Abstract
The liver is the major site of metastasis of primary colorectal cancer. Hepatic resection (HR) is considered the standard treatment for colorectal liver metastasis. In high-risk cases, radiofrequency ablation (RFA) can be attempted as an alternative treatment. This study compared the clinical profiles and overall and disease-free survival rates of patients with colorectal liver metastasis undergoing HR and RFA. From 1995 to 2009, we retrospectively analyzed clinical experiences of 43 and 17 patients who had undergone HR and RFA for primary colorectal cancer, respectively. To compare outcomes, we investigated the 3-year overall and disease-free survival rates. The 3-year overall survival rates of patients treated with HR and RFA were 53.5 and 47.1 %, respectively (p = 0.285); the disease-free survival rates were 35.0 and 26.9 %, respectively (p = 0.211). In the HR and RFA groups, 30 (60.2 %) and 13 (76.5 %) patients developed recurrence, respectively (p = 0.604). In the HR group, 1 patient died from postoperative liver failure, and 9 (20.9 %) developed postoperative complications, including wound infection, biliary leakage, intra-abdominal abscess, and pneumonia. In the RFA group, 1 patient (5.9 %) required prolonged inpatient care because of a procedure-related liver abscess. Although HR should be considered the first option for colorectal liver metastasis, RFA can be regarded as a primary treatment modality depending on the patient’s characteristics, especially when a patient refuses surgery or has comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cancer statistics from the Ministry for Health, Welfare, and Family Affairs of South Korea indicate that the incidence rate of colorectal cancer has increased over recent years. Colorectal cancer was the fourth most frequently occurring cancer until 2004 but has been the second most frequently occurring cancer since 2005. In 2007, colorectal cancer was the third and fourth most common cause of death among women and men in Korea, respectively [1]. The liver is the most common site of metastasis in patients with colorectal cancer, and liver metastasis is the predominant cause of death in such patients [2, 3]. Untreated colorectal liver metastasis has a very poor prognosis; the average survival time is 6–12 months, and the 5-year survival rate is as low as 10 % [4]. However, the 5-year survival rate with hepatic resection (HR) is reported to range from 23 to 58 %. Therefore, HR has been recognized as the standard treatment for liver metastasis [5–7]. Nevertheless, some clinical conditions such as unfavorable tumor location, insufficient hepatic reserve, disease extent, and medical comorbidities make HR difficult. Alternative methods such as transcatheter arterial chemoembolization and radiofrequency ablation (RFA) can be performed in patients who are not indicated for hepatectomy [8–10]. In particular, many researchers report RFA to be less invasive as well as more safer than HR; the advantage of RFA lies in the fact that it can be performed repeatedly for recurrence or new metastases [11–13]. However, the therapeutic outcomes of RFA are reported to be inferior to those of HR [7, 14], and studies published hitherto report the use of RFA primarily for surgically unresectable cases. Several recent studies reported the practice of performing RFA for resectable colorectal liver metastasis [15]. However, there is a lack of reliable data on the results of RFA in patients with resectable colorectal liver metastases. Thus, the effectiveness of RFA should not be considered equivalent to that of HR. Accordingly, this study compared the survival and clinical features of patients with resectable lesions of colorectal liver metastasis treated with HR and RFA.
Methods
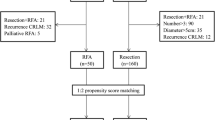
A total of 701 patients were diagnosed with colorectal liver metastasis between January 1995 and December 2009 at our hospital. The presence of liver metastasis was confirmed by preoperative abdominal computed tomography (CT), intraoperative diagnosis, and postoperative follow-up CT. Of the 100 patients who were selected after excluding cases of unresected primary tumors, untreated liver metastasis due to distant metastasis to another organ, multiple metastases, and incomplete follow-up, 60 patients were finally selected after the secondary exclusion of patients who had received single-agent chemotherapy or transcatheter arterial chemoembolization (Fig. 1). The medical records of the 60 patients were analyzed retrospectively. Percutaneous RFA was performed in cases involving comorbidities, when patients refused surgery, or in cases of inadequate liver remnant. Patients were divided into the HR (n = 43) and RFA (n = 17) groups (Fig. 1). The following clinicopathological characteristics were compared between groups at baseline: sex, age, chemotherapy, location and size of the primary tumor, detection time of metastasis, number and maximal size of liver metastatic sites, and preoperative carcinoembryonic antigen levels. The disease-free and overall survival rates were compared between groups to assess treatment outcomes. Among the 60 patients, 45 received chemotherapy. Chemotherapy included a variety of regimens, including 5-fluorouracil (5-FU), FOLFIRI (5-FU, folinic acid, and irinotecan), FOLFOX (5-FU, folinic acid, and oxaliplatin), capecitabine (Xeloda®; Hoffman-La Roche Canada, Mississauga, ON), Xeloda® plus irinotecan, and Xeloda® plus oxaliplatin. The follow-up imaging studies included ultrasonography, CT, or MRI performed every 3 months after surgery for 2 years and every 6 months thereafter. The first follow-up imaging study was performed 1 month after RFA, and further follow-up studies were performed every 3 months for 2 years and every 6 months thereafter.
Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA). Intergroup comparisons were made using the Mann–Whitney U test for continuous variables and the χ 2 test for categorical variables. Kaplan–Meier analysis was performed to determine survival durations, and the log-rank test was used to compare survival rates. The level of significance was set at p < 0.05.
Results
The mean baseline values of the 60 subjects (40 men and 20 women) were as follows: age, 58 years (range, 21–82 years); primary tumor size, 5.6 cm (range, 3.0–9.5 cm); metastatic site size, 2.9 cm (range, 1.0–8.0 cm); and preoperative carcinoembryonic antigen level, 42.85 ng/mL (range, 0.70–479.0 ng/mL). During the observation period, 37 patients died (61.7 %), including 29 from tumor progression. Of the 23 patients (38.3 %) who were alive at the end of the observation period, 10 (16.7 % of all patients) had no detectable tumors.
The 1- and 3-year overall survival rates for all 60 patients were 93.3 and 51.7 %, respectively, with a median survival period of 36 months. The 1- and 3-year disease-free survival rates were 54.6 and 32.3 %, respectively, with a median of 16 months.
Clinicopathological Analyses
The clinicopathological characteristics of the patients are presented in Table 1. The HR and RFA groups did not differ significantly with respect to sex, age, chemotherapy, location or size of the primary tumor, number or size of liver metastatic sites, or preoperative carcinoembryonic antigen level. The HR group had a significantly larger proportion of metachronous cases (13/30) than the RFA group (14/3; p = 0.001).
Treatment Outcomes
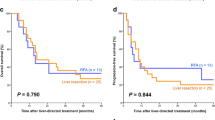
The median survival periods for patients in the HR and RFA groups were 57 and 30 months, respectively. The median times to disease-free status for patients in the HR and RFA groups were 22 and 11 months, respectively. The 3-year overall survival rates for patients in the HR and RFA groups were 53.5 and 47.1 %, respectively (p = 0.285, Fig. 2), whereas the 3-year disease-free survival rates were 35.0 and 26.9 %, respectively (p = 0.211, Fig. 3); thus, there were no significant differences between groups with respect to overall or disease-free survival rates. In the HR and RFA groups, 30 (60.2 %) and 13 (76.5 %) patients developed recurrence, respectively (p = 0.604).
Sex, age, chemotherapy, location and size of the primary tumor, detection time of metastasis, number and size of liver metastatic sites, and preoperative carcinoembryonic antigen level were not significantly associated with the 3-year overall and disease-free survival rates. In the HR group, 1 patient died from postoperative liver failure and 9 (20.9 %) developed postoperative complications including wound infection (5), biliary leakage (1), intra-abdominal abscess (2), and pneumonia (1). In the RFA group, 1 patient (5.9 %) required prolonged inpatient care because of procedure-related liver abscess.
Discussion
This study compared the therapeutic outcomes of HR and percutaneous RFA for resectable lesions of colorectal liver metastases. Although the HR group had a slightly higher survival rate than the RFA group, the differences were not significant. The results demonstrate that RFA can confer overall and disease-free survival outcomes for up to 3 years equivalent to those of HR.
HR has been traditionally considered the standard treatment for colorectal liver metastasis. Many studies indicate HR is superior to RFA [14–16]. Unfortunately, only 10–25 % of patients with colorectal liver metastases are candidates for HR; the others are not because of systemic conditions, underlying diseases, multiple liver metastases, and other problems [8–10, 17]. Therefore, alternative treatments are necessary for colorectal liver metastasis. The morbidity and mortality of the treatment modality are important to consider when selecting a treatment. Weng et al. [18] report that postoperative morbidity is significantly higher in patients receiving HR than RFA (24.10 vs. 9.98 %, p = 0.009). A large multicenter study demonstrates that RFA is a relatively safe procedure for treating focal liver tumors, with a very low mortality rate of 0.2 % and a major complication rate of 2.2 % [2, 12]. Therefore, RFA is recommended as the primary alternative therapy for colorectal liver metastasis. In the present study, 1 patient died from postoperative liver failure, 9 (20.9 %) developed postoperative complications in the HR group, and 1 (5.9 %) developed procedure-related complications in the RFA group. Thus, the present results are similar to those of other studies. As a result of recent technological progress and the development of improved imaging devices, the outcomes of RFA have become comparable to those of HR. Oshowo et al. [12] report that the preliminary survival curves for patients with solitary colorectal liver metastases treated by RFA (3-year survival rate, 52.6 %) are similar to those for patients who underwent liver resection (3-year survival rate, 55.4 %). These results indicate that RFA is an effective local treatment for patients considered unsuitable for conventional surgical treatment. Meanwhile, Sørensen et al. [19] report that the 5-year survival rate of 102 patients treated with RFA was 44 %. They claim RFA is an effective method for treating liver metastases from colorectal carcinoma and that survival has improved to a level comparable to that following surgical resection. Meanwhile, some studies report good outcomes with resection and report the effectiveness of the RFA in special circumstances. Hur et al. [16] report that the 5-year survival rates of 38 patients with small tumors (<3 cm) treated with HR and RFA were similar, including overall (56.1 vs. 55.4 %, p = 0.451) and local recurrence-free (95.7 vs. 85.6 %, p = 0.304) survival rates. Hence, RFA can be recommended as an alternative treatment for patients who are not candidates for surgery. Kim et al. [20] claim that RFA is a safe alternative treatment for solitary colorectal liver metastases <3 cm, with outcomes equivalent to those achieved with HR. Furthermore, Gillams et al. [21] report that RFA for solitary liver metastases ≤4 cm can be performed with minimal morbidity and results in excellent long-term survival, approaching that of surgical resection, even in patients who are not candidates for surgery.
On the other hand, several studies report better outcomes with HR than RFA. Hur et al. [16] analyzed 42 and 25 patients who underwent HR and RFA, respectively, for the treatment of solitary colorectal liver metastases; the 5-year overall and local recurrence-free survival rates after HR (50.1 and 89.7 %, respectively) were significantly higher than those after RFA (25.5 and 69.7 %, respectively; p = 0.0263 and 0.028, respectively). In addition, recurrence was significantly lower after HR. Lee et al. [15] compared the outcomes after HR and RFA; although the exact survival rates are not mentioned, the disease-free and overall survival periods were significantly longer in the HR group (p = 0.008 and 0.017, respectively); they report that RFA resulted in poorer outcomes than HR. These findings suggest that RFA should be considered only for selected patients with unresectable disease or high operative risk. Meanwhile, McKay et al. [14] also report that RFA is inferior to resection. In their study, the 5-year survival rate after resection was 43 % compared to 23 % after RFA; for patients with solitary lesions, the 5-year survival was 48 % after resection and 15 % after RFA. Therefore, the results of the present study corroborate the consensus that RFA cannot be considered an equivalent procedure to HR with respect to outcomes.
The recent meta-analyses of Weng et al. [18] and Wu et al. [22] further demonstrate that HR is significantly superior to RFA for the treatment of colorectal liver metastases. Wu et al. [22] report that patients with solitary colorectal metastasis treated with HR had significantly better 5-year survival outcomes than those treated by RFA (odds ratio, 0.41; 95 % confidence interval, 0.22–0.90; p = 0.008). They state that RFA should be reserved for patients who are suboptimal candidates for resection rather than being used as a first-line therapeutic option. Meanwhile, Weng et al. [18] report that despite the multiple confounders in clinical trials, HR is significantly superior to RFA for the treatment of colorectal liver metastasis, even when limited to tumors <3 cm, solitary tumors, open surgery, or laparoscopic approach. However, in these two meta-analyses, the RFA groups in several studies included unresectable liver metastases; therefore, the RFA groups had more severe disease progression. This may be an important source of bias, consequently limiting the interpretation of the results of studies reporting superior results with HR in patients with colorectal liver metastasis compared to RFA. In order to accurately report the results of the treatment outcomes between HR and RFA, another meta-analysis targeting studies involving patients receiving RFA as the only possible treatment is required.
The major limitations of the present study are the lack of randomization of treatment modalities, the small numbers of patients, and the short follow-up duration. Because of small sample size, it seems that difference in survivals of both groups did not reach statistical significance. Therefore, larger multicenter studies with longer follow-up periods are required to yield more objectively applicable data to confirm the present results. Although RFA has been investigated as an alternative to surgery because of its safety and feasibility, the comparative effectiveness of RFA and HR has yet to be clearly demonstrated.
Conclusion
In the present study, the 3-year overall and disease-free survival of patients with colorectal liver metastasis treated with RFA were similar to those treated with HR. However, this does not mean RFA is an appropriate substitute for HR. Although HR should be considered the first option, RFA can be regarded as a primary treatment modality depending on the patient’s characteristics; in particular, aggressive RFA treatment is a good option when a patient refuses surgery or has comorbidities. Nevertheless, a larger multicenter study with a longer follow-up period is required to clarify the effectiveness of HR and RFA for colorectal liver metastasis.
References
Annual Report of the Korea Central Cancer Registry. Cancer Registry Center of the Ministry for Health, Welfare and Family Affairs, 2008
Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS (2003) Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer 97:3027–3035
Bradley AL, Chapman WC, Wright JK, Marsh JW, Geevarghese S, Blair KT, Pinson CW (1999) Surgical experience with hepatic colorectal metastasis. Am Surg 65:560–566
Bengmark S, Hafstrom L (1969) The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 23:198–202
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S (2006) Survival after hepatic resection for colorectal metastases: a 10 years experience. Ann Surg Oncol 13:668–676
Aldrighetti L, Castoldi R, Di Palo S, Arru M, Stella M, Orsenigo E et al (2005) Prognostic factors for long-term outcome of hepatic resection for colorectal liver metastases. Chir Ital 57:555–570
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–827
Geoghegan JG, Scheele J (1999) Treatment of colorectal liver metastases. Br J Surg 86:158–169
Chiappa A, Zbar AP, Biella F, Staudacher C (1999) Survival after repeat hepatic resection for recurrent colorectal metastases. Hepatogastroenterology 46:1065–1070
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P et al (1999) Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 230:1–8
Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I (2003) Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 90:1240–1243
Gillams AR, Lees WR (2005) Radiofrequency ablation of colorectal liver metastases. Abdom Imaging 30:419–426
McKay A, Fradette K, Lipschitz J (2009) Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg ID 346863:1–8
Lee KH, Kim HO, Yoo CH, Son BH, Park YL, Cho YK et al (2012) Comparison of radiofrequency ablation and resection for hepatic metastasis from colorectal cancer. Korean J Gastroenterol 59:218–223
Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK et al (2008) Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 197:728–736
Silen W (1989) Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg 124:1021–1022
Weng M, Zhang Y, Zhou D, Yang Y, Tang Z, Zhao M et al (2012) Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. Open Access 7:1–8
Sørensen SM, Mortensen FV, Nielsen DT (2007) Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 48:253–258
Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN et al (2011) Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 81:25–34
Gillams AR, Lees WR (2008) Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 19:712–717
Wu YZ, Li B, Wang T, Wang SJ, Zhou YM (2011) Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol 17:4143–4148
Acknowledgments
This work was supported by a grant from Inje University, 2011.
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, WW., Kim, K.H., Kim, S.H. et al. Comparison of Hepatic Resection and Radiofrequency Ablation for the Treatment of Colorectal Liver Metastasis. Indian J Surg 77 (Suppl 3), 1126–1130 (2015). https://doi.org/10.1007/s12262-015-1211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-015-1211-1