Abstract
Spontaneous adrenal hemorrhage is a rare clinical entity which can lead to an adrenal crisis, shock, and death. We conducted this study to analyze the etiopathogenesis, clinical presentation, imaging modality, and treatment of patients with spontaneous adrenal hemorrhage. Retrospective analysis of 18 patients with spontaneous adrenal hemorrhage managed between 2005 and 2017 at a single institution was performed. The most common presenting symptom was abdominal pain in 10 (55.6%) patients. Contrast-enhanced computerized tomography (CECT) was the most commonly used diagnostic modality (16/18 [88%]). Bilateral adrenal hemorrhage was seen in 6 patients (33%). Adrenal insufficiency was detected in 7 (38.9%) and these patients received steroid therapy. Etiology of the bleed included idiopathic (5 [27.8%]), antiphospholipid antibody syndrome (APLA) (5 [27.8%]), and pheochromocytoma (4 [22.2%]), associated with pregnancy, anticoagulant use, pseudoaneurysm, and myelolipoma in 1 (5.5%) each. Nine (50%) patients could be managed conservatively, and 8 (44.4%) required adrenalectomy while embolization was performed in one (5.6%). There were no mortalities. In this series, the most common clinical presentation of a patient with spontaneous adrenal hemorrhage was abdominal pain. The detection of an unexplained adrenal hemorrhage should prompt one to look for predisposing conditions like APLA. Patients with adrenal hemorrhage should be evaluated for adrenal insufficiency and if present, steroid therapy needs to be initiated. As seen in this series, not all patients with adrenal hemorrhage require an adrenalectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous adrenal hemorrhage is a rare clinical entity which was previously diagnosed primarily at post-mortem. Reported incidence in various autopsy studies ranges from 0.14 to 1.1% [1]. The advancements in imaging technology and their increased usage have resulted in the increased frequency of diagnosis of adrenal hemorrhage (1.5–5%) [2]. The risk factors for adrenal hemorrhage are based on case reports/series and include trauma, sepsis, and severe stress, following major surgeries, anticoagulant therapy, chronic nonsteroidal anti-inflammatory abuse, antiphospholipid antibody (APLA) syndrome, tumors of the adrenal gland, and pregnancy [3,4,5,6]. The clinical presentation of these patients varies depending upon the rate and degree of hemorrhage and the volume of adrenal cortex affected by the bleed. If left untreated, this may lead to adrenal crisis, shock, and death. Therefore, a high level of suspicion for patients at increased risk for adrenal hemorrhage may lead to early diagnosis and improved survival.
Material and Methods
This was an institution review board, and ethics committee approved the retrospective study of patients with spontaneous adrenal hemorrhage managed either in the departments of endocrinology, endocrine surgery, urology, and obstetrics or in the surgical intensive care unit (ICU) at a single institution between January 2005 and December 2017. Patients managed in general medical wards or in the medical ICU as well as those in whom complete data was unavailable were excluded. Data was collected from the computerized hospital information system and was summarized using mean ± SD/median (min, max) for continuous variables and frequency with percentage for categorical variables. The etiopathogenesis, clinical presentation, imaging modality used, treatment, and outcome were analyzed.
Results
Eighteen patients with spontaneous adrenal hemorrhage were managed during the study period. The mean age of presentation was 39 years (range 27–59 years) with eight males and ten females. The clinical presentation and etiology of the bleed for these patients are depicted in Tables 1 and 2.
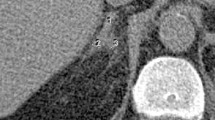
Contrast-enhanced computer tomography (CECT) was the most common imaging modality used for evaluation (16/18 [88%]) (Fig. 1) while in the remaining two patients, magnetic resonance imaging (MRI) was used. The average tumor size on imaging was 6.4 cm (range 3.8–15 cm). Imaging revealed bilateral adrenal hemorrhage in 6 patients (33.3%) (Fig. 2). Among them, four were diagnosed with APLA syndrome, one patient had an associated pregnancy, and one was associated with a pheochromocytoma. Twenty-four-hour urine catecholamines and 8 am cortisol levels were checked in all patients for functional assessment. The 8 am cortisol level was low in 8/18 (44.4%) patients, seven of whom were hyponatremic (S. sodium < 135 meq/L) and two were hyperkalemic (S. potassium > 5 meq/L). Seven (38%) of these patients required steroid replacement in view of adrenal failure. Nine out of 18 (50%) patients were managed with supportive therapy; this included 5 patients with bilateral hemorrhage. Eight patients (44.4%) underwent an adrenalectomy, and seven were unilateral while one patient with a bilateral pheochromocytoma underwent a bilateral operation—this was the only patient with bilateral hemorrhage that was surgically managed. The indications for surgery were pheochromocytoma (4), myelolipoma (1), and an extensive retroperitoneal hemorrhage (3). The surgical approach included transperitoneal laparoscopy in four, retroperitoneoscopic adrenalectomy in one, and transperitoneal open in three. One patient (5.6%) with uncontrolled hypertension and dialysis-dependent chronic kidney disease developed a right adrenal hemorrhage secondary to a bleed from a pseudoaneurysm. This patient was hemodynamically stabilized and underwent embolization of the pseudoaneurysm (Fig. 3). Twelve patients were reviewed following treatment. The mean duration of follow-up among them was 2.3 years (1–9 years). There were no deaths among any of the patients during the management of their acute episodes nor in the 12 patients reviewed.
Discussion
The most common reported presenting symptom of adrenal hemorrhage is abdominal/flank pain [3, 7,8,9]. A similar presentation was witnessed in the majority of patients (55%) in this cohort. Hypertensive crisis defined as systolic blood pressure > 180 mmHg or diastolic blood pressure > 120 mmHg; a rare presentation of adrenal hemorrhage was seen in four patients (22%) [10]. Among these 4 patients, two were due to a pheochromocytoma, one was due APLA, and one was idiopathic. Adrenal hemorrhage leading to hypertensive crisis in the absence of a pheochromocytoma has been reported previously [1, 11]. The proposed etiology being intermittent excessive secretion of catecholamines due to the raised intra-adrenal pressure secondary to the hematoma [11].
The largest series of spontaneous adrenal hemorrhage (141 cases) has been reported from Mayo Clinic [3]. Their series excluded hemorrhage secondary to an adrenal mass. In their cohort, sepsis/stress was the most common cause (39.7%); other causes included idiopathic (31.2%) and heparin-associated thrombocytopenia (HAT)/APLA (14.2%), following major surgery (9.9%) and patients on anticoagulant therapy/trauma (< 5%) [3]. Our patient cohort did not include patients admitted in medical intensive care units/medical wards. This may account for the majority of adrenal hemorrhages in this series being idiopathic (28%) while there were no hemorrhages due to sepsis. APLA accounted for 28% and among these five patients, four had bilateral adrenal hemorrhage. Spontaneous adrenal hemorrhage in a preexisting adrenal mass is rare. A recent review of 133 cases reported the most common cause to be due to a pheochromocytoma (48%). Malignant adrenal lesions (20%), pseudocyst/adenoma (17%), and myelolipoma (10%) were the other causes detected [7]. Five (27.8%) patients in this series had an adrenal hemorrhage secondary to an underlying mass; among them, 4 (80%) were due to a pheochromocytoma while the remaining one (25%) was due to a myelolipoma.
CECT of the abdomen is a good modality to evaluate patients with an adrenal hemorrhage. Adrenal hemorrhage appears as a round to oval lesion with focal heterogenous high density (50–70 Hounsfield units) and a lack of contrast enhancement. Periadrenal fat stranding may also be seen. In addition, adrenal hematomas decrease in size and attenuation over time and hence, CECT is a useful imaging modality to assess the lesion on follow-up [12,13,14,15,16]. Magnetic resonance imaging (MRI) is another modality commonly used for the evaluation of patients suspected of having an adrenal hemorrhage. The appearance of hemorrhage on MRI depends on the age of the bleed with signal intensity changing with the progressive degradation of hemoglobin. Therefore, this modality is useful in differentiating acute from chronic bleeds. In the acute phase (< 2 days), hemorrhage is hypointense on T1-weighted image while in 2–7 days, it becomes hyperintense on T1. Chronic hematoma demonstrates a rim of low T1 and T2 signals due to intracellular hemosiderin with central T2 hyperintensity [17]. CECT was adequate modality in the majority of patients (88%) in this series. In view of pregnancy-related adrenal hemorrhage, MRI was performed in the remaining two patients.
Bilateral adrenal hemorrhage is associated with several predisposing factors such as stress caused by surgery, severe illness, overwhelming sepsis, burns, hemorrhagic diatheses, and thromboembolic disease (including antiphospholipid antibody syndrome) [18]. There were 6 patients with bilateral adrenal hemorrhage in this study. Four were due to APLA and one was associated with pregnancy. Interestingly one other patient had bilateral adrenal hemorrhage secondary to bilateral pheochromocytomas. Presotto et al. reported sixteen cases of adrenal hemorrhage due to APLA; fourteen were bilateral whereas two were unilateral [19]. In addition, Espinosa et al. reported 40 patients with APLA and adrenal hemorrhage [20]. These reports indicate the increased risk of adrenal hemorrhage in patients with APLA. This is due to a hypercoagulable state leading to adrenal vein thrombosis. The unique vascular structure of adrenal glands with a rich arterial supply and a limited venous drainage predisposes this gland for secondary hemorrhagic infarction after venous thrombosis [21]. Hence, bilateral adrenal hemorrhages are commonly seen in these patients. Bilateral adrenal hemorrhage can be potentially life threatening as these patients may develop acute adrenal insufficiency. A mortality rate of 15% has been reported in spite of steroid therapy, and this rate varies according to the severity of the underlying predisposing illness [18]. Therefore, it would be prudent to evaluate for APLA in all patients with bilateral adrenal hemorrhage as well as those with spontaneous adrenal hemorrhage as these patients can avoid surgery. Further, work-up for associated rheumatological disorder can be initiated, and the patient can be started on anticoagulation to prevent further thrombotic episodes. Eight patients (44.4%) in this study developed adrenal insufficiency, seven of whom required steroid supplements.
The management of adrenal hemorrhage depends upon the etiology. Surgery is indicated in patients where the bleed is secondary to an underlying adrenal mass (pheochromocytoma, myelolipoma, adenoma, or malignancy), in whom the hemorrhage is due to trauma, in whom there is a fall in hematocrit or remains unstable despite resuscitation, and in those with extensive retroperitoneal hemorrhage. The surgical approach could be either laparoscopic or open depending upon the nature of the bleed/lesion, hemodynamic stability of the patient, and the experience of the surgeon. Eight patients in this series needed surgery; five were performed laparoscopically while three were open. All other causes of adrenal hemorrhage may be managed conservatively with supportive therapy and specific treatment for the underlying cause. Steroid replacement must be initiated in patients with bilateral adrenal hemorrhage and in those detected to have adrenal failure. Embolization is an alternative strategy reported in literature to control the hemorrhage. This modality has been described in patients with traumatic adrenal hemorrhage with a few cases reporting its use in those with an underlying tumor [22,23,24,25,26]. In our series, embolization was performed successfully in one patient with adrenal hemorrhage secondary to a pseudoaneurysm who presented in shock.
Conclusion
The most common presenting symptom of adrenal hemorrhage is abdominal pain. A high index of suspicion of adrenal hemorrhage should be raised in the chronically ill, septic, postoperative patients and those on anticoagulant therapy presenting with severe acute abdominal pain. The detection of an unexplained adrenal hemorrhage should prompt one to look for predisposing conditions like APLA. Patients with adrenal hemorrhage should be evaluated for adrenal insufficiency and if present, supplementary steroid therapy needs to be initiated. Abdominal CECT is a good imaging modality for evaluation of this condition. As seen in our cohort, not all patients with adrenal hemorrhage require an adrenalectomy and with multi-departmental coordination, mortality can be prevented.
References
Charalampakis V, Stamatiou D, de Bree E et al (2018, 2018) Spontaneous adrenal hemorrhage. Report of two cases and review of pathogenesis, diagnosis and management. J Surg Case Rep. https://doi.org/10.1093/jscr/rjy129
Simon DR, Palese MA (2009) Clinical update on the management of adrenal hemorrhage. Curr Urol Rep 10:78–83. https://doi.org/10.1007/s11934-009-0014-y
Vella A, Nippoldt TB, Morris JC (2001) Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 76:161–168. https://doi.org/10.4065/76.2.161
Pushkar P, Agarwal A (2015) Spontaneous massive adrenal hemorrhage: a management dilemma. J Endourol Case Rep 1:52–53. https://doi.org/10.1089/cren.2015.0003
Gowda D, Shenoy V, Malabu U, Cameron D, Sangla K (2014) Bilateral adrenal gland haemorrhage: an unusual cause. Endocrinol Diabetes Amp Metab Case Rep 2014. https://doi.org/10.1530/EDM-14-0058
Harper JR, Ginn WM, Taylor WJ (1962) Bilateral adrenal hemorrhage—a complication of anticoagulant therapy: case report and review of the literature. Am J Med 32:984–988. https://doi.org/10.1016/0002-9343(62)90045-1
Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R (2012) Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg 36:75–82. https://doi.org/10.1007/s00268-011-1338-6
Clark OH, Hall AD, Schambelan M (1974) Clinical manifestations of adrenal hemorrhage. Am J Surg 128:219–224. https://doi.org/10.1016/0002-9610(74)90096-8
Souiki T, Tekni Z, Laachach H, Bennani A, Zrihni Y, Tadmori A, Ajdi F, Bouazzaoui A, Chbani L, Akoudad H, Mazaz K, Aitlaalim S (2014) Catastrophic hemorrhage of adrenal pheochromocytoma following thrombolysis for acute myocardial infarction: case report and literature review. World J Emerg Surg 9:50. https://doi.org/10.1186/1749-7922-9-50
Rodriguez MA, Kumar SK, De Caro M (2010) Hypertensive crisis. Cardiol Rev 18:102–107. https://doi.org/10.1097/CRD.0b013e3181c307b7
Schmidt J, Mohr VD, Metzger P, Zirngibl H (1999) Posttraumatic hypertension secondary to adrenal hemorrhage mimicking pheochromocytoma: case report. J Trauma 46:973–975
Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P (1995) Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol 154:1647–1651. https://doi.org/10.1016/S0022-5347(01)66738-7
Kawashima A, Sandler CM, Ernst RD, Takahashi N, Roubidoux MA, Goldman SM, Fishman EK, Dunnick NR (1999) Imaging of nontraumatic hemorrhage of the adrenal gland. RadioGraphics 19:949–963. https://doi.org/10.1148/radiographics.19.4.g99jl13949
Hiroi N, Yanagisawa R, Yoshida-Hiroi M, Endo T, Kawase T, Tsuchida Y, Toyama K, Shibuya K, Nakata K, Yoshino G (2006) Retroperitoneal hemorrhage due to bilateral adrenal metastases from lung adenocarcinoma. J Endocrinol Investig 29:551–554. https://doi.org/10.1007/BF03344146
Goldman HB, Howard RC, Patterson AL (1996) Spontaneous retroperitoneal hemorrhage from a giant adrenal myelolipoma. J Urol 155:639
Tan GXV, Sutherland T (2016) Adrenal congestion preceding adrenal hemorrhage on CT imaging: a case series. Abdom Radiol N Y 41:303–310. https://doi.org/10.1007/s00261-015-0575-9
Hammond NA, Lostumbo A, Adam SZ, Remer EM, Nikolaidis P, Yaghmai V, Berggruen SM, Miller FH (2015) Imaging of adrenal and renal hemorrhage. Abdom Imaging 40:2747–2760. https://doi.org/10.1007/s00261-015-0453-5
Di Serafino M, Severino R, Coppola V et al (2017) Nontraumatic adrenal hemorrhage: the adrenal stress. Radiol Case Rep 12:483–487. https://doi.org/10.1016/j.radcr.2017.03.020
Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M (2005) Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 153:507–514. https://doi.org/10.1530/eje.1.02002
Espinosa G, Santos E, Cervera R, Piette JC, de la Red G, Gil V, Font J, Couch R, Ingelmo M, Asherson RA (2003) Adrenal involvement in the antiphospholipid syndrome: clinical and immunologic characteristics of 86 patients. Medicine (Baltimore) 82:106–118
Khare S, Patel H, Sutaria G, Sharma R (2017) Primary antiphospholipid antibody syndrome presenting as unilateral adrenal hemorrhage. Indian J Endocrinol Metab 21:932. https://doi.org/10.4103/ijem.IJEM_474_17
Igwilo OC, Sulkowski RJ, Shah MR, Messink WF, Kinnas NC (1999) Embolization of traumatic adrenal hemorrhage. J Trauma 47:1153–1155
Kim DG, Jung HS (2016) Endovascular treatment of a post-traumatic adrenal hemorrhage in a pediatric patient: a case report. J Korean Soc Radiol 75:508–511. https://doi.org/10.3348/jksr.2016.75.6.508
Patel A, Downing R, Vijay S (2013) Spontaneous rupture of the adrenal artery successfully treated using the endovascular approach: a report of 2 cases. Vasc Endovasc Surg 47:124–127. https://doi.org/10.1177/1538574412469284
O’Dell MC, Liu B, Fursevich D, Contreras FJ (2015) Embolization of spontaneous hemorrhagic adrenal myelolipoma with variant adrenal arteries. J Vasc Interv Radiol 26:1086–1088. https://doi.org/10.1016/j.jvir.2015.03.003
Habib M, Tarazi I, Batta M (2010) Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol 17:65–70
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, V., Cherian, A.J., Thomas, S.S. et al. Spontaneous Adrenal Hemorrhage—a Mixed Bag: 18 Cases from a Single Institution. Indian J Surg 82, 382–386 (2020). https://doi.org/10.1007/s12262-019-01969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-019-01969-2