Abstract
Necrotizing pancreatitis is the most dreadful evolution of acute pancreatitis. Intervention is generally required for infected pancreatic necrosis. Traditionally, the most widely used approach has been open pancreatic necrosectomy (OPN), but it is burdened by high morbidity and mortality. Recently, there has been a paradigm shift in the management (step-up approach) evolving towards minimal invasive techniques depending upon availability of resources and expertise. However, OPN still remains the technique of choice in selected cases and in centers where multidisciplinary team is not available. The aim of the study was to analyze the treatment outcome of pancreatic necrosis by step-up approach and OPN. Retrospective analysis of all patients with pancreatic necrosis requiring intervention during 2015–2019 was done. Patient’s demographics, etiology, CT severity scoring, organ failure, and operative complications were analyzed. A total of fifteen patients (out of 80 necrotizing pancreatitis) with suspected or proven infected walled-off necrosis were enrolled. Twelve (80%) were male with mean age of 43.2 years. The most common etiology was alcohol. The mean APACHE II and modified CTSI score was 10.6 and 8.5 respectively. Three (20%) patients had isolated, and eight (53.3%) had multiple organ failure. Two (13.3%) patients were exclusively managed with percutaneous catheter drainage. OPN was performed in the remaining thirteen (86.6%) patients. The overall morbidity and mortality was seen in 46.6% and 26.6% patients respectively. In resource-limited setting, OPN is still safe and a feasible option with acceptable morbidity and mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic necrosis occurs in 20 to 30% of patients following acute pancreatitis. It is an indicator of severe pancreatitis and is associated with organ failure and infections, which per se is a risk for high associated morbidity and mortality [1]. Infection of pancreatic necrosis occurs in approximately 30% of patients, usually following 2 weeks of onset of pancreatic necrosis. Infection in necrosis further complicates the disease and leads to persistent organ failure, sepsis, failure to thrive, and ultimately death if left untreated [2, 3].
Traditionally, the most widely used tool for treatment of infected pancreatic necrosis (IPN) was open pancreatic necrosectomy. This was found to be associated with high morbidity (34–95%) and mortality (11–40%) rates [4, 5]. In recent years, there has been a paradigm shift in the management of IPN with the advent of step-up approach. It is based on the principle of initially percutaneous catheter drainage (PCD) of IPN, followed if necessary by minimally invasive approach, with the open necrosectomy used as a last resort [6]. This step-up approach led to the management of IPN primarily by PCD in 35% of cases. The remaining patients who underwent surgery by a minimally invasive approach had lower morbidity compared with the open approach, with no change in the mortality rates (19%) [6].
There is no doubt that some form of intervention is required in IPN, other than the rare 3% of patients, who can be successfully managed with antibiotics alone [7, 8]. Step-up approach, with PCD, as initial treatment is most widely used, after 2–3 weeks of onset of pancreatitis. However, a multidisciplinary team comprising of an interventional radiologist, gastroenterologist, intensivists, endoscopists, and an experienced pancreatic surgeon is the pre-requisite for the management of this complex pancreatic disease [3, 9, 10]. This team approach with the specialists may not be available at all centers due to the limited resource set-up. Furthermore, the patients in low-income country like ours present in delayed fashion with organ failure, and because of the financial constraints demands a one-time procedure.
With this background, we planned to review our prospective database to see the outcomes of pancreatic necrosis that were managed with percutaneous catheter drainage and open necrosectomy.
Methods
The study is a retrospective analysis of prospectively maintained database of patients diagnosed with pancreatic necrosis over a 4-year period between April 2015 to March 2019. Those patients requiring intervention (step-up approach) in the form of percutaneous catheter placement and open pancreatic necrosectomy for sterile or infected pancreatic necrosis were included in the study. The data included clinical profile of the patients, American Society of Anesthesiologists (ASA) grade, etiology of pancreatitis, APACHE II, BISAP score, and modified CT severity index (CTSI) score. Contrast-enhanced computed tomography (CECT) was used as an imaging modality to see the extent of pancreatic and extrapancreatic necrosis and the presence of infection.
The data also included time to presentation, time to first intervention, time to surgery, presence of organ failure, microbiological profile, and patients managed primarily by PCD. Open pancreatic necrosectomy was done by limited subcostal incision (Fig. 1). PCD catheter tract was used as a guide to enter the pancreatic necrosis which was removed with sponge forceps and cavity copiously lavaged. The pancreatic bed was drained with two 18 Fr Foley’s catheter for postoperative irrigation. Feeding jejunostomy was placed for postoperative nutrition. Cholecystectomy for gallstone-related pancreatitis was performed later on as second-stage surgery.
The primary outcome of the study was postoperative complications and death (30 and 90-days). Postoperative outcomes were measured for any pancreas-specific complications (pancreatic fistula, enteric fistula, bleeding, and wound infections), re-surgery, and postoperative length of hospital stay. Delayed complications in terms of recurrent pancreatitis, exocrine and endocrine deficiency, or incisional hernias were also documented. Factors predicting the mortality were analyzed. The study was approved by the Institute Review Board (IRB-1481/018).
All the data were entered into a Microsoft Excel sheet, and statistical analysis (SPSS version 11.0) was carried out. Results were presented in terms of mean, median, mode, and standard deviation as appropriate. Comparison of outcome variables between two groups (mortality vs. no mortality) was done with chi square (X2) test and Fischer’s exact test as required for qualitative variables. P value of less than 0.05 was considered significant.
Results
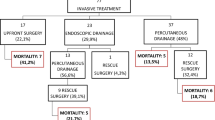
A total of 80 patients were admitted with a diagnosis of pancreatic necrosis during the study period. Among them, sixteen (20%) patients were diagnosed with suspected or proven infected pancreatic necrosis and required intervention. One patient with spontaneous colonic fistula due to severe necrotizing pancreatitis deferred surgery and therefore excluded. Finally, 15 patients were enrolled in the study and further analyzed.
The mean age of the patient was 43.2 years with a predominance of male population (80%). The most common etiology was alcohol (46.6%), followed by gallstone (33.3%), idiopathic (13.3%), and trauma (6.6%). The mean Acute Physiology and Chronic Health Evaluation (APACHE) II score at presentation was 10. The majority (80%) of our patients were in the high ASA grade (III/IV). CECT showed pancreatic parenchymal necrosis in four (26.6%), extrapancreatic necrosis in five (33.3%), and both pancreatic and extrapancreatic necrosis in the remaining six (40%) patients. Gas in pancreatic necrosis was seen in 8 (53.3%) patients. The median modified CTSI score was 8.
At presentation, three (20%) patients had isolated organ failure, whereas eight (53.3%) had multiple organ failure. Renal failure was the most common organ failure seen. The mean time to first presentation and intervention at our center was 44.2 and 57 days respectively.
A total of 10 (66.6%) patients underwent image-guided PCD placement. PCD was not feasible in the remaining four (26.6%) due to the difficult access and peritonitis requiring upfront surgery (n = 1). Two (13.3%) patients were exclusively managed with PCD (number of pigtail, 3 each). Open pancreatic necrosectomy was performed in the remaining eight (53.3%) patients as a part of the step-up protocol. Ultimately, a total of 13 (86.6%) patients underwent open pancreatic necrosectomy. The pancreatic necrosum sent for the culture was positive in 10 (66.6%) cases. The most common organism isolated was Escherichia coli (50%). Fungal culture was positive in 2 (13.3%) cases. The details of demographics and clinical profile are shown in Table 1.
The overall postoperative morbidity was seen in seven (46.6%) patients. Colonic fistula, pancreatic fistula, and surgical site infection was each seen in two (13.3%) patients. One patient developed controlled duodenal fistula. Diversion loop ileostomy was performed in two patients for colonic fistula. Pancreatic fistula was managed conservatively with intraoperatively placed drain which subsequently closed at 3 and 12 months.
Delayed complications, in patients who survived, were seen in seven (63.6%) patients; incisional hernia in 2, endocrine insufficiency in 3, and recurrent pancreatitis in 2 patients (Table 2). None of the patients required intervention for recurrent pancreatitis.
There were four (26.6%) postoperative mortalities. One patient was an old gentleman with diabetes mellitus, who succumbed to death due to pulmonary embolism on the 7th postoperative day. Another was an elderly male with duodenal fistula who developed massive myocardial infarction at 3 weeks post-surgery. The remaining two patients had progressive sepsis with superadded fungal infection, at the 2nd and 4th week. On univariate analysis, early surgery (less than 8 weeks from onset of pancreatitis) was significant predictors of mortality (early vs late, 100% vs 10%, p = 0.014). Moreover, there was trend towards higher mortality with multi-organ failure (50% vs 0%, p = 0.077) group (Table 3). The factors affecting mortality in the study was surgery performed less than 8 weeks of onset of pancreatitis.
Discussion
This study is one of the preliminary reports, evaluating the step-up approach and open pancreatic necrosectomy, from an academic tertiary care referral center (750-bedded) of Nepal, which cater up to 2.0 million populations. In this study, the overall postoperative complications and deaths occurred in 46.6% and 26.6% of patients respectively, which is in line with that of the international standards for morbidity (34 to 95%) and mortality (10.5 to 30%) [1, 9, 11].
The most important and independent factor predicting mortality in infected pancreatic necrosis at time of surgical intervention is the persistent organ failure. This has been addressed in various studies [12,13,14]. Surgical trauma, during open necrosectomy, leads to a surge of cytokines and inflammatory mediators, further propagating the SIRS and multi-organ failure and death. In our cases, persistent organ failure was seen in three out of four patients at the time of death. In the recent study by the Dutch Pancreatitis study group, on the impact of organ failure on mortality, it was found that there is an increased mortality in patients with necrotizing pancreatitis, who develop persistent organ failure late (> 3 weeks) in the course of disease [12]. The organ failure was seen in 38% (240/639) of patients, with mortality being higher in the organ failure group (35% vs. 2%). This was attributed to the need for prolonged hospital and ICU stay, older age of the patients, presence of infection in the necrosis, and the increased requirement of intervention in this group of patients. Similarly, the dynamics of the organ failure in pancreatic necrosis was studied from a tertiary care center of India, by Thandassery et al. [13], where the persistent and the deteriorating organ failure indicated the poor prognosis. The mortality in patients with early-onset transient organ failure was 0%, while the mortality was higher in persistent, and persistent and deteriorating organ failure group patients (12.5% vs. 78.9%). Moreover, the mortality was higher too in the late-onset organ failure group patients (33%).
PCD as the first step in the management of infected pancreatic necrosis leads to complete resolution of the disease, without the need for surgical intervention in 35 to 50% of patients [3, 15]. In the landmark trial by the Dutch group, PCD was the primary treatment modality in 35% of the patients, with the mean time to first intervention being 4 to 6 weeks [6]. In our study, the PCD as the primary treatment was seen in only 13% of patients, with the majority of patients undergoing first intervention at a mean time of 8 weeks. The first intervention was prolonged because of the delayed presentation of our patients. Recently, there is enough evidence on proactive catheter drainage for (suspected) infected pancreatic necrosis, with organ failure [16, 17]. There is no need to wait for the necrosis to become walled-off, at 4 weeks or later to intervene, which was in fact based on the principle of surgical intervention only after 30 days. Early pigtail drainage of peripancreatic collections after 2 weeks (before severe sepsis) with frequent drain revising and upsizing is safe and effective, and leads to lower incidence of organ failure, need for necrosectomy, and in-hospital mortality. In the near future, the results of the ongoing POINTER trial will further enlighten us [18].
The most common early postoperative complications following intervention in pancreatic necrosis are pancreatic fistula, enteric fistula, and hemorrhage [1]. It depends on the type of intervention (percutaneous, endoscopic, minimally invasive, or open necrosectomy). However, the morbidity ranges from 19 to 62% and seems to be lesser for endoscopic and minimally invasive approach than that of the open approach [7, 10]. The rates of pancreatic fistula (41–50%), enteric fistula (10%), and bleeding (3–20%) is higher in the open group, compared with the minimally invasive retroperitoneal drainage (vs. 10%, 5% and 10% respectively) [1, 19]. Endoscopic step-up approach, which nowadays is the preferred option, is associated with similar overall complication compared with surgical step-up approach; however, it has lower rates of pancreatic fistula [20, 21]. In our study, the pancreatic fistula rate was 13%, which matches with the published standard. However, the enteric fistula rate was higher (20%), which may be because of the large size of walled-off necrosis, delayed presentation leading to formation of one of the walls of the necrosis by the bowel. The fistula, hence, became evident postoperatively upon removal of the necrosis, which was probably compressed by the necrosum.
The best part of this study is that it forms the foundation of the management of infected pancreatic necrosis with step-up approach in our part. It can serve as a benchmark and a starting point for further multi-institutional studies from Nepal. Although the morbidity and mortality were slightly higher, it matches with that of the published international standard [6, 22]. In a low- and middle-income country (LMIC) like ours, with limited resource, expertise, infrastructure, geographic barrier, financial constraints, and lack of health insurance to cover expenditures, open necrosectomy as a second step towards management remains a preferred one-time treatment modality. However, there is no debate that the minimally invasive surgical and endoscopic necrosectomy is better than open necrosectomy. In a recent study by van Brunschot et al. [23], open pancreatic necrosectomy still remains the common procedure performed in the world at most centers, other than the few referral hospitals, who frequently performs minimally invasive/endoscopic step-up approach. In their large observational study in 2017 from 51 hospitals across 8 countries and 3 continents, the majority of the patients (n = 1167) underwent open necrosectomy as primary or secondary treatment, versus only 813 who underwent minimally invasive surgical or endoscopic necrosectomy. Similarly, a recent multicenter study from Japan found no significant increase in mortality rates with secondary open necrosectomy for infected pancreatic necrosis compared with the minimally invasive treatment (48.5% vs 29.2%. p = 0.23). Moreover, the high mortality rates seen in open necrosectomy group were because there were more sick patients and severe underlying pancreatitis with extensive retroperitoneal necrosis [24].
The study has several limitations. First, the study was retrospective in nature. Second, the sample size is small; hence, limited power of statistical analysis could be performed. Third, there was limited use of CT-guided PCD, due to the lack of the expertise. However, if available, the percentage of patients, managed primarily by PCD, might have increased, hence decreasing the rates of necrosectomy and mortalities.
Conclusion
The management of infected pancreatic necrosis needs to be tailored for each patient depending on the anatomy, location of collection, and the local expertise of the interventional endoscopists, radiologists, and surgeons. Although endoscopic or minimally invasive step-up approach remains the preferred treatment standard in recent days, open pancreatic necrosectomy, as a step-up approach, still remains safe, in a resource-limited setting, provided an experienced pancreatic surgeon and team is available, with an acceptable morbidity and mortality rates.
References
Martin RF, Hein AR (2013) Operative management of acute pancreatitis. Surg Clin North Am 93:595–610
Vege SS, Baron TH (2005) Management of pancreatic necrosis in severe acute pancreatitis. Clin Gastroenterol Hepatol 3(2):192–196
da Costa DW, Boerma D, van Santvoort HC, Horvath KD, Werner J, Carter CR, Bollen TL, Gooszen HG, Besselink MG, Bakker OJ (2014) Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg 101(1):e65–e79
Velagapudi A, McKay M, Barry T, Bann S, Wickremesekera SK (2016) A low impact approach to infected pancreatic necrosis: review of a case series. Surg Infect 17(6):749–754
Bezmarević M, Van Dijk SM, Voermans RP, Van Santvoort HC, Besselink MG (2019) Management of (peri)pancreatic collections in acute pancreatitis. Visc Med 35(2):91–96
Van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH et al (2010) A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med
Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck C, Fockens P, van Goor H, van Grevenstein W, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven C, Laméris JS, van Leeuwen M, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling G, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort H, Dutch Pancreatitis Study Group (2019) Superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 156(4):1016–1026
Van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM et al (2011) A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 141(4):1254–1263
Babu BI, Sheen AJ, Lee SH, O’Shea S, Eddleston JM, Siriwardena AK (2010) Open pancreatic necrosectomy in the multidisciplinary management of postinflammatory necrosis. Ann Surg 251(5):783–786
Thomson JE, Van Dijk SM, Brand M, Van Santvoort HC, Besselink MG (2018) Managing infected pancreatic necrosis. Chirurgia (Romania) 113:291–299
Werner J, Hartwig W, Hackert T, Büchler MW (2005) Surgery in the treatment of acute pancreatitis - open pancreatic necrosectomy. Scand J Surg 94:130–134
Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort H, Bruno MJ, Dutch Pancreatitis Study Group (2019) Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 68(6):1044–1051
Thandassery RB, Yadav TD, Dutta U, Appasani S, Singh K, Kochhar R (2013) Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. Hpb. 15(7):523–528
Petrov MS, Shanbhag S, Chakraborty M, Phillips ARJ, Windsor JA (2010) Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 139(3):813–820
Van Baal MC, Van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG (2011) Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg 98:18–27
Van Grinsven J, Timmerman P, Van Lienden KP, Haveman JW, Boerma D, Van Eijck CHJ et al (2017) Proactive versus standard percutaneous catheter drainage for infected necrotizing pancreatitis. Pancreas 46(4):518–523
Sugimoto M, Sonntag DP, Flint GS, Boyce CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Bell DA, Barton JG, Traverso LW (2016) Better outcomes if percutaneous drainage is used early and proactively in the course of necrotizing pancreatitis. J Vasc Interv Radiol 27(3):418–425
Van Grinsven J, Van Dijk SM, Dijkgraaf MG, Boermeester MA, Bollen TL, Bruno MJ et al (2019) Postponed or immediate drainage of infected necrotizing pancreatitis (POINTER trial): study protocol for a randomized controlled trial. Trials 20(1)
Rasch S, Phillip V, Reichel S, Rau B, Zapf C, Rosendahl J et al (2016) Open surgical versus minimal invasive necrosectomy of the pancreas - a retrospective multicenter analysis of the German pancreatitis study group. PLoS One 11(9):1–12
van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck C, Erkelens WG, van Goor H, van Grevenstein W, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden K, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P, Dutch Pancreatitis Study Group (2018) Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 391(10115):51–58
van Brunschot S, van Grinsven J, Voermans RP, Bakker OJ, Besselink MGH, Boermeester MA et al (2013) Transluminal endoscopic step-up approach versus minimally invasive surgical step-up approach in patients with infected necrotising pancreatitis (TENSION trial): design and rationale of a randomised controlled multicenter trial [ISRCTN09186711]. BMC Gastroenterol 13(1)
Vasiliadis K, Papavasiliou C, Al Nimer A, Lamprou N, Makridis C (2013) The role of open necrosectomy in the current management of acute necrotizing pancreatitis: a review article. ISRN Surg 2013:1–10
Van Brunschot S, Hollemans RA, Bakker OJ, Besselink MG, Baron TH, Beger HG et al (2018) Minimally invasive and endoscopic versus open necrosectomy for necrotising pancreatitis: a pooled analysis of individual data for 1980 patients. Gut 67(4):697–706
Minami K, Horibe M, Sanui M, Sasaki M, Iwasaki E, Sawano H et al (2019) The effect of an invasive strategy for treating pancreatic necrosis on mortality: a retrospective multicenter cohort study. J Gastrointest Surg. https://doi.org/10.1007/s11605-019-04333-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The study was approved by the Institute Review Committee.
Rights and permissions
About this article
Cite this article
Pandit, N., Yadav, T.N., Awale, L. et al. Open Pancreatic Necrosectomy Is Still Safe and Effective Treatment for Pancreatic Necrosis Managed by Step-Up Approach. Indian J Surg 83 (Suppl 3), 743–748 (2021). https://doi.org/10.1007/s12262-020-02157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02157-3