Abstract
Severe acute pancreatitis represents about 20% of all pancreatitis diagnosis and it has a high mortality rate when associated with a lung dysfunction. The aim of this research was to investigate the use of a wild spectrum metabolic drug (Cytoflavin®) for the treatment of acute lung injury in experimental pancreatitis. A l-arginine-induced acute pancreatitis (groups II and III) was simulated experimentally in a rat population. A combined metabolic drug was used as treatment. We determined the levels of amylase, medium molecules (MM 254 and 280), malonic aldehyde (MA), diene conjugates (DC), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) in the blood and we performed a histologic examination of the lungs and pancreas of the rat population. The activation of lipid peroxidation peaks at 24–48 h from the onset of the disease. The level of malonic aldehyde in groups II and III was higher (39.09% and 30.99%, respectively) than that in the control group I (p < 0.02; p < 0.01), the diene conjugates level was higher by 43.66% and 42.03% respectively (p < 0.01). With medical correction after 72 h (group III), the level of malonic aldehyde and diene conjugates decreased by 17.0% and 30.5% when compared with group II (no medical correction) (p < 0.05). The level of medium molecules peaked after 72 h of the disease induction (p < 0.001). A correlation was established between the level of endogenous intoxication with the membrane-destructive processes in the lung tissue 48 h after modeling of acute experimental pancreatitis. The use of the proposed medicament correction reduces the manifestations of endogenous intoxication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is one of the most common causes of emergency surgery [1]. Although, 80% of cases are mild, the remaining 20% are classified as a severe form [2]. The mortality in patients with severe form can reach up to 50% [3, 4]. Mortality in AP has two peaks: the first occurs within 7 days after the onset of the pancreatitis symptoms and it is associated with the development of organ dysfunction; the second develops later when a multiple organ failure syndrome occurs generally complicated by the development of sepsis [5, 6]. The multiple organ failure syndrome includes disorders of the cardiovascular, respiratory, and excretory systems and especially of the liver. Once started, the severity of the pathological process is linked to the synthesis of a complex chain of inflammatory mediators such as cytokines, proteolytic enzymes, free oxygen radicals, complement fragments, eicosanoids that lead to a systemic process and a multiple organ dysfunction [7, 8].
In recent decades, the use of modern medical technologies has significantly improved the effectiveness of the treatment in patients with various forms of AP [9, 10], but in the case of an acute respiratory distress syndrome, the mortality reaches up to 48–86% without a significant trend towards improvement [11]. Experimental and clinical studies have shown the increased activity of pancreatic enzymes in the blood and cytokine content is responsible for pulmonary injury development in the setting of acute pancreatitis during the first hours [12, 13].
The severity of AP depends in fact on the relationship between pro- and anti-inflammatory cytokines, including also interleukin-10 (IL-10), which inhibits the phagocytic activity of macrophages and the synthesis of other inflammatory mediators [14]. The tumor necrosis factor-α (TNF-α) instead has a cytotoxic effect on parenchymal target cells enhancing interleukin-8 (IL-8) synthesis.
The purpose of the following study is to investigate the effectiveness of a drug/medication combination “Cytoflavin®” (in 1 ml: 100 mg of succinic acid, 10 mg of nicotinamide, 20 mg of riboxin inosine and 2 mg of riboflavin sodium phosphate), for the treatment of acute lung injury induced by acute pancreatitis in a rat population.
Literature data already demonstrated that Cytoflavin® have shown clear benefits in the case of cerebrovascular or neuro-degenerative diseases and neuroasthenia. Recently, it has been successfully tested in the intensive care unit for the treatment of metabolic disorders associated with acute purulent-destructive lung diseases, peritonitis, or for the correction of enteral dysfunctions in patients with AP [15].
The drug components are inducers of the major metabolic pathways in pulmonary cells, they act as activators of several energy-generating processes that promote the utilization of free oxygen radicals, thereby reducing the level of peroxide processes and tissue ischemia. The components of Cytofalvin® are natural metabolites of the body which stimulate tissue respiration and improve the metabolism of the central nervous system. The succinic acid is an endogenous intracellular metabolite of the Krebs cycle, during which it is transformed into fumaric acid. It stimulates aerobic glycolysis and the synthesis of ATP in cells. Inosine is a derivative of purine that has the ability to activate a number of enzymes of the Krebs cycle increasing ATP production. Riboflavin (B2 vitamin) activates the enzyme succinate dehydrogenase and other redox reactions of the Krebs cycle. Nicotinamide (vitamin PP) is crucial for the activation of nicotinamide-dependent enzymes of the Krebs cycle required for cellular respiration.
Methods
The experimental study was conducted in 105 white Wistar rats weighting 190–230 g, kept in a standard diet in the vivarium of the Ivano-Frankivsk National Medical University (Ukraine). The mean age of the rat population was 11 weeks (range, 8–12 weeks). Sixty rats were female (57.1%), forty-five male (42.9%).
The rats were divided into three groups: group I—control group (n = 10), in which a 0.9% isotonic NaCl solution was injected intraperitoneally (after overnight fasting); group II—experimental (n = 51), in which we induced an AP by two intraperitoneal injections of 20% l-arginine (total dose of 5 g / kg, at 1-h interval) [16].
Finally, group III—experimental (n = 44), in which rats were injected with “Cytoflavin®” at a dose of 0.21 ml/kg at 5 min after AP simulation. A general anesthesia was induced for each rat during the course of the study. The timeframe of the experiment was 12, 24, 48, 72, and 96 h after AP induction time. All animals were euthanized at the timeframe specified in the experiment.
Tissue sampling from the lungs and pancreas of the rats was performed 20–40 min after euthanasia. Blood tests in the experimental induced-AP population were performed after the death of the animals. For histological examination, sections were stained with hematoxylin and eosin and they were investigated by the method of elective detection of fibrin in modifications [17]. All sections were examined using a microscope “Leica DME™” (Leica Microsystems CMS GmbH, Germany, 2008) with a digital camera “Nikon Coolpix 5100™” (Japan, 2008). The blood amylase activity was determined by the amyloclastic method [18]. The level of malonic aldehyde was investigated according to the modified method of lipid peroxidation product determination [19].
The method for the detection of diene conjugates (DCs) was the formation of a conjugated double bond system, which was accompanied by the appearance of a new absorption maximum band in the spectrum range (λ max = 233 nm) [20]. The method of determining the content of medium molecules (MM) was based on the deposition of high molecular weight peptides and proteins of biological fluids using trichloroacetic acid in the obtained centrifugation of the supernatant of medium molecular weight peptides by the absorption in monochrome light flux at a wavelength of 254 nm and 280 nm [21]. We analyzed the cytokine levels in serum by enzyme immunoassay (analyze “StatFax 303 Plus”) and we used the enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of TNF-α, interleukin-8 (IL-8) and interleukin-10 (IL-10) by close actioners corporation “Vector-Best.”
The study had the approval of the Institutional Ethical Committee of Ivano-Frankivsk National Medical University (Ukraine) and it was performed according to the Helsinki Declaration guidelines. (Directives EU № 609 from 24/11/1986 and the Law of Ukraine № 3447-1 from 21/02/2006).
Statistical analysis was performed using SPSS 19 (Statistical Version 6, StatSoft, Inc.). Quantitative data were expressed as mean values ± standard deviation (SD). Fisher’s exact test was used to compare the quantitative variables. Spearman’s (rank) correlation coefficient (nonparametric) was used to establish correlations among biochemical variables. A p value < 0.05 indicated a statistical significance.
Results
The highest level of amylase activity (threefold) was found 24 h after the study starting time (Table 1). Activation of lipid peroxidation by AP in experimental conditions reaches its peak at 24–48 h from the beginning of the disease: malonic aldehyde content in the group II and III exceeded the control values by 39.09% and 30.99%, whereas the level of diene conjugates by 43.66% and 42.03% (p < 0.001). In the subsequent course of AP (for 72 h), the malonic aldehyde content decreased by 18.65%, and diene conjugates continued to increase by 18.34% in comparison with the indicators at 24 h of the experiment (p < 0.05), whereas with medication correction for 72 h, the diene conjugates decreased by 11.29% and the malonic aldehyde by 11.58% when compared with the 24 h time value (p < 0.05).
However, during the first 72 h, we observed a decrease of malonic aldehyde and diene conjugates by 17.0% and 30.5%, respectively, due to medical corrections, in comparison with similar indicators in group II (p < 0.05).
After 72 h after AP development, the lipid peroxidation processes slowly decreased, the level of endogenous pathogens—medium molecules (MМ254 and MМ280)—instead reached its peak: in group II—it increased by 39.52% and 50.00%, meanwhile in group III—it increased by 33.33% and 36.41% when compared with group I (p < 0.001).
At 72 h from the experiment start time, the content of IL-8 in group II was 31.72% higher than group III (p < 0.02) (Table 2). The IL-10 count continued to decline after 72 h and 96 h in group II, and it remained stable at group III. When comparing IL-8 and TNF-α after 48 h in group II, the content of IL-8 and TNF-α was 27.29% and 60.42% higher than that in group III, indicating a high therapeutic efficacy for the course of the pathological process (p < 0.01). After 24 h from the development of the experimental AP, the concentration of cytokines increased in groups II and III: TNF-α by 13.23 and 17.04 times respectively (p < 0.001), IL-8 by 4.77 and 5.61-fold (p < 0.001), and IL-10 decreased by 56.71% and 36.89% compared with the control group (p < 0.01). After 48 h, TNF-α levels began to decrease in both groups, but it remained higher by 9.37 and 5.01 times relative to the control group (p < 0.001), while in the group III the IL-8 content maintained at the previous level (58.54 SD 4.30 pg/ml), and in group II it reached its peak 80.51 SD 3.79 pg/ml (p < 0.01), whereas the concentration of IL-10 at 48 h from the experiment starting time reached its peak 63.50% higher than the same indicator in group I (p < 0.05) in the treated AP group.
After 24 h from treatment administration, the level of ММ280 decreased by 17.7%, malonic aldehyde by 13.3%, and diene conjugates by 14.5% (p < 0.05); after 48 h, the content of interleukin-8 decreased by 37.5% and tumor necrosis factor-α by 58.6% (p < 0.01), while the concentration of interleukin-10 increased by 63.5% (p < 0.02) when compared with their values in group II.
Discussion
These data seem to confirm the overall concentration of inflammatory molecules (medium molecules, malonic aldehyde, diene conjugates) increased by 1.2–1.5 times in the blood of the bronchopulmonary system of rats with induced AP. This seems to be caused by the disbalance in pro- and anti-inflammatory cytokine production [22].
Conversely, in the rat population treated, the levels of IL-8 and TNF-α were lower thus indicating a good therapeutic efficacy during the course of the pathological process.
Our results are comparable with another study where in genetically modified mice lacking the ability to synthesize IL-10, the course of acute pulmonary injury due to AP was significantly more severe than that in not modified animals, while the severity of AP in both groups was similar [23]. TNF-α has cytotoxic effects on the parenchymatous target cells and it increases IL-8 synthesis; moreover, it directly damages the endothelial cells and it causes the activation of coagulative hemostasis, microvascular thrombosis; finally, it also stimulates neutrophil cell accumulation in the lungs [24, 25].
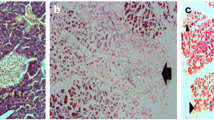
During our experience, some pathological changes occurred in the lungs of the rats with an experimentally induced-AP (Fig. 1): at the final histological examination, in fact, we observed multiple areas of atelectasis, the presence of hyaline membrane disease, and several microcirculatory disorders (microthrombosis) characterized by the accumulation of neutrophilic leukocytes and alveolar macrophages in the pulmonary vessels and parenchyma. The revealed changes in the lung tissue of rats with AP-induced lung injury caused an impairment of the pulmonary hemoperfusion with a venous-arterial bypass, which lead to deterioration of the alveolocyte function due to the anomalous perfusion.
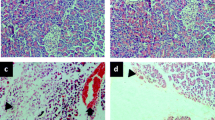
We also established a positive correlation between the levels of medium molecules MМ254 and MМ280 (r = 0.59 and r = 0.56, р < 0.01) and the membrane-destructive processes in lung tissue 48 h after the induction of AP in group II (Fig. 2); this correlation indicated a worsening of the endogenous intoxication. This process appears to be related to increased permeability of the alveolar-capillary membrane induced by the mediators of inflammation of the endothelium. The increased thickness of the aero-hematic barrier significantly impaired the oxygen and CO2 diffusion. In the treated rat population after the experimentally induced AP, the positive effect of the therapy on the state of the structural components of the aero-hematic barrier was revealed at 72 h from the onset of the AP (Fig. 3). We noticed in fact a reduction in alveolocyte swelling, and a clear contour of the blood capillaries in the treated population; no sign of stasis was observed.
Based on the data obtained during the experimental study, we can conclude “Cytoflavin®” contributes to the reduction of endogenous intoxication due to its ability to limit the membrane-destructive processes in lung tissues. These findings, in our opinion, could open the way for the use of this drug for the treatment of AP in the near future.
Conclusions
The main alterations we observed in the lungs of rats with experimentally reproduced-acute pancreatitis were areas of microatelectasis, the development of a hyaline membrane disease, episodes of microthrombosis, appearance of neutrophilic leukocytes, and alveolar macrophages in the lungs tissue. The correlation between the levels of medium molecules (М254 and MМ280) with the membrane-destructive processes 48 h after the modeling of acute pancreatitis (group II) indicated an increase of the endogenous intoxication.
The application of the proposed medication (Cytoflavin®) for the treatment of experimental acute pancreatitis reduced the manifestations of the endogenous intoxication and it promoted the equilibrium of redox reactions and cytokine network.
References
Yadav D, Lowenfels AB (2013) The epidemiology of pancreatitis and pancreatic cancer. Gastroenterol. 144:1252–1261
Párniczky A, Lantos T, Tóth EM, Szakács Z, Gódi S, Hágendorn R, Illés D, Koncz B, Márta K, Mikó A, Mosztbacher D, Németh BC, Pécsi D, Szabó A, Szücs Á, Varjú P, Szentesi A, Darvasi E, Erőss B, Izbéki F, Gajdán L, Halász A, Vincze Á, Szabó I, Pár G, Bajor J, Sarlós P, Czimmer J, Hamvas J, Takács T, Szepes Z, Czakó L, Varga M, Novák J, Bod B, Szepes A, Sümegi J, Papp M, Góg C, Török I, Huang W, Xia Q, Xue P, Li W, Chen W, Shirinskaya NV, Poluektov VL, Shirinskaya AV, Hegyi PJ, Bátovský M, Rodriguez-Oballe JA, Salas IM, Lopez-Diaz J, Dominguez-Munoz JE, Molero X, Pando E, Ruiz-Rebollo ML, Burgueño-Gómez B, Chang YT, Chang MC, Sud A, Moore D, Sutton R, Gougol A, Papachristou GI, Susak YM, Tiuliukin IO, Gomes AP, Oliveira MJ, Aparício DJ, Tantau M, Kurti F, Kovacheva-Slavova M, Stecher SS, Mayerle J, Poropat G, Das K, Marino MV, Capurso G, Małecka-Panas E, Zatorski H, Gasiorowska A, Fabisiak N, Ceranowicz P, Kuśnierz-Cabala B, Carvalho JR, Fernandes SR, Chang JH, Choi EK, Han J, Bertilsson S, Jumaa H, Sandblom G, Kacar S, Baltatzis M, Varabei AV, Yeshy V, Chooklin S, Kozachenko A, Veligotsky N, Hegyi P, Hungarian Pancreatic Study Group (2019) Antibiotic therapy in acute pancreatitis: from global overuse to evidence-based recommendations. Pancreatology 19(4):488–499. https://doi.org/10.1016/j.pan.2019.04.003
Yokoe M, Takada T, Mayumi T (2015) Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci 22(6):405–432
Kui B, Balla Z, Vasas B, Végh ET, Pallagi P, Kormányos ES, Venglovecz V, Iványi B, Takács T, Hegyi P, Rakonczay Z (2015) New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS One 10(2):e0117588. https://doi.org/10.1371/journal.pone.0117588
Zhang H, Neuhöfer P, Song L (2013) IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest 123(3):1019–1033
Maheshwari N, Kumar A, Iqbal ZA, Mandal AK, Vyas A, Wig JD (2015) Organ failure in acute pancreatitis and its impact on outcome in critical care. Southwest J Pulm Crit Care 10:253–264
Campos A (2017) Plasmapheresis in the management of acute pancreatitis due to severe hypertriglyceridemia-reporting new cases. J Ren Hepat Disord 1(1):29–34
Dedemadi G, Nikolopoulos M, Kalaitzopoulos I, Sgourakis G (2016) Management of patients after recovering from acute severe biliary pancreatitis. World J Gastroenterol 22(34):7708–7717
Baal MC, Santvoort HC, Bollen TL, Dutch Pancreatitis Study Group (2011) Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. BJS. 98:18–27
Pezzilli R, Zerbi A, Campra D, Italian Association for the Study of the Pancreas (AISP) (2015) Consensus guidelines on severe acute pancreatitis. Dig Liver Dis 47(7):532–543
Costa DW, Boerma D, van Santvoort HC (2014) Staged multidisciplinary step-up management for necrotizing pancreatitis. BJS. 101:65–79
Jaswal DS, Leung JM, Sun J (2014) Tidal volume and plateau pressure use for acute lung injury from 2000 to present: a systematic literature review. Crit Care Med 42(10):2278–2289
Zhou MT, Chen CS, Chen BC (2010) Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol 16(17):2094–2099
Deng LH, Hu C, Cai WH (2017) Plasma cytokines can help to identify the development of severe acute pancreatitis on admission. Medicine 96(28):E7312
Orlov YP, Gorova NV, Glushchenko AV (2016) Acute pancreatitis by the view of anesthesiology – reanimatology: comments to the Russian recommendations for the treatment of acute pancreatitis. Bulletin of Intensive Therapy 4:34–40
Czako L, Takacs T (1998) Involvement of oxygen-derived free radicals in L-arginine-induced acute pancreatitis. Dig Dis Sci 43(8):1770–1777
Bahrii VA, Dibrova (2016) Methods of morphological research: monograph / M.M. Vinnytsya: New Book
Fedorkiv MB (2015) Prevention and correction of pulmonary complications for severe acute pancreatitis. Klin Khir (6):22–24 Ukrainian
Korobeinykova YN (1989) Modification of the determination of lipid peroxidation products in reaction with thiobarbituric acid. Laboratory work 7:8–10
Havrylov VB, Havrylova AR, Khmara YF (1988) Measurement of diene conjugates in blood plasma by IF absorption of heptane and isopropanol extracts. Laboratory Work 2:60–63.22
Gabrielyan NI, Dmitriev AA, Kulakov GP (1981) Diagnostic value of determination of average molecules in blood plasma in nephrologic diseases. Clin Med 10:38–42
Potanina OK, Dorfman AG, Ogurtsova EV (2011) Comparison of efficiency of prognostic scales of an estimation of gravity of a condition of resuscitation patients of a surgical profile. Doctor and IT 6:50–61
Ozkardeş AB, Bozkurt B, Dumlu EG (2015) Effects of everolimus on a rat model of cerulein-induced experimental acute pancreatitis. Ulus Cerrahi Derg 31:185–191
Sargin G, Coşkum A, Akin HŞ, Karaoğlu AŐ (2013) Recurrent acute pancreatitis due to hypertriglyceridemia and acute lung injury. Istanbul Med J 14:143–145
Swaroopa D, Bhaskar K, Mahathi T (2016) Association of serum interleukin-6, interleukin-8, and Acute Physiology and Chronic Health Evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J Crit Care Med 20(9):518–525
Author information
Authors and Affiliations
Contributions
Mariana Fedorkiv and Marco V. Marino analyzed the data and drafted the article; Mariana Fedorkiv, Roman Kuzenko, and Sergiy Gvozdyk participated in the experiment simulation and data collection; Mykola Bagrii performed histological exams, histological photography, and data collection; Galyna Shabat performed study design and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the Institutional Ethical Committee of Ivano-Frankivsk National Medical University (Ivano-Frankivsk, Ukraine) and performed according to the Helsinki Declaration guidelines. (Directives EU № 609 from 24/11/1986 and the Law of Ukraine No. 3447-1 from 21/02/2006).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fedorkiv, M., Marino, M.V., Kuzenko, R. et al. Experimental Acute Pancreatitis-Induced Lung Injury—Prevented with “Cytoflavin®”. Indian J Surg 83, 720–725 (2021). https://doi.org/10.1007/s12262-020-02475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02475-6