Abstract
The aim of this study was to evaluate the clinicopathologic characteristics and pattern of lymph node (LN) metastasis in papillary thyroid cancer (PTC) located in the isthmus. A systematic review of the relevant electronic databases was conducted between January 2000 and December 2019, including Pubmed, Web of Science, and the China Journal Net. Outcomes of interest included gender, age, tumor size, multifocality, capsule invasion, extrathyroidal extension, and lymph node metastasis. We calculated the pooled odds ratios (ORs) with 95% confidence intervals (CIs) for each study using a random or fixed effect model. Nine studies with a total of 4541 patients were included. Patients with isthmic PTC were 565 (12.4%). Our meta-analysis revealed that there was a significant association between the isthmus location and multifocality (OR = 1.50; 95% CI = 1.18–1.90), capsule invasion (OR = 1.53; 95% CI = 1.17–1.99), extrathyroidal extension (ETE) (OR = 1.95; 95% CI = 1.34–2.86), and central LN metastasis (OR = 1.53; 95% CI = 1.17–1.99). For patients with solitary nodule, our meta-analysis illustrated that there was a significant association between the isthmus location and ETE (OR = 1.65; 95% CI = 1.11–2.46), and central LN metastasis (OR = 2.50; 95% CI = 1.82–3.44). However, the meta-analysis suggested that there was no correlation between the isthmus location and lateral LN involvement (OR = 1.04; 95% CI = 0.58–1.87). PTCs located in the isthmus were associated with multifocality, capsule invasion, ETE, and more likely to involve the central lymph node.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Thyroid cancer rarely occurs in the isthmus. The isthmus is located in front of the second and third tracheal rings connecting the two lateral lobes and has a length of about 20 mm, a width of about 20 mm, and a thickness of 2 to 6 mm [1]. The incidence of papillary carcinoma arising in the thyroid isthmus is low (1 to 9.2%) [2,3,4,5].
Previous studies have shown that papillary thyroid cancer (PTC) located in the isthmus are more likely to exhibit extrathyroidal extension and multiple lesions than those located in the other parts of the thyroid [2, 4, 6]. In addition, isthmus PTC is more strongly associate with central lymph node metastasis than lobar tumors [5].
Surgical treatment of PTCs located in the isthmus remains controversial due to its anatomical and biological characteristics. The American Thyroid Association (ATA), the British Thyroid Association (BTA), and the European thyroid Association (ETA) provided recommendations for well-differentiated thyroid cancer, but there are no accurate guidelines for the management of patients with dominant thyroid nodules of the isthmus [7,8,9]. To determine the optimal extent of prophylactic lymph node dissection, it is crucial to establish a pattern of lymph node metastasis in isthmus PTC.
In the present study, we analyzed the clinicopathologic features in patients with papillary carcinoma of the thyroid isthmus and compared the findings with those for patients with tumors in other parts of the thyroid.
Materials and Methods
Search Strategy
Pubmed, Web of science, and the China Journal Net were searched for publications from January 2000 to December 2019. The search terms used were “Papillary Thyroid Cancer,” “Isthmus,” and “Clinicopathologic Characteristics.” The reference lists of relevant studies were checked manually to locate any missing studies.
Inclusion and Exclusion Criteria
Criteria for eligibility of a study included in this meta-analysis were as follows: (1) Detection of the papillary thyroid cancer in the isthmus. (2) The studies were published in English, Chinese, and Korean. (3) When several studies were reported from the same authors or organizations, the meta-analysis enrolled the most recent or highest quality study only if the most recent one did not fit the inclusion criteria. Studies were excluded if (1) studies were case reports, letters, and reviews without original data; animal or laboratory studies; (2) the studies without control data were excluded; (3) repeated studies were based on the same database or patients.
Data Extraction
Two review authors (L.Y. and L.H.) independently selected studies for inclusion and extracted the data. A third researcher (X.J.) arbitrated in the event of any disagreement. The decision for inclusion in the analysis was made by consensus. Full-text copies of potentially relevant studies were obtained. The following variables were recorded: authors, sex, number of patients, age of patients, and clinicopathological characteristics. The dominant nodule was considered as the primary carcinoma when multifocal disease was found. Patients with dominant nodule in isthmus were classified as isthmus group, and patients with dominant nodule located in lobes were classified as the non-isthmus group.
Statistical Analysis
A formal meta-analysis was made for all studies. The statistical analysis was carried out using the Review Manager 5.0. Pooled estimates of the complications were calculated using a fixed effects model, but a random effects model was used according to heterogeneity. The test of effect homogeneity was performed using χ2 tests, with p ≤ 0.05 indicating significant heterogeneity. When the hypothesis of homogeneity was not rejected, the fixed effects model was used to estimate the pooled effect of the outcomes; when the reverse was true, the random effects model was also calculated. For the pooled analysis of the correlation between isthmus location and clinicopathological features (sex, tumor size, multifocality, capsule invasion, presence of extrathyroidal extension, lymph node metastasis), odds ratios (ORs) and 95% CIs were combined to estimate the effect.
Results
Study Selection
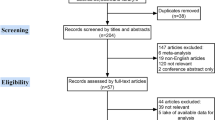
We identified 19 potentially relevant articles (Fig. 1). After exclusion of duplicate references, non-relevant literature, and those manuscripts that did not satisfy the inclusion criteria, 9 candidate articles [2, 5, 6, 10,11,12,13,14,15] were considered for the meta-analysis. The study characteristics are summarized in Table 1.
A total of 4541 patients who underwent thyroidectomy due to papillary thyroid cancer (PTC) were enrolled. Of them, most cases of PTC were located in the lobe (n = 3976, 87.6%), and a total of 565 PTC cases had tumors located at the isthmus. The patient demographics for the 9 studies are presented in Table 1. All papers were retrospective chart reviews. The publication dates ranged from 2010 to 2018. The study sizes ranged from 70 to 1973 patients.
Five studies demonstrated that there was a significant association between the isthmus location and multifocality (OR = 1.50; 95% CI = 1.18–1.90) (Fig. 2). Except this abovementioned parameter, controversies also existed on the correlation among capsule invasion, extrathyroidal extension, and lymph node metastasis. Four studies including 2712 patients were analyzed for the association between the isthmus location and capsule invasion. There was a significant association between the isthmus location and capsule invasion (OR = 1.53; 95% CI = 1.17–1.99) (Fig. 3). Four studies including 760 patients were analyzed for the association between the isthmus location and extrathyroidal extension (ETE). There was a significant association between the isthmus location and extrathyroidal extension (OR = 1.95; 95% CI = 1.34–2.86) (Fig. 4a). In addition, there was a significant correlation between the isthmus location and lymph node metastasis (OR = 1.83; 95% CI = 1.03–3.25) (Fig. 5a) and central lymph node metastasis (OR = 2.03; 95% CI = 1.28–3.23) (Fig. 6a). However, the meta-analysis suggested that there were no correction between the isthmus location and lateral lymph node involvement (OR = 1.04; 95% CI = 0.58–1.87) (Fig. 7a). When we focused on the solitary thyroid nodule, we also discovered that there was a significant association between the isthmus location and extrathyroidal extension (OR = 1.95; 95% CI = 1.34–2.86) (Fig. 4b), lymph node metastasis (OR = 1.81; 95% CI = 1.18–2.75) (Fig. 5b), and central lymph node metastasis (OR = 2.50; 95% CI = 1.82–3.44) (Fig. 6b). On the contrary, there were no significant differences between the isthmus location and lateral lymph node involvement for solitary isthmus nodule (OR = 1.53; 95% CI = 0.87–2.71) (Fig. 7b).
Discussion
A solitary PTC of the isthmus is an uncommon lesion and requires surgical evaluation. Despite its low incidence, isthmic PTC is associated with more aggressive clinical and pathological features. Previous studies have indicated that PTCs located in the isthmus are more likely be multifocal compared with PTC located in other parts of the gland [4, 16]. Arora [17] have reported that multifocal tumors are common in PTC, and their incidence is not related to tumor size. The multifocality seems to be related to the midline position of the tumors, which easily spreads to the thyroid bilobes.
The capsule invasion rate was 59.9% in the isthmic PTC group, which was significantly higher than the 51.8% rate reported in the control group. The main reason for this difference might be the special anatomical structure with tissue that is 2 to 6 mm thick and is covered by strap muscles [3], even with smaller tumors, the isthmic PTC is more likely to invade the capsule, and capsule invasion cases are more likely to invade the surrounding tissue, which translates into a higher rate of extrathyroidal extension.
Several reports have suggested that tumor location is associated with neck metastasis [18,19,20]. However, the controversy remains regarding whether PTC located in the isthmus is correlated with central lymph node or lateral lymph node metastasis. In our results, isthmus PTC were more likely to involve the central lymph nodes, and there was no significant difference in the frequency of lateral lymph node involvement. This result seems to be due to differences in the lymphatic system according to the anatomical locations of the thyroid gland. Although the isthmus has poor lymphatic channels, lymphatics from the isthmus usually drain into the prelaryngeal and pretracheal regions [2].
Prelaryngeal LN are also called Delphian, from “the Oracle of Delphi,” a Greek legend, predicting an unfavorable prognosis [21]. Furthermore, prelaryngeal lymph node metastasis in PTC has been associated with poor prognostic markers, such as higher rates of extrathyroidal extension, capsule invasion, and multifocality [22,23,24]. Lateral node involvement was also commonly observed with isthmus PTC in our study at 14.6% (59/405), which is comparable with other previous studies [25]. However, there was no significant difference in the frequency of lateral LN involvement. This difference may be explained by differences in the extent of neck dissection performed.
There are some limitations in our meta-analysis. Firstly, the pooled studies differed in inclusion and exclusion criteria. These may be the major source of heterogeneity. Second, the data included in some studies may have been too crude and also subject to measurement error. Finally, the sample size of the included studies was too small to exclude beta error. Hence, our findings must be interpreted with caution.
Conclusions
The location of the cancer in the isthmus was associated with multifocality, capsule invasion, ETE, and central lymph node metastasis at the time of diagnosis. Thus, patients with PTC originating in the isthmus should be performed with total thyroidectomy and possible central node dissection.
References
Hoyes AD, Kershaw DR (1985) Anatomy and development of the thyroid gland. Ear Nose Throat J 64:318–333
Lee YS, Jeong JJ, Nam KH, Chung WY, Chang HS, Park CS (2010) Papillary carcinoma located in the thyroid isthmus. World J Surg 34:36–39
Nixon IJ, Palmer FL, Whitcher MM, Shaha AR, Shah JP et al (2011) Thyroid Isthmusectomy for well-differentiated thyroid cancer. Ann Surg Oncol 18:767–770
Sugenoya A, Shingu K, Kobayashi S, Masuda H, Takahashi S, Shimizu T, Onuma H, Asanuma K, Ito N, Iida F (1993) Surgical strategies for differentiated carcinoma of the thyroid isthmus. Head Neck 15:158–160
Karatzas T, Charitoudis G, Vasileiadis D, Kapetanakis S, Vasileiadis I (2015) Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: a case control study. Int J Surg 18:64–68
Hahn SY, Han BK, Ko EY, Shin JH, Ko ES (2014) Ultrasound findings of papillary thyroid carcinoma originating in the isthmus: comparison with lobe-originating papillary thyroid carcinoma. AJR Am J Roentgenol 203:637–642
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and differentiated thyroid Cancer, D.S. Cooper, G.M. Doherty, B.R. Haugen, Kloos RT, Lee SL, et al., Revised American. Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer, Thyroid. 2009;19:1167–214
Watkinson JC The British Thyroid Association guidelines for the management of the thyroid cancer in adults. Nucl. Med. Commun. 25:897–900
Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thryoid Cancer Taskforce (2006) European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:787–803
Lee YC, Na SY, Chung H, Kim SI, Eun YG (2016) Clinicopathologic characteristics and pattern of central lymph node metastasis in papillary thyroid cancer located in the isthmus. Laryngoscope. 126:2419–2421
Chang YW, Lee HY, Kim HS, Kim HY, Lee JB, Son GS (2018) Extent of central lymph node dissection for papillary thyroid carcinoma in the isthmus. Ann Surg Treat Res 94:229–234
Song CM, Lee DW, Ji YB, Jeong JH, Park JH, Tae K (2016) Frequency and pattern of central lymph node metastasis in papillary carcinoma of the thyroid isthmus. Head Neck 38(Suppl 1):E412–E416
Li G, Lei J, Peng Q, Jiang K, Chen W, Zhao W et al (2017) Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine (Baltimore) 96:e7143
Xiang D, Xie L, Xu Y, Li Z, Hong Y, Wang P (2015) Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery. 157:526–533
Choi SY, Kim JS, Soh EY, Park CH (2010) Clinicopathologic characteristics of papillary carcinoma in the thyroid isthmus. J Korean Surg Soc 78:77–81
Goldfarb M, Rodgers SS, Lew JI (2012) Appropriate surgical procedure for dominant thyroid nodules of the isthmus 1 cm or larger. Arch Surg 147:881–884
Arora N, Turbendian HK, Kato MA, Moo TA, Zarnegar R, Fahey TJ (2009) Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid 19:473–477
Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, Nam KH (2009) Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol 16:1348–1355
Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, Shen Q, Li DS, Wu Y et al (2012) Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 97:1250–1257
Lee YS, Shin SC, Lim YS, Lee JC, Wang SG, Son SM, Kim IJ, Lee BJ (2014) Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck 36:887–891
Chai YJ, Kim SJ, Choi JY, do Koo H, Lee KE, Youn YK (2014) Papillary thyroid carcinoma located in the isthmus or upper third is associated with Delphian lymph node metastasis. World J Surg 38:1306–1311
Lee YC, Shin SY, Kwon KH, Eun YG (2013) Incidence and clinical characteristics of prelaryngeal lymph node metastasis in papillary thyroid cancer. Eur Arch Otorhinolaryngol 270:2547–2550
Isaacs JD, Lundgren CI, Sidhu SB, Sywak MS, Edhouse PJ, Delbridge LW (2008) The Delphian lymph node in thyroid cancer. Ann Surg 247:477–482
Kim WW, Yang SI, Kim JH, Choi YS, Park YH, Kwon SK (2012) Experience and analysis of Delphian lymph node metastasis in patients with papillary thyroid carcinoma. World J Surg Oncol 10:226
Lei J, Zhu J, Li Z, Gong R, Wei T (2016) Surgical procedures for papillary thyroid carcinoma located in the thyroid isthmus: an intention-to-treat analysis. Onco Targets Ther 22:5209–5216
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Li, Y. & Xiang, J. Papillary Carcinoma of Thyroid Nodule if Located in Isthmus Is Associated with Greater Disease Progression: a Systematic Review and Meta-analysis. Indian J Surg 82, 1212–1218 (2020). https://doi.org/10.1007/s12262-020-02279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02279-8