Summary
Background
Discovery of calreticulin (CALR) mutations are considered essential in understanding the molecular basis of myeloproliferative neoplasms (MPNs). These mutations have been demonstrated to be the second driver mutation in MPNs especially essential thrombocythemia (ET) and primary myelofibrosis (PMF). The diagnostic and prognostic significance of these mutations, in addition to the geographical and ethnic heterogeneity, were previously reported. Molecular profiling also guides the selection of therapy for patients with MPNs. Therefore, we investigated the frequency and clinical and demographic characteristics of CALR mutations in Egyptian patients with MPNs.
Methods
We investigated CALR mutations in 84 Egyptian patients with MPNs using florescent polymerase chain reaction followed by fragment analysis. Then, we analyzed the relationship between the mutations and patient clinical data.
Results
The frequency of CALR mutations was 13% (type 2 mutations, 55%; mix or complex mutations, 27%; type 1 mutations, 18%). CALR mutations were most frequent in ET (21.4%), followed by PMF (15.4%) and were not found in polycythemia vera (PV). Clinically, patients with mutated CALR had significantly lower levels of leukocytes (P = 0.042) and higher platelet count (P = 0.04) compared to those with nonmutated CALR and lower risk of thrombosis although a statistical difference was not detected.
Conclusions
CALR mutations were found frequently in Egyptian MPNs patients with ET and PMF especially in those without JAK2 mutation. Patients with type 2 and complex mutations were more frequent in our cohort than the previously known published data. Thus, CALR is a potentially valuable marker for stratification of JAK2-negative MPN patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myeloproliferative neoplasms (MPNs) are chronic myeloid cancers characterized by overproduction of mature blood cells with increased risk of transformation into acute myeloid leukemia (AML) [1, 2]. Philadelphia chromosome-negative MPNs include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Thus far, both JAK2V617F mutations and MPL mutations have helped stratify and prognosticate patients. JAK2V617F mutations are detected in approximately 95% of patients with PV and in 50–60% of ET and PMF patients [2]. Rarely, patients carry other JAK2 mutations, such as insertions or deletions in exon 12 [3]. Activating mutations of MPL, the thrombopoietin receptor gene, are present in 5–10% of patients with ET and PMF with nonmutated JAK2 [5, 6]. Nearly one-third of patients with ET or PMF do not carry any JAK2 or MPL mutations [5, 7].
The identification of calreticulin (CALR) mutations has changed the molecular diagnostic algorithm for MPNs and improves the diagnostic accuracy of JAK2V617F-negative ET and PMF cases and influence prognosis [4].
At the end of 2013, two studies identified recurrent mutations in the CALR gene using whole-exome sequencing. They detected recurrent CALR mutations in 60–88% of patients with ET and PMF who were negative for JAK2 and MPL mutations [8, 9].
Mutant CALR activates JAK/STAT signaling leading to downstream proliferative signaling [10]. CALR is required for downstream JAK-STAT activation and oncogenic transformation through the C‑terminal domains of the mutant protein [11, 12]. Additionally, expression of mutant CALR in Ba/F3 cells results in cytokine-independent proliferation with increased phospho-STAT5 expression and sensitivity to JAK2 inhibition [10].
CALR mutations are either insertions or deletions in exon 9 (type 1: a 52-bp deletion, 45–53%, or type 2: a 5-bp insertion (ins TTGTC), 32–41%). Other less frequent mutations in exon 9 account for up to 15% of CALR mutations [13]. All CALR mutations lead to a frame shift generating a common 36 amino acid C‑terminal end with the loss of the KDEL motif, and the KDEL endoplasmic reticulum (ER) retention signal generating an abundance of positively charged amino acids [13].
Patients carrying calreticulin mutations have been reported to be at lower risk for disease complications including thrombosis in ET or massive myelofibrosis with transfusion dependency and symptomatic splenomegaly in PMF as compared to patients with JAK2 V617F mutations [14]. It was recently reported that antiplatelet therapy did not affect thrombosis risk in CALR-mutated ET patients but was associated with a higher incidence of bleeding [15]. Also, the best posthematopoietic cell transplantation (HCT) outcome was observed with CALR-mutated PMF [16]. Given the above findings, it is important to study CALR mutations to further stratify and determine the clinical therapeutic strategy for CALR-mutated patients.
Very few studies have investigated CALR mutations in Egyptian patients with MPNs. Therefore, in this study, we analyzed the status and frequency of CALR mutations in a cohort of Egyptian patients with MPNs correlating that with clinical and laboratory characteristics to better guide therapeutic decisions in routine clinical practice.
Materials and methods
The study was approved by the institutional review board of the faculty of medicine, Mansoura University, Egypt. Between 2009 and 2017, patients were selected from the Oncology Center database according to availability of archived peripheral blood DNA for mutation screening. Patients were diagnosed as MPNs based on the criteria of diagnosis established by the WHO 2008 [17]. A second revision of diagnostic criteria was performed based on the new WHO 2016 revision for MPNs [4]. A total of 84 patients with MPN (53 males and 31 females), including cases with ET (n = 42), PV (n = 20), PMF (n = 13), MDS/MPN (n = 3), and chronic myeloid leukemia (CML) (n = 6) were selected. The patient samples were chosen to represent different MPN subtypes and not the incidence proportions. We focused our selection on JAK2V617F-negative patients with an additional small number of JAK2V617F-positive patients to be used as a comparative group. We also included 11 healthy controls (7 males and 4 females). Demographic and clinical information at the time of presentation, from patients and healthy controls, was obtained from medical records and is summarized in Table 1. Each patient provided informed consent for sample collection in accordance with the Declaration of Helsinki.
Janus kinase 2 and calreticulin mutation analyses
DNA was extracted from peripheral blood using the DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed for detection of JAK2V617F mutations as previously described [18] as a part of the routine diagnostic workup for MPN patients.
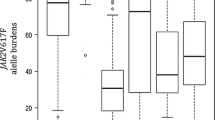
CALR mutations were detected in archived genomic DNA from peripheral blood using fluorescent PCR followed by fragment length analysis. Detection of CALR mutations in exon 9 was performed as previously described [19]. Briefly, 50 ng of genomic DNA was amplified with 7.5 pmol each of forward and reverse primers to amplify a 293-bp product. The forward primer was (CALR-F) 5′–6 FAM (6-carboxyfluorescein) -TAACAAAGGTGAGGCCTGGT-3′ and the reverse primer was (CALR-R) 5′GCCTCTCTACAGCTCGTCCTT-3′. The reaction was carried out in a total of 25 μl using 1 × Hot Star Taq Master Mix (Qiagen). PCR conditions were as follows: 10 min denaturation at 95 °C, then 40 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s followed by 10 min incubation at 72 °C. PCR products were separated by electrophoresis on a 3% agarose gel (supplementary Figs. 1and 2). PCR products were analyzed by capillary electrophoresis on an ABI-3500 genetic analyzer followed by fragment analysis on Gene Mapper Software 4.1 (Applied Biosystems, Forest City, CA, USA) (Fig. 2). Products’ peak heights were analyzed, and the ratio of mutant to wild-type plus mutant peaks was calculated in order to define % CALR mutation burden [20].
Statistical analysis
Statistical analyses were performed with the statistical software GraphPad Prism 5.0 (GraphPad Software, USA). Continuous variables including age and peripheral blood count were summarized by median and range and analyzed using the Mann–Whitney test. Overall survival (OS) analysis was considered from the date of diagnosis to the date of death or last contact and was prepared by the Kaplan–Meier method. A value of P < 0.05 was considered statistically significant.
Results
Frequency and pattern of the calreticulin mutation
Among the 84 MPN cases, CALR mutations were identified in 11 patients (13%), 23 patients (27%) were positive for JAK2 V617F, and 50 patients (60%) were negative for both mutations (wild-CALR and JAK2V617F). In patients without JAK2V617F (wild-type), the frequency of CALR mutations was slightly higher at 18% (11/61) (Fig. 1).
All 11 CALR mutations were detected in ET and PMF cases (11/55, 20%). No CALR mutations were found in patients with PV, CML or MDS/MPN.
Type 2 CALR mutations were the most prevalently found in 55% (6/11) of patients. Type 1 mutations were found in 18% (2/11) of patients. The remaining 3 cases, harbored two or more mutated clones (3/11, 27%) (Table 2, Fig. 2). Type 2 mutations were more frequent in ET patients (6/9, 67%) and type 1 mutations represented 50% of the 2 CALR-mutated PMF patients (Fig. 3).
Clinical characteristics of patients with CALR mutations
All of the 20 PV, 6 CML and 3 MDS/MPN patients had wild-type CALR. Table 3 shows the clinical and laboratory characteristics of the CALR-mutated and -nonmutated cases. As shown in Table 3, patients with mutated CALR had a significantly lower white blood cell (WBC) count than those with nonmutated CALR (P = 0.042). A significantly higher platelet count was also observed in the CALR-mutated group (P = 0.04) which may be due to the large number of ET patients in our cohort. No significant differences were observed in Hb level, LDH level or age between the CALR-mutated and -nonmutated group. As CALR mutation is observed only in ET and PMF patients, we compared the clinical characteristics of ET and PMF patients in Table 4. ET patients with mutant CALR had lower WBC counts (P = 0.015) than those with mutant JAK2V617F. Similarly, PMF patients with mutant CALR showed lower WBC counts (P = 0.009) than mutant JAK2 patients. No significant difference was identified between ET patients with mutant CALR and mutant JAK2 in terms of age, hemoglobin level and platelet count. Additionally, there was no significant difference in age, WBC count, or platelet count between PMF patients with mutant CALR and mutant JAK2.
Thrombotic event, splenomegaly and overall survival
According to our data, there were no significant differences in thrombotic events or splenomegaly between the CALR-mutated and -nonmutated patient groups.
CALR-mutant patients had a lower risk of death than JAK2-mutant patients (P = 0.186, median survival months 37.5 and 25 months, HR: 0.29 and 3.35, respectively; Fig. 4). Additionally, the median survival months were higher in CALR-mutated ET and PMF patients (37.5 vs 33 months, HR: 0.22 vs 4.58 and 63 vs 40.5 months, HR: 0.25 and 4.05, respectively) when compared to JAK2-mutated patients but significant level was not reached (Table 4).
Discussion
CALR mutations play an important role in MPN patients without JAK2V617F and MPL mutations [9]. These mutations are increasingly established as valuable molecular markers for confirming the diagnosis of MPN [13]. Somatic mutations in CALR exon 9 are considered the second most common mutation in JAK2- and MPL-negative PMF and ET patients [21, 22].
Due to the diagnostic and prognostic value of these mutations, in addition to the geographical and ethnic variation previously reported [23, 24], the frequency of CALR mutations was investigated in a retrospective cohort of Egyptian MPN patients to determine the existing mutational spectrum. Although a number of methodological approaches used to detect CALR mutations exists, fragment length analysis was found to be sufficient to detect relevant mutations [20, 25] so was the method of choice to investigate CALR mutations in our patients.
Previous studies showed that CALR mutations are present almost exclusively in JAK2V617F-negative patients with ET and PMF [9, 26]. This was confirmed in our study where CALR mutations were detected only in ET and PMF cases without JAK2V617F mutation. According to multiple studies, the incidence of CALR mutations are observed in 9–25% of MPN patients [8, 9, 13, 26], being detected in approximately 20–25% of patients with ET and PMF and not in patients with PV [8, 9, 13, 26]. In our study, CALR mutations were detected in 11 of 84 (13%) patients with MPNs and in 11 of 61 (18%) MPN patients without JAK2V617F mutations. This was consistent with the El Nahass et al. [27] study of Egyptian MPN patients that found CALR mutation in 15% (14/93) of Egyptian MPN patients. Furthermore, they reported CALR mutation in 24% of ET patients and in 17% of PMF patients and not detected in PV patients [27].
In our patients CALR mutations were detected in 21.4% of ET and 15.4% of PMF patients, with no mutation detected in PV patients. This is consistent with what was observed by El Nahass et al. in Egyptian MPN patients [27].
Also, our results were consistent with the Kim et al. study on Korean patients with MPNs that found CALR mutations in 12.6% of patients with MPNs (17.7% of ET patients, 14.8% of PMF patients and not in PV patients) [28] and with the Wang et al. study on Chinese patients with Ph-negative MPNs that reported CALR mutations in 10.6% [29].
It was previously observed that type 1 mutations are associated with PMF and ET with an increased risk of myelofibrotic transformation, while type 2 mutations are associated with ET, low risk of thrombosis and an indolent clinical course [22].
In our study, the majority (9/11, 82%) of the CALR mutation pattern was an insertion and complex (mix) mutation pattern while 18% (2/11) were a type 1 mutation. Interestingly, type 2 mutations were the most prevalent (55%) in our patients followed by complex mutations (27%) which is noticed to be the higher reported in literature then type 1 mutations (18%). Type 2 mutations were more frequent in ET patients (6/9, 67%).
Many studies have reported that patients with CALR mutations were preferentially male and younger in age [26, 30]; however, no male preference was observed in our CALR-mutated patient group. Additionally, there were no age difference between our patients with CALR and JAK2V617F mutations which is similar to what was previously reported in Egyptian MPN patients [27].
Previous reports demonstrated that CALR mutations were associated with higher platelet count, lower thrombosis risk and less leukocytosis [22, 31]. Our patients with CALR mutations also showed significantly higher platelet counts (P = 0.04) and lower WBC counts (P = 0.042) compared to CALR-negative patients, which is consistent with reported by El Nahass et al. [27].
In previous studies, CALR mutations have been associated with longer survival times in PMF cases [32]. In our cohort, there was no difference in overall survival between CALR-mutated and -nonmutated cases (P = 0.186). Survival of CALR-mutated versus -nonmutated patients was 100% versus 82% (P = 0.186) consistent with the 100% and 92% (P = 0.197) that was reported by the Egyptian MPN study [27].
In conclusion, the frequency and spectrum of CALR mutations in our Egyptian MPN patients are comparable to previously published reports, thus, confirming the importance of CALR as a driver mutation in ET and PMF. However, in our patient population type 2 and complex mutations are more frequent than in previously published studies. Therefore, further research on a larger patient cohort and more deep molecular profiling are required to confirm these findings and help to establish better treatment guidelines.
Take Home Message
The frequency and spectrum of CALR mutations in our Egyptian MPN patients are comparable to previously published reports, confirming the importance of CALR as a driver mutation in ET and PMF. Type 2 and complex mutations are more frequent in our study than in previously published studies. Further research on a larger patient cohort and more deep molecular profiling are required to confirm these findings and help to establish better treatment guidelines.
References
Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–8. https://doi.org/10.1182/blood-2008-03-077966.
Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–66. https://doi.org/10.1056/NEJMra063728.
Jeong JH, Lee HT, Seo JY, Seo YH, Kim KH, Kim MJ, et al. Screening PCR versus sanger sequencing: detection of CALR mutations in patients with thrombocytosis. Ann Lab Med. 2016;36:291–9. https://doi.org/10.3343/alm.2016.36.4.291.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. https://doi.org/10.1371/journal.pmed.0030270.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. https://doi.org/10.1182/blood-2006-04-018879.
Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Jelena D, et al. JAK2 or CALR mutation status de fi nes subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–51. https://doi.org/10.1182/blood-2013-11-539098.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. https://doi.org/10.1182/blood-2016-03-643544.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–405. https://doi.org/10.1056/NEJMoa1312542.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90. https://doi.org/10.1056/NEJMoa1311347.
Kollmann K, Warsch W, Gonzalez-Arias C, Nice FL, Avezov E, Milburn J, et al. A novel signalling screen demonstrates that CALR mutations activate essential MAPK signalling and facilitate megakaryocyte differentiation. Leukemia. 2017;31:934–44. https://doi.org/10.1038/leu.2016.280.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood. 2016;127:1307–16. https://doi.org/10.1182/blood-2015-09-671172.
Shide K, Kameda T, Yamaji T, Sekine M, Inada N, Kamiunten A, et al. Calreticulin mutant mice develop essential thrombocythemia that is ameliorated by the JAK inhibitor ruxolitinib. Leukemia. 2017;31:1136–44. https://doi.org/10.1038/leu.2016.308.
Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL, et al. Type 1 versus type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89:121–4. https://doi.org/10.1002/ajh.23743.
Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–66. https://doi.org/10.1042/BJ20081847.
Falchi L, Kantarjian HM, Verstovsek S. Assessing the thrombotic risk of patients with essential thrombocythemia in the genomic era. Leukemia. 2017;31:1845–54. https://doi.org/10.1038/leu.2017.150.
Salit RB, Deeg HJ. Transplant decisions in patients with myelofibrosis: should mutations be the judge? Biol Blood Marrow Transplant. 2018;24:649–58. https://doi.org/10.1016/j.bbmt.2017.10.037.
Vardiman JW, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2019;114:937–52. https://doi.org/10.1182/blood-2009-03-209262.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. https://doi.org/10.1016/S0140-6736(05)71142-9.
Chi J, Nicolaou KA, Nicolaidou V, Koumas L, Mitsidou A, Pierides C, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28:1152–4. https://doi.org/10.1038/leu.2013.382.
Jones AV, Ward D, Lyon M, Leung W, Callaway A, Chase A, et al. Evaluation of methods to detect CALR mutations in myeloproliferative neoplasms. Leuk Res. 2015;39:82–7. https://doi.org/10.1016/j.leukres.2014.11.019.
Verger E, Cassinat B, Chauveau A, Dosquet C, Giraudier S, Schlageter M‑H, et al. Clinical and molecular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126:2585–91. https://doi.org/10.1182/blood-2015-07-659060.
Guglielmelli P, Rotunno G, Bogani C, Mannarelli C, Giunti L, Provenzano A, et al. Ruxolitinib is an effective treatment for CALR-positive patients with myelofibrosis. Br J Haematol. 2016;173:938–40. https://doi.org/10.1111/bjh.13644.
Mansier O, Migeon M, Etienne G, Bidet A, Lippert E. JAK2V617F and CALR double mutations are more frequently encountered in patients with low JAK2V617F allelic burdens. Leuk Lymphoma. 2016;57:1949–51. https://doi.org/10.3109/10428194.2015.1116122.
Broseus J, Park J‑H, Carillo S, Hermouet S, Girodon F. Presence of calreticulin mutations in JAK2-negative polycythemia vera. Blood. 2014;124:3964–6. https://doi.org/10.1182/blood-2014-06-583161.
Langabeer SE, Andrikovics H, Asp J, Bellosillo B, Carillo S, Haslam K, et al. Molecular diagnostics of myeloproliferative neoplasms. Eur J Haematol. 2015;95:270–9. https://doi.org/10.1111/ejh.12578.
Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552–5. https://doi.org/10.1182/blood-2013-11-538983.
ElNahass YH, Mahmoud HK, Mattar MM, Fahmy OA, Samra MA, Abdelfattah RM, et al. MPN10 score and survival of molecularly annotated myeloproliferative neoplasm patients. Leuk Lymphoma. 2018;59:844–54. https://doi.org/10.1080/10428194.2017.1365852.
Kim SY, Im K, Park SN, Kwon J, Kim J‑A, Lee DS. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015;143:635–44. https://doi.org/10.1309/AJCPUAAC16LIWZMM.
Wang J, Hao J, He N, Ji C, Ma D. The mutation profile of calreticulin in patients with myeloproliferative neoplasms and acute leukemia. Turk J Haematol. 2016;33:180–6. https://doi.org/10.4274/tjh.2015.0220.
Pietra D, Rumi E, Ferretti VV, Di Buduo CA, Milanesi C, Cavalloni C, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia. 2016;30:431–8. https://doi.org/10.1038/leu.2015.277.
Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–7. https://doi.org/10.1038/leu.2014.3.
Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1:97–105. https://doi.org/10.1001/jamaoncol.2015.89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. M. Saleh, R. Algamal, H. Abd Elmasseh, E. Barber, and H. Abdel-ghaffar declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author contributions
Hasan Abdel-ghaffar and Hanaa Abd Elmasseh designed the study. Layla M. Saleh and Reem Algamal performed experiments. Layla M. Saleh and Reem Algamal analyzed the data and wrote the manuscript. Layla M. Saleh, Reem Algamal, Hanaa Abd Elmasseh, Emily Barber and Hasan Abdel-ghaffar assisted in editing the manuscript.
Caption Electronic Supplementary Material
12254_2020_584_MOESM1_ESM.docx
Supplementary figure 1: PCR amplification of exon 9 of CALR gene loaded on 3% agarose gel; lane M: 100 bp marker, MPN patients # 5, 10, 11, 42, 67 and 80 type‑2 CALR mutation. Patients # 49 and 58 type‑1 CALR mutation. Patients # 16, 19 and 24 mix CALR mutation.
12254_2020_584_MOESM2_ESM.docx
Supplementary figure 2: a PCR amplification of exon 9 of CALR gene loaded on 3% agarose gel; lane M: 100 bp marker, a subset of healthy controls # 90:96. b Results of PCR fragment analysis of CALR exon 9 from healthy controls that were tested.
Rights and permissions
About this article
Cite this article
Saleh, L.M., Algamal, R., Abd Elmasseh, H. et al. Different CALR mutation subtypes in essential thrombocythemia and primary myelofibrosis patients without JAK2 mutation. memo 13, 235–243 (2020). https://doi.org/10.1007/s12254-020-00584-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-020-00584-2