/10.1016/S0140-6736(11)60993-8
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/JCO.2018.77.8738
Marrazzo E, Frusone F, Milana F, Sagona A, Gatzemeier W, Barbieri E, Bottini A, Canavese G, Rubino AO, Eboli MG, Rossetti CM, Testori A, Errico V, De Luca A, Tinterri C (2020) Mucinous breast cancer: a narrative review of the literature and a retrospective tertiary single-centre analysis. Breast 49:87–92. https://doi.org/10.1016/j.breast.2019.11.002
Hanagiri T, Ono K, Baba T, So T, Yamasaki M, Nagata Y, Uramoto H, Takenoyama M, Yasumoto K (2010) Clinicopathologic characteristics of mucinous carcinoma of the breast. Int Surg 95(2):126–9
Memis A, Ozdemir N, Parildar M, Ustun EE, Erhan Y (2000) Mucinous (colloid) breast cancer: mammographic and US features with histologic correlation. Eur J Radiol 35(1):39–43. https://doi.org/10.1016/s0720-048x(99)00124-2
Lam WW, Chu WC, Tse GM, Ma TK (2004) Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol 182(4):1069–1074. https://doi.org/10.2214/ajr.182.4.1821069
Linda A, Zuiani C, Girometti R, Londero V, Machin P, Brondani G, Bazzocchi M (2010) Unusual malignant tumors of the breast: MRI features and pathologic correlation. Eur J Radiol 75(2):178–184. https://doi.org/10.1016/j.ejrad.2009.04.038
Bitencourt AG, Lima EN, Chojniak R, Marques EF, Souza JA, Andrade WP, Guimarães MD (2014) Can 18F-FDG PET improve the evaluation of suspicious breast lesions on MRI? Eur J Radiol 83(8):1381–1386. https://doi.org/10.1016/j.ejrad.2014.05.021
Chaudhry AR, El Khoury M, Gotra A, Eslami Z, Omeroglu A, Omeroglu-Altinel G, Chaudhry SH, Mesurolle B (2019) Imaging features of pure and mixed forms of mucinous breast carcinoma with histopathological correlation. Br J Radiol 92(1095):20180810. https://doi.org/10.1259/bjr.20180810
Cyrta J, Andreiuolo F, Azoulay S, Balleyguier C, Bourgier C, Mazouni C, Mathieu MC, Delaloge S, Vielh P (2013) Pure and mixed mucinous carcinoma of the breast: fine needle aspiration cytology findings and review of the literature. Cytopathology 24(6):377–384. https://doi.org/10.1111/cyt.12016
Shield PW, Ribu DL, Cominos D (2016) The significance of extracellular mucin in breast fine needle aspiration specimens. Cytopathology 27(3):185–192. https://doi.org/10.1111/cyt.12257
Walsh MM, Bleiweiss IJ (2001) Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol 32(6):583–589. https://doi.org/10.1053/hupa.2001.24988
Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J (2010) Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol 63(12):1043–1047. https://doi.org/10.1136/jcp.2010.082495
Budzik MP, Fudalej MM, Badowska-Kozakiewicz AM (2021) Histopathological analysis of mucinous breast cancer subtypes and comparison with invasive carcinoma of no special type. Sci Rep 11(1):5770. https://doi.org/10.1038/s41598-021-85309-z
Didonato R, Shapiro N, Koenigsberg T, D’Alfonso T, Jaffer S, Fineberg S (2018) Invasive mucinous carcinoma of the breast and response patterns after neoadjuvant chemotherapy (NAC). Histopathology 72(6):965–973. https://doi.org/10.1111/his.13451
Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N (2010) Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med 1(5):747–754. https://doi.org/10.3892/etm.2010.133
Lei L, Yu X, Chen B, Chen Z, Wang X (2016) Clinicopathological characteristics of mucinous breast cancer: a retrospective analysis of a 10-year study. PLoS ONE 11(5):e0155132. https://doi.org/10.1371/journal.pone.0155132
Zhang M, Teng XD, Guo XX, Zhao JS, Li ZG (2014) Clinicopathological characteristics and prognosis of mucinous breast carcinoma. J Cancer Res Clin Oncol 140(2):265–269. https://doi.org/10.1007/s00432-013-1559-1
Erhan Y, Ciris M, Zekioglu O, Erhan Y, Kapkac M, Makay O, Ozdemir N (2009) Do clinical and immunohistochemical findings of pure mucinous breast carcinoma differ from mixed mucinous breast carcinoma? Acta Chir Belg 109(2):204–8. https://doi.org/10.1080/00015458.2009.11680406
Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, Ditkoff BA, Schnabel FR (2004) Pure mucinous carcinoma of the breast. Am J Surg 187(4):528–532. https://doi.org/10.1016/j.amjsurg.2003.12.039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were following the ethical standards of the institutional and national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmer, A.C., Tazeoğlu, D. & Dağ, A. Comparison of Clinical, Histopathological, and Survival Outcomes of Pure and Mixed Mucinous Breast Carcinoma. Indian J Surg 85, 802–808 (2023). https://doi.org/10.1007/s12262-022-03573-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-022-03573-3