Abstract
Atresiaplasty is still a challenge for otolaryngologist, although the operation technique has been modified several times over the past decades. This study describes a reliable flap technique for combining atresiaplasty with total auricular reconstruction by performing the total auricular reconstruction with the MEDPOR framework at the same time as the atresiaplasty for patients with microtia and congenital aural atresia. We performed a prospective study of the medical records of 18 consecutive patients with congenital aural atresia. All the patients had a Jahrsdoerfer grade of 6 or higher, and the mean age was 9.7 years. Atresiaplasty with the new flap technique was performed in 19 ears in our department from January 1, 2011, through July 31, 2013. Among the postoperative ears, 17 ears (89.5 %) had the excellent outcomes, without infections and stenosis. Only two ears (10.5 %) had postoperative stenosis and atresia after infection, respectively. There were no other complications. We concluded that the new flap technique combining atresiaplasty and total auricular reconstruction yielded satisfactory canal patency, low infection rates, and favorable cosmetic results, with acceptable rates of complications. This novel flap technique is a valuable option in the armamentarium of microtia and atresia surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtia or pinna deformities with congenital aural atresia (CAA) are severe conditions that significantly affect the quality of life. The purpose of correcting CAA is to gain functional hearing and to maintain the patency of the reconstructed auditory canal without complications. Accordingly, several surgical methods for creating an external auditory canal (EAC) including skin grafts, vascularized temporoparietal fascia with skin grafts, and pedicled/local flaps have been proposed [1–3]. Among these, a split-thickness skin graft laid onto deep temporal fascia (DTF) or the vascularized temporoparietal fascia (TPF) flap is often used for the reconstruction of the EAC [1, 3].
The most important surgical goals for CAA are rehabilitation of hearing via restoring the normal sound-conducting mechanism of the ear and creating a clean, patent, well-epithelialized EAC, and closure of the air bone gap. However, a high frequency of postoperative EAC stenosis and unsatisfactory hearing results remain a significant challenge. As reported, approximately one third of patients need a revision procedure due to the postoperative meatal stenosis and another one third suffer from being unable to gain proper hearing [4, 5]. A recent study has revealed that EAC stenosis or chronic drainage due to stenosis or unhealthy canal skin accounts for 77 % of the main indications for revision surgery in patients with CAA [6]. Consequently, prevention of postoperative EAC stenosis is imperative to reduce the rate of revision surgery. Most complications from conventional surgical correction stem from the use of skin grafts. Skin contracture, necrosis, and the development of atheromas occur as a result of skin grafting in the EAC. We assumed that these problems could be resolved by using vascularized skin. The conventional skin flap is generally much thicker than skin graft because it contains adipose layers, which thus renders the insertion of a flap into the EAC nearly impossible. Here, we report a new surgical procedure for the reconstruction of congenital aural atresia using the pedicled postauricular skin (PPS) flap, pedicled superficial temporal fascia (PSTF) flap, and pedicled periosteal (PP) flap. The flaps are robust, with a dependable blood supply mainly based on the branches of the superficial temporal artery. We discuss the new flap technique and the advantages of it. We believe that this new surgical procedure, which has not been described before, has the potential to replace other flaps in the reconstruction of congenital aural atresia.
Patients and Methods
A prospective study was performed of all patients undergoing the surgery combining atresiaplasty with total auricular reconstruction by performing the total auricular reconstruction with the MEDPOR framework at the same time from January 1, 2011, to July 31, 2013. The age of the initial surgery ranged from 5.7 to 18.5 years, with an average age of 9.7 years. There were 16 male patients and 2 female patients. The follow-up periods ranged from 12 to 42 months, with a mean period of 26.6 months. All the surgeries were performed at the Department of Otorhinolaryngology, Shanghai Ninth People’s Hospital Affiliated Shanghai Jiao Tong University School of Medicine, Shanghai, China. The informed consent was obtained from all participants. All 18 patients who were included in this series had complete congenital aural atresia and a Jahrsdoerfer [7] grade of 6 or more. Table 1 shows the age at atresiaplasty surgery, sex, laterality of aural atresia, Jahrsdoerfer atresia grading, follow-up periods, and complications for the 18 atresiaplasty patients (19 ears). Patient number 2 had bilateral atresiaplasties. All other patients had unilateral atresiaplasties.
Surgical Techniques
The surgical procedure is divided into three stages: (1) separation and preparation of the thin pedicled postauricular skin flap and pedicled superficial temporal fascia flap; (2) preparation of the pedicled periosteal flap, canaloplasty, and tympanoplasty; and (3) coverage of the bony external auditory meatus with the previously created flaps.
First Stage
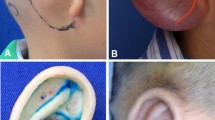
The first stage involves lining out the location of the reconstructing auricle, including the bottom of the lobule, vertex of helix, and horizontal length between the front end of the auricle and paropia (Fig. 1a). According to the preoperative design, a thin and anteriorly based PPS flap (2.5 × 6.0 cm) was elevated, leaving the periosteum on the mastoid cortex (Fig. 1b). After confirming the scope of the superficial temporal fascia flap, a superiorly based at the auricle PSTF flap (12.0 × 12.0 cm) was elevated, which deeps reaching fascia temporalis profunda and superficial pericranium containing the superficial temporal vessels (Fig. 1e). The temporalis muscle fascia graft which was harvested from the temporal parietal will be used as the tympanic membrane fascia graft.
The operation process. a Preoperative photograph; the real line indicates flap incision, and the dotted line represents the trend of superficial temporal artery. b Exposure of superficial temporal fascia. PPS, anteriorly based pedicled postauricular skin flap. c View of malleus/incus complex following completion of drilling a new external auditory. PP, anteriorly based pedicled periosteal flap. d Postoperative view of canaloplasty and tympanoplasty. The temporalis muscle fascia flap was placed directly over the malleus/incus complex. e MEDPOR framework implanting. PSTF, superiorly based at the auricle pedicled superficial temporal facial flap. f View of the newly formed EAC with the pedicled postauricular skin (PPS) flap folded into a sac, which was inserted into it
Second Stage
Using the temporal line and temporomandibular joint as surface landmarks, the ideal position of the external acoustic pore is posterosuperiorly at the temporomandibular joint, inferiorly at the temporal line, posteriorly at the roots of zygoma, or at the cribriform area. An anteriorly based PP flap (1.5 × 2.5 cm) was carefully prepared at the canal opening (Fig. 1c). Drilling was initiated to create a new auditory canal to the anterior epitympanic space; the atretic plate was removed with a diamond burr with great caution not to injure the inner ear structure and the facial nerve until the ossicular mass was identified. The pneumatic mastoid air cells were as little disturbed as possible during drilling. The anterior surface of the bony canal was removed completely with care not to injure the temporomandibular joint. The canal was constructed with an inner hatch diameter of 1.0–1.5 cm and outer hatch diameter of 1.5–2.0 cm, large enough to allow for later narrowing from scar formation. Subsequently, the entire ossicular mass and the middle ear space were exposed. After assessing the ossicular chain mobility and the tympanic cavity status, tympanoplasty was performed with the use of various techniques according to the status of the ossicular mass and the middle ear. The temporalis muscle fascia graft was placed directly over the malleus/incus complex (Fig. 1d). Exposed tympanic antrum and air cells were covered with the edge of the graft.
Third Stage
The anteriorly based PP flap was inverted into the newly made external auditory canal to cover the anterior portion of it. After inverting the PSTF flap to envelop the entire MEDPOR framework, the remaining PSTF flap was used to cover the superior, inferior, and posterior portion of the newly formed canal, and the end edge was overlapped on the surface of the temporalis muscle fascia graft. Then, a vacuum-assisted drain was placed under the framework. Finally, the anteriorly based PPS flap was folded into a sac, which was inserted into the newly formed EAC (Fig. 1f). By this procedure, it is possible to avoid the denuded portion of the newly formed EAC in most cases. If there was any denuded portion, a split-thickness skin graft was used to cover the bare area. After skin closure, the canal was packed with antibiotic-soaked gauze.
Results
Postoperative surgical outcomes, including EAC patency, texture, and color match, were evaluated at the regular follow-up visits. Postoperative EAC stenosis was defined as follows: (1) minimum meatal diameter of less than 3 mm in all dimensions [6] or (2) narrow meatus with persistent discharge. A narrow canal larger than 3 mm in any dimension and favorable epithelialized EAC skin was not considered postoperative EAC stenosis. All the patients had been followed up for 12 to 42 months, and every ear had been medically cared with antibiotic and dexamethasone-soaked gauze in the EAC about 1–3 months. Descriptive findings in patients with postoperative EAC stenosis or atresia are summarized in Table 1. Postoperative EAC stenosis or atresia only occurred in two ears (10.5 %). Patient number 6 with postoperative EAC stenosis has physical scars. As medical care irregularly, atresia occurred in the ear after infection in patient number 9. Most of the ears (89.5 %) have favorable outcomes, and the reconstructed EAC showed excellent patency, obvious hearing improvements, and good color match and texture (Fig. 2). All the patients were satisfied with their reconstructed ear and external auditory canal.
Discussion
The surgery for congenital aural atresia remains one of the most challenging procedures in otology, mainly due to the difficulty of the technique and the relatively high postoperative complication rates. Revision surgery is needed in about one third of cases. Postoperative EAC stenosis or atresia is the most common complication following canaloplasty, with a reported rate of 14 to 31 %, and is also the most common indication for revision surgery [5, 6, 8–10]. According to the report of Oliver [6], chronic drainage is also the common indication (about 19 %) for revision surgery after atresiaplasty. With severe canal stenosis or chronic otorrhea, the EAC cannot be cleaned and/or squamous debris is trapped in the canal, causing infection and discharge. Accordingly, it is essential in canaloplasty to reduce the rate of postoperative EAC stenosis, chronic drainage, and need for revision surgery.
At the early time, De la Cruz et al. showed that there was a significant decrease in postoperative canal stenosis when a split-thickness skin graft was used as opposed to a full-thickness skin graft [11]. However, the bone of the EAC is dense cortical bone, which is certainly not an ideal surface for a skin graft, and it can be anticipated that the graft-take will be unideal in many instances. When skin grafting for canal stenosis, especially when accompanied by a bony stenosis, the successful rate of the grafted skin is sometimes unsatisfactory because of poor blood supply from the exposed bone. Although the created canal initially appears adequate with a split-thickness or full-thickness skin graft, cicatricial shrinking occurs during the postoperative period, which greatly reduces the cavity size [12]. Numerous techniques have been described for the reconstruction of congenital aural atresia. However, the number of the CAA cases is still small and there is a lack of long-term follow-up. Most of them did not report simultaneous reconstruction of microtia [2, 12, 13].
With our new technique for combining atresiaplasty with total auricular reconstruction at the same time, the patients with microtia and congenital aural atresia can avoid the above complications as far as possible. The PSTF flap is a superiorly based flap, supplied by superficial temporal vessels. The PP flap is a subcutaneous pedicled flap, mainly supplied by the branches or perforators of the arteria meningea media. Two large reliable flaps can fully cover the entire surface of the newly created canal, thus can avoid the exposure of the air cells and tympanic antrum and the problems related to skin graft. As the size of the two flaps can be adjusted, we can enlarge the canal amply to avoid the postoperative stenosis. The PPS flap, which is supplied by the preauricular artery, can be easily folded like a skin graft to cover the surface of the PSTF and PP flaps. With the dermal and subdermal plexus of the skin flap and enough blood supply, it causes less contracture and prevents the postoperative stenosis of the EAC that is frequently observed when a split-thickness skin graft is used.
The advantages of the new flap technique are as follows: (1) the three flaps are easy to design and easy to harvest in the operative field according to the anatomic variation of the patients; (2) the size of the PSTF flap and PP flap can be adjusted according to the canal to cover the exposed mastoid air cells and tympanic antrum, thus possibly avoiding postoperative stenosis and chronic drainage as far as possible; (3) the fascia flap and periosteal flap are relatively less deformed than the skin or other soft tissues, therefore reducing the possibility of canal stenosis; (4) the periosteum may induce new bone formation to its undersurface and some site-specific regulation, timely contouring the newly formed canal and inhibiting bony overgrowth [14, 15]; (5) the PPS flap with saccate form can maintain the exact shape of the new canal with less inflammation and less flap displacement in the period of resurfacing; (6) these flaps are supplied by vessels through their pedicles, thus enhancing their attachment; (7) these flaps can cover all the bare bony surfaces; and (8) the PPS flap derives from neighboring skin, thus avoiding the color and texture mismatch that resulted from the previous atresiaplasty using skin graft.
The use of PPS flap, PSTF flap, and PP flap, which we now call “three flaps technique,” is a useful method for atresiaplasty simultaneous with the total auricular reconstruction with the MEDPOR framework and can produce a more natural appearance by providing full skin coverage of bony surface of the reconstructed external auditory canal.
References
Manolopoulos L, Papacharalampous GX, Yiotakis I, Protopappas D, Vlastarakos PV, Nikolopoulos TP (2010) Congenital aural atresia reconstruction: a surgical procedure with a long history. J Plast Reconstr Aesthet Surg 63:774–781
Chang SO, Jeon SJ, Jeong HS, Kim CS (2002) Prevention of postoperative meatal stenosis with anteriorly and inferiorly based periosteal flaps in congenital aural atresia surgery. Otol Neurotol 23:25–28
Haginomori S, Nonaka R, Takenaka H, Ueda K (2008) Canal wall-down tympanoplasty with soft-wall reconstruction using the pedicled temporoparietal fascial flap: technique and preliminary results. Ann Otol Rhinol Laryngol 117:719–726
Schuknecht HF (1989) Congenital aural atresia. Laryngoscope 99:908–917
Lambert PR (1998) Congenital aural atresia: stability of surgical results. Laryngoscope 108:1801–1805
Oliver ER, Hughley BB, Shonka DC, Kesser BW (2011) Revision aural atresia surgery: indications and outcomes. Otol Neurotol 32:252–258
Jahrsdoerfer RA, Yeakley JW, Aguilar EA, Cole RR, Gray C (1992) Grading system for the selection of patients with congenital aural atresia. Am J Otol 13:6–12
Chang SO, Lee JH, Choi BY, Song JJ (2007) Long term results of postoperative canal stenosis in congenital aural atresia surgery. Acta Otolaryngol Suppl 558:15–21
De la Cruz A, Teufert KB (2003) Congenital aural atresia surgery: long-term results. Otolaryngol Head Neck Surg 129:121–127
Nishizaki K, Masuda Y, Karita K (1999) Surgical management and its post-operative complications in congenital aural atresia. Acta Otolaryngol Suppl 540:42–44
De la Cruz A, Linthicum FH Jr, Luxford WM (1985) Congenital atresia of the external auditory canal. Laryngoscope 95:421–427
Yotsuyanagi T, Urushidate S, Nihei Y, Sawada Y (1998) Reconstruction of congenital stenosis of external auditory canal with a postauricular chondrocutaneous flap. Plast Reconstr Surg 102:2320–2324
Narushima M, Yamasoba T, Iida T, Sakamoto T, Kashio A, Karino S, Yamamoto T, Kikuchi K, Mihara M, Koshima I (2013) Supermicrosurgical reconstruction for congenital aural atresia using a pure skin perforator flap: concept and long-term results. Plast Reconstr Surg 131:1359–1366
McCulloch CA, Tenenbaum HC, Fair CA, Birek C (1989) Site-specific regulation of osteogenesis: maintenance of discrete levels of phenotypic expression in vitro. Anat Rec 223:27–34
Imai S, Kaksonen M, Raulo E, Kinnunen T, Fages C, Meng X, Lakso M, Rauvala H (1998) Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM). J Cell Biol 143:1113–1128
Acknowledgments
We thank all the patients and their families for taking part in our new flap technique study. Dr. Shi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
None.
Authors’ Contributions
All authors contributed to the study concept and design and to the critical revision of the manuscript for important intellectual content and have provided administrative, technical, or material support. Acquisition, analysis, or interpretation of data was done by Shi, Chen, and Jiang. Shi and Chen were responsible for the drafting of the manuscript. Study supervision was done by Shi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Jiang, C., Wu, Q. et al. A New Flap Technique for Reconstruction of Microtia and Congenital Aural Atresia. Indian J Surg 77 (Suppl 3), 1237–1241 (2015). https://doi.org/10.1007/s12262-015-1263-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-015-1263-2