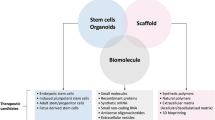
Abstract
Congenital anomalies account for more than one-third of all pediatric hospital admissions annually. The defect in most cases is absence of normal functioning tissue or dysfunctional tissue. Cure in such cases often requires excision of dysfunctional tissue and replacement by healthy functioning tissue. Such tissues, however, are seldom available. Using the regenerative potential of multipotent stem cells which have the ability to self-renew, differentiate into specialized tissue, and protect healthy tissues against ischemia and inflammation-induced injury represents a lucrative option available to the pediatric surgeons for the management of such cases. Actual clinical use, however, has been limited by stem cell availability and lack of required expertise for their optimal usage. Pediatric surgeons are best-positioned for optimal utilization of stem cells for congenital anomalies, and in this article, the author has reviewed the latest research on stem cell usage in difficult to treat pediatric surgical diseases and its future implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Congenital anomalies occur as the result of aberrant organogenesis in utero. Possible etiologies include genetic defects, exposure to environmental teratogens, ischemia, and infection [1]. The morbidity and mortality caused by these anomalies is quite high, and up to 25% of cases require termination of pregnancy in utero because of anomalies incompatible with postnatal survival [1]. The defect in most cases is deficient or defunctionalized tissue, often requiring out of the box creativity by the pediatric surgeons for tissue regeneration. For a long time, the clinical application of stem cells was limited to human leukocyte antigen (HLA)-matched bone marrow transplantation. Now, umbilical cord stem cells have been effectively used in the treatment of sickle cell anemia, leukemia, non-Hodgkin’s lymphoma, malignancies, and various autoimmune diseases [2,3,4]. We reviewed the pediatric surgical procedures, where stem cells can give promising results.
Sources of Stem Cells
Stem cells were historically of two subtypes: pluripotent embryonic stem cells and adult stem cells. The former arises from a fertilized egg and gives rise to whole-organisms, while the latter represents tissue-specific multipotent cells that reside in adult tissues, and maintain their homeostatic balance. In 1998, James Thompson et al. described human embryonic stem cells (hESCs), extracted from inner cell mass at day 5–7 of in vitro fertilization. However, due to ethical, legal, and political issues, their use is restricted to study purpose prior to August 9, 2001 [5]. Ferraris and colleagues first used the bone marrow stem cells to regenerate the injured muscle tissue [6]. Bone marrow stem cells (BM-SC) can be hematopoietic (HSC) or mesenchymal stem cells (MSC). The drawback of adult stem cells is limited differentiation capability, contrary to ESCs [6].
Perinatal stem cell-based therapies using amniotic fluid-derived stem cells (AF-MSC and AF-ESC) can be collected during routine amniocentesis or at planned cesarean section [4, 7]. The advantages of autologous amniotic fluid (AF) as a source of stem cell are their apparent enhanced plasticity and ready availability [8]. Amniocentesis is done in second trimester as a standard diagnostic procedure to rule out lethal chromosomal and genetic defects. After 15 weeks of gestation, amniocentesis is safe with less than 1% rate of fetal loss [9]. By contrast, harvesting stem cells prenatally from the placenta, cord blood, bone marrow, and liver is much more difficult and is associated with higher fetal morbidity and fetal loss [10, 11]. AF-MSC is an ideal vehicle for high-efficiency gene transduction [12]. Because of their tremendous immunomodulatory potential, AF-MSC is used in autoimmune inflammatory viz. graft- vs. -host disease [2, 13]. Amniotic fluid derived-neural stem cell (AF-NSC) can be used in neuron regeneration [14].
The umbilical cord and placenta is another good source of autologous mesenchymal stem cells available at the time of birth. Umbilical cord mesenchymal stem cells (U-MSC) can be isolated with relatively high efficiency through cell culture, with best results noted from blood that is less than 15 h old and with a total volume of over 33 ml [2]. U-MSC has a high potential for proliferation and differentiation in all 3 germ cells [15]. The umbilical cord blood banks have facilitated their therapeutic use.
Placental MSC (P-MSC) is also relatively easy to obtain through culture of the products of placenta tissue digestion [16].
Enteric neural stem cells (E-NSC) are in experimental protocols obtained from mechanical and enzymatic digestion of gut from its muscular layer. Gastrointestinal stem cells (+ 4 label-retaining cells and crypt-based columnar cells can differentiate into enterocytes, endocrine cells, and goblet cells [5].
Stem cell-based regenerative medicine approach requires multidisciplinary coordination among surgeons, maternal-fetal medicine specialists, neonatologists, cell biologists, and material scientists and continues to provide opportunities for significant clinical success in the future.
Methods
The PubMed database was searched up to July 2018 by using the following keywords: stem cell, mesenchymal stem cell, mesenchymal stromal cell, amniotic fluid-derived stem cell, umbilical stem cell, placental stem cell, pediatric patients, congenital anomalies, pediatric surgical diseases, necrotizing enterocolitis, and neural tube defect. Articles with patient population including children from birth to 16 years of age and animal models were included. These included case reports, clinical trials, controlled clinical trials, observational studies, experimental studies, randomized control trials, and pragmatic clinical trials. The data were reviewed, and disease-specific utility and adverse effects would summarize as below.
Spinal Dysraphism
Spinal dysraphism/spina bifida/meningomyelocele (MMC) includes neural tube defects resulting from defective neurulation in utero. The incidence is approximately 1 in 1500 live births [17]. Postnatal closure often results in significant morbidity caused by associated hydrocephalus, bowel and bladder incontinence, and loss of lower limb function post surgical repair. Fetal surgical repair has now become the standard of care as a result of MOMS trial done in the USA demonstrating reduced incidence of hydrocephalus and modest gains in ambulation after antenatal repair [17]. The early results are promising; however, a significant proportion of spinal cord damage has already occurred in the first half of pregnancy before the planned surgical repair [18]. Several investigators have studied the role of AF-SC therapies in animal models of fetal spinal dysraphism [18,19,20], primarily as a means to provide skin coverage in utero to protect the exposed spinal cord from ongoing mechanical and chemical damage. In these cases, AF-SC was injected in the epidural space and also directly in the defective spinal cord at the time of surgical repair of the defect. There were improved short- and long-term functional outcomes [19, 20]. Fauza and colleagues explored the efficacy of intra-amniotic delivery of high doses of amniotic fluid-based stem cells (termed trans-amniotic stem cell therapy, TRASCET) in a mouse model [20]. In their study, these cells were shown to preferentially home to the neural tube defect to provide partial coverage of the exposed neural tube [19, 21]. Another group of investigators has demonstrated the therapeutic efficacy of amniotic fluid-based tissue engineering by repairing fetal rat neural tube defects using three-dimensional tissue-engineered skin from amniocytes. In this pilot study, epidermis was generated from keratinocytes derived from human amniotic fluid-derived iPSCs, whereas dermis was created from cultured human fibroblasts and type I collagen [20]. Although the early results were promising, the long-term effectiveness of these stem cell-based strategies on functional spinal cord regeneration is yet to be determined, mostly because postnatal survival is not feasible in any of the available rodent MMC models. Despite this, preclinical experiments have not shown good results in human clinical trials (Kim et al.) [22]. Carefully conducted animal studies using fetal lambs with a large sample size will likely be required before this technique can be considered for a clinical trial [21]. Evolving strategies such as the transplantation of AF-NSC into the spinal cord at the time of surgical repair may offer additional benefits in terms of facilitating regeneration of neural derivatives and re-innervation of denervated tissues [14]. This represents a fertile area of research and is likely to result in significant breakthroughs in the future.
Congenital Heart Disease
Congenital heart defects represent the most common major birth defects seen in children worldwide. Those at the most severe end of the spectrum are often diagnosed early during pregnancy by fetal echocardiography. An alternative, regenerative medicine-based approach for these cases would include generation of autologous replacement cardiomyocytes specific to each patient. AF stem cells have been shown to have tremendous cardiomyogenic differentiation potential when cultured with the appropriate paracrine mediators of tissue regeneration [23]. Bone marrow MSCs have been shown to facilitate remodeling of myocardial function in animal models of acute myocardiac infarction [24]. Although research in this area is still quite preliminary, future work has enormous potential in helping to circumvent the scarcity of heart donors for infants born with severe heart failure caused by hypoplastic left heart syndrome and other similar conditions.
Another use for stem cell-based therapy is in children with severe valvular heart disease. Surgical reconstruction of the heart valves and great vessels in these children is currently performed using prefabricated acellular prosthetic implants. Although successful in the short term, these implants fail to grow with the child into adulthood, resulting in the need for multiple surgeries in the future to preserve heart valve function. By contrast, theoretically, a cellularized, tissue-engineered prosthetic would have the ability to grow and remodel over time, thereby avoiding the need for serial revisional procedures. Such an implant would also obviate the need for long-term anticoagulation [25, 26]. To date, multiple animal models have been developed to study the properties of tissue-engineered heart valves [25,26,27,28]. Dijkman et al. beautifully described functional heart valves derived from human AF-SC [29]. The cells were seeded onto synthetic heart valve leaflet scaffolds and conditioned within a pulse bioreactor to facilitate their maturation. Microscopic analyses revealed endothelialized tissue formation as well as biomechanical properties sufficient for implantation in vivo. Subsequent work in large animal models has demonstrated short-term in vivo functionality with intact valvular integrity and absence of thrombus formation [27]. If such promising results continue, clinical trials will not be far behind, providing the opportunity for definitive cure in long-term sufferers of valvular heart disease.
Congenital Diaphragmatic Hernia (CDH)
Congenital diaphragmatic hernias often require use of synthetic patch for surgical repair due to large size of defect and paucity of native tissue available for repair. The commonest complication of using synthetic acellular prosthetics for diaphragmatic repair is recurrent herniation, occurring in up to 50% of survivors [30]. This re-herniation is attributed to the inability of the prosthesis to cover the resultant diaphragmatic defect as the child grows and requires surgical revision [30]. Since most cases of CDH are now diagnosed prenatally, there remains considerable interest in harvesting AF cells at that time in preparation for implantation of a better, long-term diaphragmatic prosthesis which will be available at birth. In an animal model, recurrent herniation was markedly reduced in juvenile lambs when repair was done using a patch composed of AF-derived fibroblasts (termed diaphragmatic tendon) when compared with an acellular patch. The results were mainly attributed to the ability of the tissue-engineered implant to grow with age [31]. Fauza and colleagues first explored this concept in a sheep model using tissue-engineered patch made with amniotic fluid-derived fibroblasts suspended in collagen hydrogels and seeded onto decellularized dermal scaffolds [31, 32]. The results demonstrated lower rates of reherniation as well as improved modular and ultimate tensile strengths over time as compared to acellular prosthetic grafts. Clinical trials using this treatment strategy are forthcoming.
In recent years, amniotic fluid stem cell-based induction of lung growth has become another area of interest for the treatment of lung hypoplasia associated with CDH and bronchopulmonary dysplasia. Given the known association of oligohydramnios with lung hypoplasia, it was hypothesized that amniotic fluid-derived stem cells may be responsible for secretion of growth factors that promote fetal lung growth via paracrine mediators [33]. Evidence for enhanced lung growth with the use of amniotic fluid-derived stem cells in experimental CDH models has been shown in numerous studies [32,33,34]. In an ex vivo fetal lung study [35], amniotic fluid-derived MSCs were shown to augment branching morphogenesis and lung epithelial maturation in the nitrofen rat model of CDH lung hypoplasia [35]. Since nearly 30% of CDH neonates die secondary to overwhelming pulmonary hypoplasia and pulmonary hypertension, in utero augmentation of lung growth with amniotic fluid stem cell delivery directly into the lungs may have a major clinical impact in severely affected fetuses in the future [36].
Biliary Atresia
Biliary atresia (also known as progressive obliterative cholangiopathy or extrahepatic ductopenia). The liver salvage surgery Kasai’s portoenterostomy is a bridging surgery for definitive liver transplantation. Most patients are requiring transplant despite achieving adequate biliary drainage initially after portoenterostomy. Significant biochemical and scintigraphic improvements were noted following stem cell therapy in biliary atresia, in patients who received autologous mononuclear bone marrow stem cells at the time of surgery [37]. Stem cells were injected directly into the liver in infants, either via hepatic artery (20%) or the portal vein (80%) during or after portoenterostomy [38]. The positive results were attributed to anti-inflammatory actions of the stem cells and provided hope for improving patient outcome in cases with biliary atresia especially in patients presenting after 90 days of age [39].
Tracheal Anomalies
Congenital malformations involving the trachea are well-described but extremely rare clinical entities [40]. Many of these are diagnosed in utero but attempts at definitive tracheal reconstruction are currently limited by paucity of available autologous tissue that can mimic the structural and mechanical properties of native airway cartilage. Conditions like tracheal agenesis are almost uniformly fatal. Regenerative medicine using stem cells may provide hope for these kinds of patients in the future. Chondrocytes derived from AF-MSCs could serve as a viable alternative for perinatal tracheal repair and provide the necessary scaffolding [41, 42]. This therapeutic concept has been successfully used in an ovine model of tracheal agenesis [41]. In this study, cultured and GFP-labeled sheep amniocytes were seeded onto cylindrical polyglycolic acid-based scaffolds and exposed to a chondrogenic medium containing transforming growth factor beta. The constructs were then used to reconstruct partial or full circumference tracheal defects in fetal lambs. Neonatal lambs were able to breathe at birth without major respiratory distress, and there was evidence of donor cell survival as revealed by GFP+ cells within fibrous cartilage [41]. Unfortunately, these tracheal constructs were not derived from autologous amniotic fluid and were therefore prone to immunologic rejection. The successful implantation of a tissue-engineered airway structure derived from autologous BM-MSCs has already been reported in an adult [43], paving way for similar procedures in neonates in the future.
Congenital Chest Wall and Craniofacial Defects
The most severe of chest wall defects, ectopia cordis, presents with protrusion of the heart through a split sternum. This provides a unique challenge for reconstruction. Providing adequate coverage is particularly difficult because of lack of rigid, autologous tissue that can grow and remodel over time [43]. For this reason, a tissue-engineered implant composed of osteocytes derived from AF-MSCs on nanofiber scaffolds has been explored as an alternative method for major chest wall/sternal reconstruction in experimental models with some success [44, 45]. These have also shown evidence of engraftment and bony mineralization over time [45].
The same principle has also been successfully applied in a rabbit model of craniofacial defect repair [46]. There, however, have been no clinical trials till date to demonstrate efficacy in humans.
Abdominal Wall Defects
Gastroschisis is a congenital birth defect characterized by a failure of the abdominal wall to properly form during gestation. This leads to herniation of the abdominal viscera into the amniotic cavity with progressive chemical and mechanical damage to the small intestines while in utero. Similar to the case with MMC, TRASCET may have salutary, anti- inflammatory effects on fetal gastroschisis intestines once the diagnosis is made in the fetus by ultrasound at 16–20-week gestation. TRASCET has been shown to improve intestinal histology at birth in a fetal rodent model of gastroschisis [47].
Urethral Reconstruction
Congenital or acquired abnormalities may lead to urethral defects that merit complex surgical reconstruction. Free skin and vascularized skin flaps have been tried for reconstruction with limited success. Two therapeutic strategies have been developed for urethral reconstruction and urinary bladder regeneration using regenerative medicine-based approaches: based on the acellular matrix bioscaffold model and the cell-seeded bioscaffold model [48,49,50,51]. The acellular matrix bioscaffold model has been successfully used in the clinic, and the cell-seeded bioscaffold model is making its transition from laboratory to bedside providing hope for patients in the future [51].
Aganglionic Gut Disorders
Hirschsprung’s disease is caused by aganglionosis of the terminal bowel, due to incomplete colonization of the terminal embryonic hindgut by vagal neural crest-derived cells (NCC) that migrate caudally from the pharyngeal gut to the rectum during embryonic development [52]. Treatment currently consists of excision of aganglionic terminal bowel and replacement using normal ganglionic bowel. Such procedures often result in the loss of significant lengths of bowel resulting in long-term continence and absorption issues. Alternative treatment options being explored include using stem cell culture to generate ganglion-like cells and transplanting them into aganglionic bowel for definitive cure. Although full-thickness human postnatal gut tissue can be used to generate ENSSCs, reliance on its harvesting from surgical resection poses significant practical limitations [53, 54]. Postnatal human gut mucosal tissue obtained from children undergoing gastrointestinal endoscopy has been used to generate enteric nervous system stem cell (ENSSCs) within neurosphere-like bodies (NLBs). These cells, which express ENSSC markers, are bipotent and are capable of generating large colonies in clonogenic cultures and multiple ENS neuronal subtypes. Upon transplantation into aganglionic bowel, these generate ganglia-like structures, enteric neurons, and glia in recipient chick and human hindgut [55]. This research, if translated into clinical success, would be a significant upgrade over current bowel resection-based management strategies.
Inflammatory Diseases of Prematurity
AF-SC has immunomodulatory potential and has been shown to decrease T cell proliferation and polarize the Th2 subtype to secrete anti-inflammatory cytokines like IL-10 and IL-4 in animal studies [10]. This has led to speculation that stem cell-based treatment regimens could be of use in inflammatory disorders of the gastrointestinal tract in fetuses and neonates. Although not congenital anomalies per se, conditions associated with preterm infants born 4 or more weeks premature often result in profound organ-specific inflammatory and/or infectious conditions shortly after birth. The most common of these include necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and bronchopulmonary dysplasia (BPD).
NEC can result in profound sepsis and ultimately death in up to 40% of infants [56]. Even the survivors often require long-term parenteral nutrition support because of resection of large lengths of affected bowel. Enteric nervous system (ENS) abnormalities last long after recovery from the acute episode of NEC in the survivors. This led investigators to explore novel treatment modalities. In an experimental studies, intraperitoneal injection of AF-MSC instillation found to reduce the NEC severity, gut inflammation, apoptosis, and macroscopic gut damage, with improved intestinal epithelial integrity, barrier effects, absorptive capacity, enterocyte proliferation, and long-term adaptabilitity [15, 57].
In an experimental study, Amniotic fluid-derived neural stem cells (AF-NSC), also known as neurospheres also shown to decrease the severity of disease and long-term functional improvement of bowel in NEC. In animal models, AF-NSC has also shown beneficial effects in other ENS-based pediatric surgical conditions such as Hirschsprung’s disease [15].
The optimum method of stem cell delivery (intravascular delivery, intraperitoneal injections, direct infusion into ischemic tissue, enteral or transrectal) is a matter of research in the future [58]. It has been proposed that direct local application of stem cells to injured bowel could be the best option, as it will have maximum effect of injury repair via paracrine effect and bypass the systematic route (lung entrapment of stem cells) [58, 59].
Severe intraventricular hemorrhage in premature infants and the consequent post-hemorrhagic hydrocephalus causes significant mortality and neurological disabilities in a large number of preterm newborn [60]. In experimental studies on rats, human umbilical cord blood-derived MSC or fibroblasts were injected intraventricularly under stereotactic guidance. Serial brain MRI, behavior function tests, brain tissue and cerebrospinal fluid histological and biochemical analyses showed prevention of post-hemorrhagic hydrocephalus development and significant improvement in behavioral tests as compared to controls. The results were attributed to regenerative potential imparted by the injected stem cells and the plasticity of the developing infant brain [60]. As postnatal management and survival of preterm neonates continue to improve, improved management of prematurity-related sequelae is required to improve the quality of life of these patients with stem cell transplantation offering the best chance for definitive cure in patients with prematurity-related intraventricular hemorrhage.
In hypoxic animal models of BPD, bone marrow-derived MSCs have been shown to be protective against neonatal lung injury [61, 62]. The proposed mechanism involves release of pro-angiogenic factors as well as the activation of endogenous resident progenitor cells.
Childhood Malignancies
In children, hemopoietic SC transplantation (HSCT) is used as a definite treatment in hemoglobinopathies, immune deficiencies, bone marrow failure, metabolic diseases, and hematological malignancies. Only 25% of individuals have an HLA-identical sibling donor for HSCT derived from bone marrow, peripheral blood, or umbilical cord blood. Alternative stem cell sources are matched-unrelated volunteers, unrelated umbilical cord blood, and HLA haplotype-mismatched (“haploidentical”) family members. Many solid tumors exhibit a steep dose-response to alkylating agents, and autologous stem cell (obtained from long bones or iliac creast) transplantation allows escalation of the chemotherapy dosage for treatment of high risk, recurrent, and metastatic solid tumors (neuroblastoma, rhabdomyosarcoma, Ewing’s sarcoma, etc.) [63,64,65].
Large Chronic Wounds
Because of limited surface area, autologous split skin grafting could not be feasible in children. In an experimental study, local application of equine (heterologous) umbilical cord, Wharton’s jelly MSCs were used in a 6-month-old filly with a non-healing skin wound. The results were very promising [66]. Furthermore, living cryopreserved placental membrane and epidermal stem cell were also shown good and promising results in chronic and/or large wound healing [67].
Future Trends
The clinical uses of stem cell therapy could be endless. In utero, transplantation of allogenic mesenchymal cells has been shown to help patients with osteogenesis imperfecta and severe immunological deficiencies. It may also improve outcome in patients with liver cirrhosis due to biliary atresia, choledochal cysts, and sclerosing cholangitis [68,69,70]. MSCs stem cell therapy have proven useful in the management of renal dysplasias (congenital dysplastic kidneys, bilateral multicystic kidney disease, polycystic disease of the kidney, severe hydronephrosis with renal insufficiency) [71]; pancreatic insufficiency due to diabetes (following surgical resection of nesidioblastosis) [72]; Crohn’s disease (resistant to corticosteroids, anti–tumor necrosis factor drugs, and immunosuppressants) [73]; neurogenic bladder, bowel and neurological deficits after surgery for anorectal malformations with sacral agenesis; muscular dystrophy; cerebral palsy; leukemias solid tumors; primary immunodeficiency; brain damage; and cardiac disease, etc. [74, 75].
Limitations
The relative rarity of congenital anomalies prohibits the adequate study for assessing efficacy of stem cells in a single-center-controlled trial [1, 74]. Furthermore, neural stem cells, hematopoietic stem cells, marrow stromal cells, or cells derived from ESCs and induced pluripotent stem (iPS) cells, all have rapid and major cell loss shortly after delivery [75]. Intravenous injection of BM-MSC leads to significant entrapment of SC in the lungs [58]. Another limitation is that insurance agencies typically do not pay for expensive and unproven stem cell-based interventions. The patients seeking stem cell therapy must purchase them out-of-pocket or use alternative funding mechanisms [74, 75]. Stem cell preparation techniques often include use of xenogeneic reagents, which are currently prohibited from human use or require close regulation by clinical safety boards [1, 13, 76,77,78,79,80,81]. Finally, clinical translation of AF-induced pluripotent stem (AF-iPS cell-based technologies) has been hampered by the need to efficiently perform iPS cell reprogramming without the use of permanently integrating viruses for fear of oncogenic mutations frequently associated with these viruses. In particular, use of adeno- and lenti-viral-based reprogramming methods remains highly controversial because of their frequent association with insertional mutagenesis and oncogenesis [78,79,80,81]. It has been suggested that Cell-free treatment with exosomes might target specific tissues without the risk of tumor formation and immunogenicity [82].
To conclude, birth defects represent a major burden in pediatric disease and lead to significant infant morbidity and mortality worldwide. In the effort to improve clinical outcomes in neonates who would otherwise have a grim prognosis, perinatal stem cell-based therapies are being developed rapidly (Table 1). However, multicenter clinical trials with government funding and appropriate follow-up are needed to properly ascertain risks and benefits of these treatments [65].
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AF-iPS:
-
Amniotic fluid-derived induced pluripotent stem
- AF-ESC:
-
Amniotic fluid-derived embryonal pluripotent stem
- AF-MSC:
-
Amniotic fluid-derived mesenchymal stem cell
- AF-NSC:
-
Amniotic fluid derived-neural stem cell
- BPD:
-
Bronchopulmonary dysplasia
- CDH:
-
Congenital diaphragmatic hernia
- ENSSCs:
-
Enteric nervous system stem cells
- hESC:
-
Human embryonic stem cell
- iPS:
-
Induced pluripotent stem
- MMC:
-
Myelomeningocele
- MSC:
-
Mesenchymal stem cells
- NEC:
-
Necrotizing enterocolitis
- NLBs:
-
Neurosphere-like bodies
- U-SC:
-
Umbilical stem cell
- P-SC:
-
Placental stem cell
- TRASCET:
-
Trans-amniotic stem cell therapy
References
Kunisaki SM (2012) Treatment options based on amniotic fluid-derived stem cells. Organogenesis 8:89–95
Cananzi M, Atala A, De Coppi P (2009) Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod BioMed Online 18(Suppl 1):17–27. https://doi.org/10.1016/S1472-6483(10)60111-3
Shaw SW, David AL, De Coppi P (2011) Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol 23:109–116
Gupta DK, Sharma S (2005) Stem cell therapy - Hope and scope in paediatric surgery. J Indian Assoc Pediatr Surg 10:138–141
Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector AJ et al (2008) Stem cell as a potential future of pediatric intestinal disorders. J Pediatr Surg 43:1953–1963. https://doi.org/10.1016/j.jpedsurg.2008.06.019
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G et al (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279(5356):1528–1530
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R et al (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102:1548–1549
Klemmt PA, Vafaizadeh V, Groner B (2011) The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 11:1297–1314. https://doi.org/10.1517/14712598.2011.587800
Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang Q, Jiang L, Guo L, Cheng W (2012) High efficiency of reprogramming CD34(+) cells derived from human amniotic fluid into induced pluripotent stem cells with Oct4. Stem Cells Dev 21:2322–2332. https://doi.org/10.1089/scd.2011.0715
Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, Day D, Eaton JL, George LL, Naber C, Turbe-Doan A, Park PJ, Hornstein MD, Maas RL (2011) Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet 20:962–974. https://doi.org/10.1093/hmg/ddq542
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L et al (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106. https://doi.org/10.1038/nbt1274
Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, Hoopmann M (2011) Amniotic-fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int 2011:715341–715312. https://doi.org/10.4061/2011/715341
Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, Lafferty J, McGinn JT, Brodman M, Fuster V, Hajjar RJ, Polgar K (2010) Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Rep 12:117–125. https://doi.org/10.1089/cell.2009.0077
Turner CG, Pennington EC, Gray FL, Ahmed A, Teng YD, Fauza DO (2013) Intra-amniotic delivery of amniotic-derived neural stem cells in a syngeneic model of spina bifida. Fetal Diagn Ther 34:38–43 [cited 2020 Feb 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23635813
Drucker NA, McCulloh CJ, Li B, Pierro A, Besner GE, Markel TA (2018) Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr Surg 27:57–64
Lee OK, Kuo TK, Chen WM, Der Lee K, Hsieh SL, Chen TH (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103(5):1669–1675 [cited 2020 Feb 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/14576065
Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, Sutton LN, Gupta N, Tulipan NB, D’Alton ME, Farmer DL, MOMS Investigators (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004
Kunisaki SM (2012) Congenital anomalies: treat-ment options based on amniotic fluid-derived stem cells. Organogenesis 8:89–95
Dionigi B, Ahmed A, Brazzo J 3rd et al (2015) Partial or complete coverage of experimental spina bifida by simple intra-amniotic injection of concentrated amniotic mesenchymal stem cells. J Pediatr Surg 50:69–73
Fauza DO, Jennings RW, Teng YD, Snyder EY (2008) Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 144:367–373. https://doi.org/10.1016/j.surg.2008.05.009
Kajiwara K, Tanemoto T, Wada S, Karibe J, Ihara N, Ikemoto Y, Kawasaki T, Oishi Y, Samura O, Okamura K, Takada S, Akutsu H, Sago H, Okamoto A, Umezawa A (2017) Fetal therapy model of myelomeningocele with three-dimensional skin using amniotic fluid cell-derived induced pluripotent stem cells. Stem Cell Rep 8:1701–1713
Kim YH, Ha KY, Kim SIl (2017) Spinal cord injury and related clinical trials. Vol. 9, CiOS Clinics in Orthopedic Surgery. Korean Orthopaedic Association. p. 1–9
Pincott ES, Burch M (2012) Potential for stem cell use in congenital heart disease. Futur Cardiol 8:161–169. https://doi.org/10.2217/fca.12.13
Yeh YC, Lee WY, Yu CL, Hwang SM, Chung MF, Hsu LW, Chang Y, Lin WW, Tsai MS, Wei HJ, Sung HW (2010) Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 31:6444–6453. https://doi.org/10.1016/j.biomaterials.2010.04.069
Bollini S, Cheung KK, Riegler J, Dong X, Smart N, Ghionzoli M, Loukogeorgakis SP, Maghsoudlou P, Dubé KN, Riley PR, Lythgoe MF, de Coppi P (2011) Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev 20:1985–1994. https://doi.org/10.1089/scd.2010.0424
Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C et al (2010) Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139:431–436, 436, e1–2. https://doi.org/10.1016/j.jtcvs.2009.09.057
Weber B, Emmert MY, Behr L, Schoenauer R, Brokopp C, Drögemüller C, Modregger P, Stampanoni M, Vats D, Rudin M, Bürzle W, Farine M, Mazza E, Frauenfelder T, Zannettino AC, Zünd G, Kretschmar O, Falk V, Hoerstrup SP (2012) Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials 33:4031–4043
Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, Zund G, Hoerstrup SP (2007) Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 116(Suppl):I64–I70. https://doi.org/10.1161/CIRCULATIONAHA.106.681494
Dijkman PE, Fioretta ES, Frese L, Pasqualini FS, Hoerstrup SP (2016) Heart valve replacements with regenerative capacity. Vol. 43, Transfusion Medicine and Hemotherapy. S. Karger AG. p. 282–90
Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, Wilson JM, Fauza DO (2004) Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 39:834–838. https://doi.org/10.1016/j.jpedsurg.2004.02.014
Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP et al (2006) Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 41:34–39. https://doi.org/10.1016/j.jpedsurg.2005.10.011
Turner CG, Klein JD, Steigman SA, Armant M, Nicksa GA, Zurakowski D, Ritz J, Fauza DO (2011) Preclinical regulatory validation of an engineered diaphragmatic tendon made with amniotic mesenchymal stem cells. J Pediatr Surg 46:57–61. https://doi.org/10.1016/j.jpedsurg.2010.09.063
Kotecha S (2000) Lung growth: implications for the newborn infant. Arch Dis Child Fetal Neonatal Ed 82:F69–F74
Pederiva F, Ghionzoli M, Pierro A, de Coppi P, Tovar JA (2013) Amniotic fluid stem cells rescue both in vitro and in vivo growth, innervation, and motility in nitrofen-exposed hypoplastic rat lungs through paracrine effects. Cell Transplant 22:1683–1694
Di Bernardo J, Maiden MM, Hershenson MB et al (2014) Amniotic fluid derived mesenchymal stromal cells augment fetal lung growth in a nitrofen explant model. J Pediatr Surg 49:859–864
DeKoninck P, Toelen J, Roubliova X, Carter S, Pozzobon M, Russo FM, Richter J, Vandersloten PJ, Verbeken E, de Coppi P, Deprest J (2015) The use of human amniotic fluid stem cells as an adjunct to promote pulmonary development in a rabbit model for congenital diaphragmatic hernia. Prenat Diagn 35:833–840
Sharma S, Kumar L, Mohanty S, Kumar R, Gupta SD, Gupta DK (2011) Bone marrow mononuclear stem cell infusion improves biochemical parameters and scintigraphy in infants with biliary atresia. Pediatr Surg Int 27:81–89
Sharma S, Mohanty S, Das P, DattaGupta S, Kumar L, Gupta D (2013) Propitious role of bone marrow-derived mononuclear cells in an experimental bile duct ligation model: potential clinical implications in obstructive cholangiopathy. Pediatr Surg Int 29:623–632
Fujikawa T, Oh SH, Shupe T, Petersen BE (2005) Stem-cell therapy for hepatobiliary pancreatic disease. J Hepato-Biliary-Pancreat Surg 12:190–195
Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B et al (2011) Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 378:1997–2004. https://doi.org/10.1016/S0140-6736(11)61715-7
Kunisaki SM, Freedman DA, Fauza DO (2006) Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 41:675–682, discussion 675-82. https://doi.org/10.1016/j.jpedsurg.2005.12.008
Elliott MJ, De Coppi P, Speggiorin S et al (2012) Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380:994–1000
Weiss DJ (2013) Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010–2012. Ann Am Thorac Soc 10:S45–S97. https://doi.org/10.1513/AnnalsATS.201304-090AW
Steigman SA, Ahmed A, Shanti RM, Tuan RS, Valim C, Fauza DO (2009) Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J Pediatr Surg 44:1120–1126. https://doi.org/10.1016/j.jpedsurg.2009.02.038
Klein JD, Turner CG, Ahmed A, Steigman SA, Zurakowski D, Fauza DO (2010) Chest wall repair with engineered fetal bone grafts: an efficacy analysis in an autologous leporine model. J Pediatr Surg 45:1354–1360. https://doi.org/10.1016/j.jpedsurg.2010.02.116
Turner CG, Klein JD, Gray FL, Ahmed A, Zurakowski D, Fauza DO (2012) Craniofacial repair with fetal bone grafts engineered from amniotic mesenchymal stem cells. J Surg Res 178:785–790. https://doi.org/10.1016/j.jss.2012.05.017
Feng C, Graham CD, Connors JP, Brazzo J III, Pan AHS, Hamilton JR, Zurakowski D, Fauza DO (2016) Transamniotic stem cell therapy (TRASCET) miti- gates bowel damage in a model of gastroschisis. J Pediatr Surg 51:56–61
Li CL, Liao WB, Yang SX, Song C, Li YW, Xiong et al (2013) Urethral reconstruction using bone marrow mesenchymal stem cell- and smooth muscle cell-seeded bladder acellular matrix. Transplant Proc 45:3402–3407. https://doi.org/10.1016/j.transproceed.2013.07.055
Liao W, Yang S, Song C, Li X, Li Y, Xiong Y (2013) Construction of ureteral grafts by seeding bone marrow mesenchymal stem cells and smooth muscle cells into bladder acellular matrix. Transplant Proc 45:730–734. https://doi.org/10.1016/j.transproceed.2012.08.023
Iannaccone PM, Galat V, Bury MI, Ma YC, Sharma AK (2018) The utility of stem cells in paediatric urinary bladder regeneration. Pediatr Res 83:258–266. https://doi.org/10.1038/pr.2017.229
Fu Q, Cao YL (2012) Tissue engineering and stem cell application of urethroplasty: from bench to bedside. Urology 79:246–253. https://doi.org/10.1016/j.urology.2011.08.043
Sidebotham EL, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH (2002) Location of stem cells for the enteric nervous system. Pediatr Surg Int 18:581–585
Thapar N (2009) New frontiers in the treatment of Hirschsprung disease. J Pediatr Gastroenterol Nutr 48(Suppl 2):S92–S94. https://doi.org/10.1097/MPG.0b013e3181a15d62
Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N (2009) Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology 136:2214–25.e1–3. https://doi.org/10.1053/j.gastro.2009.02.048
Sommer L (2007) Stem cells of the enteric nervous system: causal therapy for Hirschsprung’s disease. Pathologe 28:125–130
Zani A, Cananzi M, Eaton S, Pierro A, De Coppi P (2009) Stem cells as a potential treatment of necrotizing enterocolitis. J Pediatr Surg 44:659–660. https://doi.org/10.1016/j.jpedsurg.2008.12.012
Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S et al (2014) Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63:300–309
McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE (2017) Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg 52:999–1005
Zhou Y, Yang J, Watkins DJ, Boomer LA, Matthews MA, Yanwei S et al (2013) Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther 4:157. https://doi.org/10.1186/scrt387
Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS (2013) Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 44:497–504. https://doi.org/10.1161/STROKEAHA.112.679092
Möbius MA, Thébaud B (2015) Stem cells and their mediators –next generation therapy for bronchopulmonary dysplasia. Front Med (Lausanne) 2:50. https://doi.org/10.3389/fmed.2015.00050
Möbius MA, Rüdiger M (2016) Mesenchymal stromal cells in the development and therapy of bronchopulmonary dysplasia. Mol Cell Pediatr 3:18. https://doi.org/10.1186/s40348-016-0046-6
Tenneti P, Zahid U, Iftikhar A, Yun S, Sohail A, Warraich Z, et al. (2018) Role of high-dose chemotherapy and autologous hematopoietic cell transplantation for children and young adults with relapsed Ewing’s sarcoma: a systematic review. Vol. 2018, Sarcoma. Hindawi Limited
Perentesis JP, Katsanis E, DeFor TE, Neglia JP, Ramsay NKC (1999) Autologous stem cell transplantation for high-risk pediatric solid tumors. Bone Marrow Transplant 24:609–615
Hale GA (2005) Autologous hematopoietic stem cell transplantation for pediatric solid tumors. Vol. 5, Expert review of anticancer therapy. [cited 2020 Feb 17]. p. 835–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16221053
Lanci A, Merlo B, Mariella J, Castagnetti C, Iacono E (2019) Heterologous Wharton’s jelly derived mesenchymal stem cells application on a large chronic skin wound in a 6-month-old filly. Front Vet Sci. Jan 30 [cited 2020 Feb 16];6(JAN):9. Available from: https://www.frontiersin.org/article/10.3389/fvets.2019.00009/full
Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K (2019) Epidermal stem cells in wound healing and their clinical applications. Vol. 10, Stem Cell Research and Therapy. BioMed Central Ltd. p. 1–14
Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H (2003) Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development 130:2513–2524
Marin JJ, Macias RI, Briz O, Banales JM, Monte MJ (2015) Bile acids in physiology, pathology and pharmacology. Curr Drug Metab 17:4–29
Cervantes-Alvarez E, Wang Y, de l’Hortet AC, Guzman-Lepe J, Zhu J, Takeishi K (2017) Current strategies to generate mature human induced pluripotent stem cells derived cholangiocytes and future applications. Organogenesis 13:1–15
Yokote S, Yamanaka S, Yokoo T (2012) De novo kidney regeneration with stem cells. J Biomed Biotechnol 2012:453519–453510. https://doi.org/10.1155/2012/453519
Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui JI, Knisely AS, Hirabayashi M, Nakauchi H (2010) Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142(5):787–799. https://doi.org/10.1016/j.cell.2010.07.039
Dalal J, Gandy K, Domen J (2012) Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr Res 71:445–451
Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, Lu H (2018) A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transplant 27:325–334. https://doi.org/10.1177/0963689717729379
Carroll J (2012) Human cord blood for the hypoxic-ischemic neonate. Pediatr Res 71:459–463
Sniecinski I, Seghatchian J (2018) Emerging stem cell based strategies for treatment of childhood diseases. Transfus Apher Sci 57:311–315. https://doi.org/10.1016/j.transci.2018.05.011
Snyder J, Turner L, Crooks VA (2018) Crowd funding for unproven stem cell-based interventions. JAMA 319:1935–1936. https://doi.org/10.1001/jama.2018.3057
Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19:1450–1456
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK et al (2007) Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 40:75–90
Heijnen CJ, Witt O, Wulffraat N, Kulozik AE (2012) Stem cells in paediatrics: state of the art and future perspectives. Paediatr Res 71:407–409
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Acknowledgments
I acknowledge to my teacher Prof Shiv Narayan Kureel, Department of Pediatric Surgery, King George Medical University, Lucknow, India for constant encouragement and knowledge.
Author information
Authors and Affiliations
Contributions
All the authors contributed to analytic framing and writing the review.
Author-1 | Author-2 | Author-3 | Author-4 | Author-5 | Author-6 | Author-7 | |
Conceptualization | √ | √ | √ | √ | √ | √ | √ |
Study design | √ | √ | √ | √ | √ | √ | √ |
Project writing and management | √ | √ | – | – | – | √ | √ |
Defining the study | √ | √ | – | √ | √ | √ | √ |
Extensive literature search | √ | √ | – | – | – | – | √ |
Actually performing the study viz. experiments, operative work, practical work | – | – | – | – | – | – | |
Data acquisition | √ | √ | – | – | – | – | √ |
Data analysis | √ | √ | – | √ | √ | – | – |
Statistical inferences, | – | – | – | – | – | – | – |
manuscript writing, and repeated editing | √ | √ | – | – | √ | – | √ |
Reviewing of the manuscript | √ | – | – | – | – | – | √ |
Read and approved the final manuscript | √ | √ | √ | √ | √ | √ | √ |
Corresponding author
Ethics declarations
Conflict of Interest
All the authors declare that they have no conflict of interest regarding funding, authorship and publication issues.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S., Varshney, A., Borkar, N. et al. Clinical Utility of Stem Cells in Congenital Anomalies: New Horizons in Pediatric Surgery. Indian J Surg 82, 1219–1228 (2020). https://doi.org/10.1007/s12262-020-02264-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02264-1