Abstract
This review discusses promising new approaches in the management of classical Hodgkin’s lymphoma that have been recently presented at the annual ASH meeting. Novel insights regarding risk-adapted, tailored therapy as well as improved treatment strategies in relapsed/refractory disease have been presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hodgkin’s lymphoma (HL) accounts for 10 % of all malignant lymphomas [1]. The histologic subtypes include classical HL or nodular lymphocyte predominant HL, which is characterized by a more indolent clinical course and will not be discussed in this review.
Before treatment, the clinical stage according the Ann Arbor classification has to be determined. However, fluorodeoxyglucose positive emission tomography (FDG-PET) leads to a change of the clinical stage in comparison to conventional computed tomography scan in10−30 % [1]. Moreover, a negative FDG-PET at the end of treatment is an important prognostic factor [2]. Interim PET has a predictive value as well, but the therapeutic consequences following a negative or positive PET after two cycles of chemotherapy still have to be determined [3].
Initial therapy
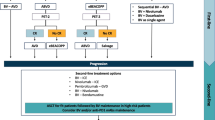
Most patients can be cured with standard chemotherapy. To achieve the highest possible cure rate and to avoid long-term toxicities, a risk-adapted management is the current standard of care. In HL patients with early-stage disease with favorable prognostic factors, a combined modality strategy using abbreviated courses of combination chemotherapy followed by radiation therapy is recommended [1]. Patients with advanced stage disease receive a longer course of chemotherapy without radiation therapy. However, some academic groups prefer the escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) protocol, leading to an improved progression-free survival (PFS) [4–6], while others prefer doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) because of the lower toxicity and lower occurrence of secondary malignancy. However, a recently published meta-analysis including 10,111 patients showed an overall survival (OS) benefit of 10 % after 5 years for BEACOPP escalated [7]. Updated results comparing upfront chemotherapy combinations for advanced HL have been presented.
Long-Term Follow-up Analysis of HD2000 Trial Comparing ABVD Versus BEACOPP Versus cyclophosphamide, vincristine, procarbazine, prednisone (COPP)/epidoxorubicin, bleomycin, vinblastine (EBV)/lomustine, doxorubicin, and vindesine (CAD)(Copp/EBV/CAD[CEC]) in Patients with Newly Diagnosed Advanced-Stage Hodgkin’s Lymphoma: A Study from the Fondazione Italiana Linfomi (Abstract#499, Merli et al.)
A total of 305 eligible patients with stage IIB, III, or IV were randomly assigned to receive six courses of ABVD (n = 103), four escalated plus two standard courses of BEACOPP (n = 100), or six courses of CEC (n = 102). The 10 years PFS was 69 %, 74 %, and 74 % for ABVD, BEACOPP, and CEC respectively (p = 0.639). The 10 years OS was 84 %, 84 %, and 86 % for ABVD, BEACOPP, and CEC respectively (p = 0.883). Of interest, the rate of secondary malignancy at 10 years was 6.7, 4.4, and 0.9 for BEACOPP, CEC, and ABVD respectively. The difference between BEACOPP and ABVD was statistically significant (p = 0.027). Therefore, this long-term analysis does not confirm the previously observed superiority of BEACOPP versus ABVD regarding PFS.
Elderly patients with comorbidities have higher rates of relapse due to chemotherapy related toxicities resulting in suboptimal dosing. Therefore, new treatment options for these patients are needed.
Brentuximab vedotin (BV) is an anti-CD30 antibody conjugated with a microtubule-disrupting agent. In a phase 2 study of BV in patients with relapsed or refractory HL after autologous stem cell transplantation (ASCT), 75 % of patients achieved an objective response [8]. High activity has also been shown in untreated patients [9]. Novel data of HL patients treated with BV have been presented.
Brentuximab Vedotin Monotherapy and in Combination with Dacarbazine in Frontline Treatment of Hodgkin Lymphoma in Patients Aged 60 Years (Abstract#294, Forero-Torres et al.)
Patients aged ≥ 60 years with HL were included in this phase 2 study, 27 patients received monotherapy, 6 combination therapy. The median age was 77 years (range, 64–92). Most patients had stage III–IV disease (70 %) and 48 % had moderate age-related renal insufficiency.
Interim results show an overall response rate (ORR) for monotherapy of 93 % with a median PFS of 10.5 months. The complete response rate (CR) was 70 %. All six patients treated with combination therapy responded. Median PFS has not been reached. Most common adverse events (AEs) were peripheral sensory neuropathy (22 %), peripheral motor neuropathy (7 %), and rash (7 %). The authors conclude that the high response rates with acceptable tolerability warrant evaluating this treatment combination in larger clinical trials.
Brentuximab Vedotin Combined with ABVD or AVD (doxorubicin, vinblastine, dacarbazine) for Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma: Long Term Outcomes (Abstract#292, Connors et al.)
In all, 51 untreated patients with advanced-stage HL were included receiving either BV-ABVD or BV-AVD. Because of two patients died due to pulmonary toxicity in the BV-ABVD-arm, BV could no longer be combined with bleomycin. The 3 years failure-free survival was 83 % for BV-ABVD and 96 % for BV-AVD respectively. The 3 years OS was 92 % and 100 %. Therefore, BV-AVD represents a highly effective treatment option without additional toxicity for patients with advanced-stage HL.
Relapsed/refractory disease
Despite the high-cure rate, 10–20 % of HL patients fail to respond or will relapse after achieving an initial CR. High-dose chemotherapy (HDCT) followed by ASCT is the standard of care for many patients with relapsed disease [1, 10, 11]. Patients who achieve CR on salvage chemotherapy prior to ASCT have better outcomes [11, 12]. However, patients relapsing after ASCT have poor prognosis and HDCT has long-term toxic effects [1, 12]. Therefore, novel treatment options are needed. Following studies concerning relapsed/refractary HL have been presented:
The Aethera Trial: Results of a Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of Brentuximab Vedotin in the Treatment of Patients at Risk of Progression Following Autologous Stem Cell Transplant for Hodgkin Lymphoma (Abstract#673, Moskowitz et al.)
A total of 329 patients with high-risk factors (refractory to frontline therapy, relapse < 12 months after frontline therapy, and relapse ≥ 12 months after frontline therapy with extranodal disease) were randomized. Patients received BV and best supportive care (BSC) or placebo and BSC for up to 16 cycles after ASCT as consolidation.
The median age was 32 years (range, 18–76). AEs of any grade in > 15 % of patients were peripheral sensory neuropathy (36 %), upper respiratory tract infection (25 %), neutropenia (24 %), fatigue (21 %), cough (19 %), and pyrexia (17 %). Grade 3 or higher AEs in ≥ 10 patients were neutropenia (20 %), peripheral sensory neuropathy (6 %), thrombocytopenia (3 %), and peripheral motor neuropathy (3 %).
Median PFS was 43 months in patients treated with BV and BSC and 24 months in patients treated with BSC alone (p = 0.001). Interim analysis of OS did not show a significant difference between treatment arms (p = 0.62).
Brentuximab Vedotin in Combination with Bendamustine for Patients with Hodgkin Lymphoma who are Relapsed or Refractory after Frontline Therapy (Abstract#293, LaCasce et al.)
A total of 45 patients (median age of 35 years, 58 % relapsed disease, 42 % refractory disease) have been enrolled. Patients received a median of 2 courses of BV/bendamustine combination.
The ORR was 96 % with 83 % CR. Adequate stem cell mobilization could be achieved in all patients who underwent this procedure. The CR rate of this combination was higher than historical data obtained by salvage chemotherapy regimens and therefore represents a promising approach for patients with planned ASCT after relapsed/refractory disease. The safety profile was manageable with premedication for infusion related reactions. Obviously, there was no negative impact on stem cell mobilization.
Nivolumab in Patients with Relapsed or Refractory Hodgkin Lymphoma - Preliminary Safety, Efficacy and Biomarker Results of a Phase I Study (Abstract#289, Armand et al.)
Nivolumab is a programmed cell death-1 (PD-1) blocking antibody. Patients with relapsed/refractory HL received nivolumab 3 mg/kg every 2 weeks. Patients were heavily pretreated, 78 % had prior ASCT, and 78 % had prior BV treatment.
Most common AEs were rash (22 %), decreased platelet count (17 %), diarrhea, nausea, pruritus, fatigue, and pyrexia (each 13 %).
Of 23 patients, the ORR was 87 % with a CR in 17 % of patients and partial response (PR) in 70 %. The remaining three patients (13 %) had stable disease. All 23 patients achieved a reduction of tumor burden. PFS at 24 weeks was 86 %, the median OS was not reached. In all, 6 patients discontinued study treatment and underwent ASCT, 2 patients discontinued because of toxicity (myelodysplastic syndrome and thrombocytopenia in 1 patient; pancreatitis in 1 patient), 4 patients progressed, and 11 patients are continuing nivolumab. In conclusion, nivolumab was highly active in this heavily pretreated patient cohort.
PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013) (Abstract#290, Moskowitz et al.)
Preliminary results from 15 patients were presented. All patients were relapsed/refractory to BV treatment, 67 % also failed prior ASCT.
Pembrolizumab was well-tolerated, most frequent AEs were respiratory events (20 %) and thyroid disorders (20 %). There were no serious AEs. In all, one patient discontinued treatment because of an AE (pneumonitis), and three patients progressed.
The ORR was 53 % with three patients (20 %) with CR at 12 weeks, and five with PR (33 %).
Conclusion
In first line treated patients, BEACOPP has been shown to be associated with a significantly higher rate of secondary malignancy in comparison to ABVD in a presented long-term follow-up. Elderly patients or patients with advanced-stage HL could benefit from incorporating BV in first line chemotherapy protocols like dacarbazine or AVD. BV shows also activity as consolidation after ASCT and in combination with bendamustine, presented data about BV in HL is summarized in Table 1. Novel drugs interacting with the PD-1 pathway show promising activity in heavily pretreated patients, also in patients pretreated with BV.
Conflict of interest
Daniel Heintel declares that there are no actual or potential conflicts of interest in relation to this article.
References
Mounier N, Gisselbrecht C. Decision-making in the management of adult classical Hodgkin’s lymphoma: determining the optimal treatment. Expert Rev Hematol. 2015;8(2):205–16.
Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52.
Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99(6):1107–13.
Mounier N, Brice P, Bologna S, Lymphoma Study Association (LYSA), et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0–2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25(8):1622–8.
Federico M, Luminari S, Iannitto E, HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27(5):805–11.
Viviani S, Zinzani PL, Rambaldi A, Michelangelo Foundation, Gruppo Italiano di Terapie Innovative nei Linfomi, Intergruppo Italiano Linfomi et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–12.
Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14(10):943–52.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9.
Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma. a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–56.
Ansell SM Hodgkin lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(7):771–9.
Moskowitz CH, Nimer SD, Zelenetz AD et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–23.
Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heintel, D. ASH 2014: novel strategies in the management of classical Hodgkin’s lymphoma. memo 8, 172–175 (2015). https://doi.org/10.1007/s12254-015-0230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-015-0230-8