Abstract
Duplication cysts of the intestine are a rare clinical entity, usually diagnosed in infancy or childhood. Cecum is the least common site of gastrointestinal duplication cysts. We are presenting a case of non-communicating cecal duplication cyst in an adult female. The cyst was excised completely laparoscopically without compromising the blood supply to the adjacent cecum. Histopathology confirmed the diagnosis. Non-communicating cecal duplication cysts can be excised without compromising the vascularity but communicating duplication cysts mandate a segmental resection of the part of the bowel involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duplication cysts of the intestine are a rare entity, usually diagnosed in infancy or childhood. The most common sites of duplication cysts are the ileum and the ileocecal junction. Duplication cysts of the caecum in adults are rare with less than 50 cases reported all over the world.
Case
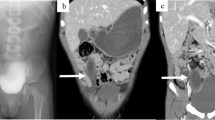
A 26-year-old healthy female presented with diffuse lower abdominal pain and no significant clinical findings. Ultrasound, and later, MRI abdomen, showed a well-defined thin-walled cyst measuring 10 * 10 cm in the right iliac fossa. No obvious connection noted to the right adnexa (Fig. 1). Laparoscopic evaluation revealed a tense thin-walled cystic swelling measuring 10 * 10 cm arising from the lateral wall of the caecum (Fig. 2a). Lateral peritoneum was incised at Line of Toldt and the caecum was mobilized medially. The cyst was tensely distended and hence was decompressed. Clear serous fluid aspirated. The cyst was excised completely after applying a purse-string suture at the base (Fig. 2b). The specimen was retrieved using a railroad technique. Histopathological evaluation revealed that the cyst was lined by colonic epithelium.
Discussion
Duplication cysts of the intestine are rare clinical entities usually presenting in infancy or early childhood [1]. Gastrointestinal duplication cysts are most commonly (75%) seen within the peritoneal cavity, the next most common site being the thorax. The ileal lesions are the most commonly encountered intraperitoneal GI (gastrointestinal) duplication cysts. The large intestine is the least common site of duplication cysts, accounting for 6.8% of all GI duplications [2].
The etiopathogenesis for the development of an intestinal duplication cyst is explained by many theories. One theory suggests it is because of the persistence of the out pouching of the developing intestines between 6 and 8 weeks of intrauterine life. Another theory suggests the failure of separation of a normal attachment between the gut endoderm and the neural tube ectoderm which occurs during the fourth week of intrauterine life [3].
They have a varied presentation with symptoms depending on its location, size, and type of the lining mucosa. The presenting features in children are intestinal obstruction, volvulus, perforation, or an asymptomatic mass. They can present as vague abdominal pain, acute abdomen, or asymptomatic masses in adults. Gastric mucosa is found in about half the cases of GI duplication cysts thereby leading to ulceration which in turn may cause perforation and/or hemorrhage [4].
Malignant transformation in a duplication cyst is a rare occurrence but is most often seen in the large intestine. Inoue and Nakamura in their review describe that although colonic duplications account for only 6.8% of all duplication cysts, the majority (67%) of the malignancies were found to occur in these cysts. The most common being adenocarcinoma, followed by squamous cell carcinoma [5].
The choice of investigation is CECT (With contrast enema) or MRI of the abdomen. Prenatally, they can be diagnosed with USG. Preoperative diagnosis in spite of the advances in the field of radiology is still uncommon.
There are two types of intestinal duplication cysts, i.e., cystic (80%) and tubular (20%) duplication cysts. They are further classified as communicating and non-communicating cysts. Communicating duplication cyst shares a wall with the normal intestine and its blood supply and hence needs resection of the adjacent bowel along with the duplication cyst. Non-communicating duplication cysts have a separate vascular pedicle and have no communication with the intestine; hence, they can be safely resected without the need of segmental bowel resection [6]. Current recommendation for asymptomatic duplication cyst is excision to prevent complications such as bleeding, perforation, obstruction, and malignant transformation [7].
The diagnosis is confirmed by histopathology of the specimen which reveals either the layers of the wall or the mucosa of the intestinal tract.
Differential diagnoses of cystic lesions in the peritoneal cavity include mesothelial cysts, cystadenoma, and intraperitoneal pseudocysts.
Mesothelial cysts or pelvic inclusion cysts are serous fluid filled cysts that are commonly seen arising on the small bowel, mesentery, and the mesocolon and are as a result of the failure of peritoneal surfaces to coalesce. Although it was morphologically similar to the lesion seen in our case histologically, these cysts are lined by mesothelial cells. Cystadenomas can present with an entirely cystic component without septations and internal debris. These are usually benign and are associated with epithelial neoplasms of the ovary and not the GI tract. Non-pancreatic intraperitoneal pseudocysts present with a thick fibrotic wall with inflammatory changes and contain hemorrhagic fluid/pus/serous fluid. In our case, the cyst was thin walled and lined by epithelium [8].
In our case, it was a non-communicating thin walled, tensely distended serous fluid-filled cyst arising from the wall of the caecum lined by colonic mucosal epithelium.
Conclusion
Non-communicating cecal duplication cysts are treated by complete excision of the cyst whereas communicating duplication cysts may need segmental bowel resection.
References
Cavar S, Bogovic M, Luetic T, Antabak A, Batinica S (2006) Intestinal duplications–experience in 6 cases. Eur Surg Res 38(3):329–332
Mourra N, Chafai N, Bessoud B, Reveri V, Werbrouck A, Tiret E (2010) Colorectal duplication in adults: report of seven cases and review of the literature. J Clin Pathol 63(12):1080–1083
Ramakrishna HK (2008) Intestinal duplication. Indian J Surg 70(6):270–273
Ricciardolo AA, Iaquinta T, Tarantini A, Sforza N, Mosca D, Serra F, Cabry F, Gelmini R (2019) A rare case of acute abdomen in the adult: the intestinal duplication cyst. Case report and review of the literature. Ann Med Surg 40:18–21
Inoue Y, Nakamura H (1998) Adenocarcinoma arising in colonic duplication cysts with calcification: CT findings of two cases. Abdom Imaging 23(2):135–137
Nichols KC, Pollema T, Moncure M (2011) Laparoscopically excised completely isolated enteric duplication cyst in adult female: a case report. Surg Laparosc Endosc Percutan Tech 21(4):e173–e175
Holcomb GW 3rd, Gheissari A, O'Neill JA Jr, Shorter NA, Bishop HC (1989) Surgical management of alimentary tract duplications. Ann Surg 209(2):167–174
Arraiza M, Metser U, Vajpeyi R, Khalili K, Hanbidge A, Kennedy E, Ghai S (2015) Primary cystic peritoneal masses and mimickers: spectrum of diseases with pathologic correlation. Abdom Imaging 40(4):875–906
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakshmikantha, N., Lakshman, K. A Rare Case of Cecal Duplication Cyst—a Case Report. Indian J Surg 83, 302–304 (2021). https://doi.org/10.1007/s12262-020-02254-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02254-3