Abstract
Similar to the mesenchymal stem cells (MSCs), dental pulp stem cells (DPSCs) also have pluripotent differentiation characteristic and may be more ideal for tissue regeneration, especially in tooth regeneration engineering. However, bacterial infection may be a powerful obstacle. Berberine (BBR), known with antibacterial effects, was recently found to play functions in bone formation through promoting osteogenic differentiation from pluripotent stem cells. However, whether BBR also function in DPSCs osteogenic differentiation has not yet been reported. Primary DPSCs were isolated from dental pulp tissues extracted from human impacted mandibular third molars, and identified by flow cytometry for cell surface antigen molecules. A dexamethasone osteogenic medium was used to induce DPSCs osteogenic differentiation. BBR (1 μM and 5 μM) was pre-added to into medium, and then cell proliferation, spheroid formation and osteogenic differentiation capacities of DPSCs were analyzed, as well as the underlying molecules modulation mechanism. Flow cytometry identified that CD44, CD90, CD81 and CD105 positively expressed in the isolated hDPSCs, with CD34 and CD45 negetively expressed. BBR enhanced the cell proliferation of hDPSCs in a dose-dependent pattern, and promoted dexamethasone-induced osteogenic differentiation via enhancing Runx2 transcription factor activity followed by upregulating osteogenesis markers expression, whereas the adipogenic differentiation of hDPSCs was suppressed dramatically by BBR. The EGFR and MAPK pathways were activated by BBR, and inhibitors for these pathways significantly suppressed the osteogenic differentiation promotion of BBR. These results have revealed a novel mechanism that berberine might promote hDPSCs osteogenic differentiation through activating EGFR-MAPK-Runx2 signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental pulp stem cells (DPSCs) are a kind of adult stem cells in dental pulp tissues, which have similar immunophenotype and pluripotent differentiation characteristics to the bone marrow mesenchymal stem cells (BMSCs) [1]. It can be differentiated into osteoblasts, odontoblasts, adipocytes, myocytes and neurocytes [2]. Because of the high proliferative capacity and easy accessibility, DPSCs become more ideal than BMSCs in tooth regeneration, nerve regeneration, bone tissue engineering and translational medicine [3, 4]. DPSCs are commonly used together with scaffold materials and cytokines to induce dental pulp regeneration and restore its structure and function [5, 6]. However, bacterial infection may be a powerful obstacle in the process of dental pulp regeneration. Finding a drug both with antibacterial and inducing effects become urgent.
Berberine (BBR) is an active alkaloid extracted from the medicinal plants of family polygonaceae, and it is a classic antipyretic and detoxifying drug in traditional Chinese medicine [7]. BBR has a significant effect on antibacterial, anti-inflammatory and anti-oxidation [8], and has characteristics of small side effects and low price. It is always a common medicine for treating bacterial diarrhea and gastroenteritis. At present, BBR has attracted attention in the field of stomatology, and subsequently its antibacterial effects on many oral pathogens have been found [9]. More interestingly, BBR not only has antibacterial effect, but also can promote bone formation [10]. Previous studies have shown that BBR promotes osteogenic differentiation of bone marrow mesenchymal stem cells (MSCs) by activating Runx2 transcriptional activity through the p38MAPK signaling pathway, or classic wnt/β-catenin signaling pathway [11, 12]. BBR can also promote osteogenic differentiation from periodontal ligament stem cells (PLSCs) via EGFR-ERK-Fos signaling pathway [13]. However, whether BBR plays a similar role in hDPSCs has not been reported.
In this study, the effects of BBR on the cell proliferation and osteogenesis of DPSCs was investigated in in vitro cultured primary human DPSCs, and the relateted signaling pathways and modulation mechanism were discussed. It will provide more research evidences for further exploration on the mechanism of dental pulp injury repair and regeneration treatment.
Materials and Methods
Human DPSCs Isolation, Identification and Osteogenic Differentiation
Fifteen impacted mandibular third molars fully extracted from healthy persons (18–29 years old) were collected for DPSCs isolation. The protocol was approved by the ethic committee of Qingdao Stomatological Hospital.
Sterilized the teeth with 75% alcohol, split crowns to open the pulp cavity and removed the dental pulp tissue, followed by washing with PBS and then cut into 1 mm3 fragments. Digested the fragments at 37 °C using 0.3% type I Collagenase (Gibco, USA) and 0.4% Dispase (Gibco, USA) for 45 min. Add alpha-mem medium (α-MEM, Gibco, USA) containing 20% fetal bovine serum (FBS, Gibco, USA) to stop digestion. After centrifugation at 1000 rpm for 5 min and resuspended with growth medium (α-MEM containing 10% FBS, 1% penicillin-streptomycin, Gibco, USA), the explanted cells were cultured at 37 °C in a humidified 5% CO2 condition. Replaced fresh medium every 48 h until the cells were approximately 80% confluent and then subcultured. The 4th generation cells were used for experiments. Cell surface antigen molecules CD34, CD44, CD45, CD80, CD90 and CD105 were identified by flow cytometry analysis with monoclonal antibodies purchased from eBioscience (USA).
To induce osteogenic differentiation, the cells were maintained in a dexamethasone osteogenic medium, which consisted of growth medium supplemented with 200 nM dexamethasone, 50 μg/ml vitamin C and 5 mM β glycerophosphate (Sigma, USA).
Alizarin Red Staining
After osteogenic differentiation induction, cells were fixed with cold methanol for 20 min, and than stained with 1% alizarin red S solution (sigma, USA) for 5 min at room temperature to visualize the calcium deposition.
Drug Preparation and Treatment
Berberine (Sigma, USA), Gefitinib (Sigma, USA), U0126 (Selleck, USA) and SB203580 (Selleck, USA) were dissolved respectively in dimethylsulfoxide (DMSO, Sigma, USA) to a stock solution of 10 mM. The stock solution was diluted according to the working solution concentration ratio before used, and the final concentration of DMSO in the medium was <0.1% (v/v).
Alkaline Phosphatase (ALP) Activity Assay
Cells were seeded in 48-well-plates (1 × 105/well). At the day 4, 7 and 14 after BBR treatment on osteogenic differentiation induction, the cells were lysed with an alkaline lysate (Thermo Scientific, USA). Protein concentrations were detected by BCA protein assay kit (Thermo Scientific, USA), and the ALP activity was performed using a PNPP (p-nitrophenyl phosphate) substrate kit according to user manual (Thermo Scientific, USA).
CCK-8 Assay
Cells were seeded in 96-well-plates (1 × 104/well). At the 1th, 3th, 5th, and 7th days after incubation with BBR, 10 μl of CCK8 solution (Solarbio, China) was added to each well followed by a further incubation for 45 min at 37 °C. Then the absorbance was measured at 450 nm and results were statistically analyzed in triplicates.
Colony Formation Assay
Cells were seeded in 35-mm dishes (1 × 104/well) and cultured in growth medium containing BBR or not for 14 days to allow colony formation. After fixed with 4.0% paraformaldehyde, the surviving colonies were stained with 0.4% crystal violet to be visible and counted.
Spheroid Formation Assay
Cells were seeded in ultralow attachment 6-wells-plates and cultred in spheroid formation medium (α-MEM containing 2% B27, 20 ng/ml EGF, 20 ng/ml bFGF and 4μg/ml heparin). After culture of 14 days, spheres with more than 100 μm in diameter were counted in random fields of vision captured by an optical microscope (Olympus, Japan).
Quantitative Polymerase Chain Reaction (qPCR)
Trizol reagent (Invitrogen, USA) was used for total RNA extraction. After concentration measurement by a micro nucleic acid analyzer (Nanodrop 2000, Thermo Fisher, USA), 2 μg of total RNA was reversely transcribed by Advantage® RT-for-PCR Kit (Clontech, USA). A SYBR green fluorescence probe (Takara, Japan) and the 2-ΔΔCt method were used to quantify the relative mRNA expression of genes normalized to internal reference GAPDH by qPCR technology on a thermocycle instrument (7500, Applied Biosystems, USA). Primers used were listed in Table 1.
Western Blot
RIPA lysis buffer (ThermoScientific, USA) was used for the extraction of total proteins and a BCA assay was performed for concentration quantitation. 30 μg total proteins were separated by Sodium Dodecyl Sulphate-PolyAcrylamide Gel Electrophoresis (SDS-PAGE), and then transfered to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Germany). After blocked with 5% skim milk (m/v) for 1 hour at room temperature, the membrane was incubated with primary antibodies at 4 °C overnight. Next day, after washed three times with TBST, the membranes were incubated with the secondary antibody incubated for 1 h at room temperature. Finally, an ECL reagent (Thermo, USA) was used to develop the protein bands. All primary antibodies used were purchased from Cell Signaling Technology (CST, USA) or Abcam (England): EGFR(#4267, CST), p-EGFR(#3777, CST), MEK(#4694, CST), p-MEK(#9154, CST), ERK1/2(#4696, CST), p-ERK1/2(#4370, CST), p38-MAPK(#8690, CST), p-p38-MAPK(#9216, CST), Tubulin(#2148, CST), Runx-2(ab76956, Abcam), OSX(ab22552, Abcam), OPN(ab69498, Abcam), OCN(ab13420, Abcam), BMP2(ab14933, Abcam).
Statistical Analysis
Mean ± standard deviation (SD) was calculated as descriptive statistics. Experiments were conducted at least 3 times independently and analyzed by One-way ANOVA or Student t test. p < 0.05 was considered statistically significance.
Results
Identification of hDPSCs and Osteogenic Differentiation Induced by Dexamethasone
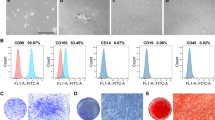
After expanded culturing, some fibroblast-like cells were observed under the optical microscope (Fig. 1a). Cell surface antigen molecules of the isolated hDPSCs were identificated by flow cytometry analysis, and results showed that the cells were positive for CD44, CD90, CD81 and CD105 but negative for hematopoietic stem cells markers CD34 and CD45 (Fig. 1b), which were similar immunophenotype to the bone mesenchymal stem cells (BMSCs). To further determine the stem cell differentiation potential of the isolated cells, dexamethasone osteogenic differentiation and adipogenic differentiation experiments were carried out. After 14 days of induction, alizarin red staining and oil red-O staining results suggested that the isolated hDPSCs could successfully induced into osteoblasts (Fig. 1c) and adipocytes (Fig. S1).
Identification of the primary isolated human DPSCs. a Representative images of hDPSCs under the optical microscope. b Cell surface antigen molecules (CD44, CD34, CD45, CD90, CD81 and CD105) identification by flow cytometry analysis. c Alizarin red staining for calcium deposition analysis of hDPSCs after osteogenic differentiation induced by dexamethasone-medium (DM). Cells cultured with routine medium (RM) was considered as control
Berberine Promotes Cell Proliferation and Self-Renewal of hDPSCs
The effect of BBR on the proliferation of hDPSCs was mainly identified by CCK8 and colony forming experiments. The cells were treated in two concentrations of BBR, 1 μM and 5 μM respectively, and the control group cells were treated with 0.1% DMSO.CCK8 results showed that the cell viability in BBR treated groups were significantly improved compared with the control group in every detected time points (Fig. 2a). After 14 days of culturing, the clone forming efficiencies of cells in BBR treated groups were significantly higher than that in DMSO control group, moreover, the 5 μM group was significantly higher than 1 μM group (Fig. 2b). The spheroid formation assay for self-renewal ability of stem cells was revealed that the spheroid forming efficiency of hDPSCs was increased in the BBR treated group compared to the DMSO group (Fig. 2c). These results indicated that BBR promotes cell proliferation and self-renewal of hDPSCs.
Berberine promoted hDPSCs proliferation and self-renewal. hDPSCs were cultured with growth medium containing 1, 5 μM Berberine or 0.1% DMSO for 2 weeks. a CCK-8 assay analysis for the cell viability at the 1, 3, 5, 7 days of induction culture. b Colony forming efficiency analysis for the cell proliferative potentials and (c) spheroid formation capacity analysis at the end of the experiments. * p < 0.05
Berberine Facilitated Dexamethasone-Induced Osteogenic Differentiation of hDPSCs
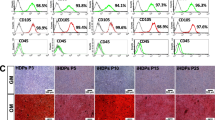
The hDPSCs were induced for osteogenic differentiation with dexamethasone medium containing Berberine (1 or 5 μM) or 0.1% DMSO. Results showed that, with induced-time going, the ALP activity was significantly enhanced by BBR in a dose-dependent pattern (Fig. 3a). Alizarin red staining analysis for cells after 14 days differentiation induction also showed that the calcium deposition was increased remarkably in BBR treated groups compared to DMSO group, especially with a higher dose of BBR (Fig. 3b). However, the oil red-O staining analysis showed that the lipid droplets in the cells treated with BBR were decreased obviously, especially in the group with a higher dose (Fig. S2), indicating a reverse anti-adipogenic activity of BBR on DPSCs. The mRNA and protein levels of osteogenic differentiation-related genes, osterix (OSX), osteopontin (OPN), osteocalcin (OCN), bone morphogenetic protein 2 (BMP-2) were significantly upregulated with BBR treatment, especially in the 5 μM BBR group (Fig. 3c and d). A transcription factor for the expression of osteogenesis genes, Runx2 was also obviously increased with BBR treatment (Fig. 3c and d). All these results suggested that Berberine facilitated dexamethasone-induced osteogenic differentiation of hDPSCs.
Berberine facilitated dexamethasone-induced osteogenic differentiation of hDPSCs. hDPSCs were cultured with dexamethasone medium containing 1, 5 μM Berberine or 0.1% DMSO for 2 weeks. a ALP activity analysis at the 4, 7, 14 days of induction culture. b Alizarin red staining for calcium deposition analysis. c qPCR analysis for the mRNA expression levels of osteogenesis-related genes. d Western blot analysis for the protein expression levels of osteogenesis-related markers. * p < 0.05, ** p < 0.01, ***p < 0.001
Berberine Increased the Runx2 Expression through Activating EGFR-MAPK Pathways
We have verified that BBR upregulated the expression of Runx2 and promoted the transcription of osteogenesis genes. Previous reports found that BBR could enhance the transcription activity of Runx2 via EGFR and MAPK pathways in PLSCs or BMSCs osteogenic differentiation [11, 13] So we also analyzed these pathways in the BBR induced osteogenic differentiation of hDPSCs. Results showed that the phosphorylation levels of EGFR, MEK, ERK1/2, and p38MAPK were significantly increased in the BBR group, indicating that the EGFR and MAPK pathways were activated by BBR treatment (Fig. 4).
Inhibitors, Gefitinib, U0126 or SB203580, Reversed the Promotion Effects of Berberine on hDPSCs Osteogenic Differentiation
To further verify the roles of EGFR and MAPK pathways in BBR treated osteogenic differentiation, we used inhibitors Gefitinib for EGFR, U0126 for MEK and SB203580 for p38 MAPK to jointly treat with BBR. Results showed all the three inhibitors effectively suppressed the upregulation of Runx2 and osteogenesis genes or proteins induced by BBR (Fig. 5b and c) through inhibiting respectively the phosphorylation levels and activation states of EGFR, MEK or p38MAPK successfully (Fig. 5a), further indicating BBR might promote hDPSCs osteogenic differentiation via EGFR-MAPK signaling pathways.
Discussion
BBR is a plant alkaloid and has a long history of medicinal use in Chinese and Ayurvedic medicine, and it is mainly used for gastroenteritis and bacillary diarrhea [14], But with the further research, BBR has been reported has broad prospects in the fields of lowering blood lipid [15], therapy for diabetes [16, 17], anti-inflammatory [18], anti-tumor [19, 20] and stomatology [9].
In this study, we focused on the role of BBR in osteogenic differentiation of hDPSCs. Previous researches indicated that BBR could promote osteogenic differentiation of BMSCs [11, 12] and PLSCs [13], and it could also promote proliferation of rat chondrocytes and osteoarthritic rat cartilage [21]. Whereas, BBR suppressed the expression of adipogenic related marker genes in BMSC [12] and efficiently inhibited adipogenesis of 3 T3-L1 cells [12]. To detect the effect of BBR on hDPSCs proliferation, osteogenic differentiation and adipogenic differentiation, we performed CCK8 assay, ALP activity assay, alizarin red staining, oil red-O staining and colony forming unit assay, spheroid formation assay, qPCR and WB. All the results indicated that BBR could significantly enhances the cell proliferation and self-renewal of hDPSCs and promotes osteogenic differentiation (from Figs. 2 to 3) but suppressed adipogenic differentiation. All these results indicate that the pluripotency of hDPSCs are very similar to the MSCs, and the effects of Berberine on osteogenic and adipogenic differentiation potential of the both are also consistent amazingly.
Runx2 is the crucial transcription factors for osteogenic differentiation especially at early stage [22,23,24], and the MAPK signaling pathways play a vital role in the activating of Runx2. MAPKs are a set of well described extracellular signal-regulated kinases (ERKs), c-Jun amino-terminal kinases (JNKs) and p38 MAPK [25]. both ERKs and p38 MAPKs are crucial in the runx2 activation process [24, 26,27,28], and some reports show JNKs also play a significant role in this process [29, 30]. As mentioned above, it was reported that BBR could promote osteogenic differentiation of PLSCs by activating the EGFR-ERK-FOS signaling pathway [13] and promote osteogenic differentiation of MSCs by activating Runx2 via the p38 MAPK signaling pathway [11]. In order to further understand the osteogenic differentiation mechanism, we detected the phosphorylation of EGFR, MEK, ERK and p38 MAPK by WB. Here we report for the first time that BBR activates the EGFR-MAPK signaling pathways in hDPSCs (Fig. 4).
Berberine activated EGFR-MAPK pathways. a Western blot analysis for proteins EGFR, MEK, ERK, p38MAPK and their phosphorylation levels in hDPSCs treated with 5 μM Berberine or 0.1% DMSO. b The relative phosphorylation levels of EGFR, MEK, ERK and p38MAPK normalized to each total protein expression levels calculated by gray scanning showed in A. * p < 0.05, ** p < 0.01
To verify whether these molecules were key molecules, we combined the EGFR inhibitor Gefitinb, MEK inhibitor U0126 and p38 inhibitor SB203580 with BBR. The results showed that all the three inhibitors alone inhibit BBR-induced osteogenic differentiation (Fig. 5). When EGFR was inhibited by gefitinb, both downstream MEK and p38 were inhibited, whereas EGFR had no effect when MEK and p38 inhibitors were incubated (Fig. 5). This suggests that EGFR might be a possible receptor for BBR on the surface of hDPSCs membrane, and BBR can activate the downstream signaling pathways through EGFR. In summary, we first discovered that BBR could interact with EGFR on the surface of hDPSCs and activate the MAPK pathways to promote osteogenic differentiation of hDPSC.
Inhibitors reversed the promotion effects of Berberine on hDPSCs osteogenic differentiation. hDPSCs were pre-treated respectively with Gefitinib (5 μM), U0126 (10 μM) and SB20580 (10 μM) for 2 h before osteogenic differentiation induction with dexamethasone combined Berberine (5 μM) for 2 weeks. a Western blot analysis for proteins EGFR, MEK, ERK, p38MAPK and their phosphorylation levels. b qPCR analysis for mRNA expression levels and (c) western blot analysis for proteins expression levels of Runx2 and osteogenesis markers. * p < 0.05, ** p < 0.01, ***p < 0.001
Periodontitis is one of the most common diseases. Controlling dental plaque and eliminating inflammation are the main treatment measures at present, but it can not achieve periodontal regeneration. Periodontal regeneration materials are placed in periodontal lesions, and bacterial infection may lead to the failure of periodontal regeneration. Therefore, controlling microbial infection and promoting periodontal regeneration is the key to periodontitis therapy. Enterococcus faecalis and Actinobacillus actinomycetes all have antibacterial effect and are excellent drugs for the treatment of periodontitis. Previous reports indicated BBR has antibacterial effect on Fusobacterium nucleatum, Enterococcus faecalis, and Prevotella intermedia [9], and hDPSCs is a potential material for bone regeneration [31]. Our study report at the first time, that BBR can enhance the osteogenic differentiation, proliferative and clonogenic of hDPSC, and BBR could interact with EGFR on the surface of hDPSCs and activate the MAPK pathways to promote osteogenic differentiation of hDPSC. In addition, BBR has antibacterial and inducing effects, it has great potential clinical application value in pulp tissue regeneration and periodonotitis treatment. The mechanism of the effect of BBR on hDPSCs needs further study.
References
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (Dpscs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Valverde Y, Narayanan R, Alapati SB, Chmilewsky F, Huang CC, Ravindran S, Chung SH (2018) Poly(adenosine phosphate ribose) polymerase 1 inhibition enhances brain-derived neurotrophic factor secretion in dental pulp stem cell-derived Odontoblastlike cells. J Endod 44:1121–1125
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83:590–595
Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S (2018) Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J Dent Res 97:1137–1143
El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK (2008) Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J 34:52–67
de Souza PV, Alves FB, Costa Ayub CL, de Miranda Soares MA, Gomes JR (2013) Human immature dental pulp stem cells (Hidpscs), their application to cell therapy and bioengineering: an analysis by systematic revision of the last decade of literature. Anat Rec (Hoboken) 296:1923–1928
Chopra RN, Dikshit BB, Chowhan JS (1932) Berberine and Berberine-containing plants in pharmacology and therapeutics. Ind Med Gaz 67:194–197
Habtemariam S (2016) Berberine and inflammatory bowel disease: a concise review. Pharmacol Res 113:592–599
Xie Q, Johnson BR, Wenckus CS, Fayad MI, Wu CD (2012) Efficacy of Berberine, an antimicrobial plant alkaloid, as an endodontic Irrigant against a mixed-culture biofilm in an in vitro tooth model. J Endod 38:1114–1117
Wei P, Jiao L, Qin LP, Yan F, Han T, Zhang QY (2009) Effects of Berberine on differentiation and bone resorption of osteoclasts derived from rat bone marrow cells. Zhong Xi Yi Jie He Xue Bao 7:342–348
Lee HW, Suh JH, Kim HN, Kim AY, Park SY, Shin CS, Choi JY, Kim JB (2008) Berberine promotes osteoblast differentiation by Runx2 activation with P38 Mapk. J Bone Miner Res 23:1227–1237
Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H (2016) Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/Beta-catenin signaling pathway. Toxicol Lett 240:68–80
Liu J, Zhao X, Pei D, Sun G, Li Y, Zhu C, Qiang C, Sun J, Shi J, Dong Y, Gou J, Wang S, Li A (2018) The promotion function of Berberine for osteogenic differentiation of human periodontal ligament stem cells via Erk-Fos pathway mediated by Egfr. Sci Rep 8:2848
Chang Y (1959) Effectiveness of Berberine in bacillary dysentery. Zhonghua Nei Ke Za Zhi 7:741–743
Dong H, Zhao Y, Zhao L, Lu F (2013) The effects of Berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med 79:437–446
Ma X, Chen Z, Wang L, Wang G, Wang Z, Dong X, Wen B, Zhang Z (2018) The pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by Berberine. Front Pharmacol 9:782
Pang B, Zhao LH, Zhou Q, Zhao TY, Wang H, Gu CJ, Tong XL (2015) Application of Berberine on treating type 2 diabetes mellitus. Int J Endocrinol 2015:905749
Wu X, Li X, Dang Z, Jia Y (2018) Berberine demonstrates anti-inflammatory properties in helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor. J Cell Biochem 119:5373–5381
Chuang TY, Wu HL, Min J, Diamond M, Azziz R, Chen YH (2017) Berberine regulates the protein expression of multiple tumorigenesis-related genes in hepatocellular carcinoma cell lines. Cancer Cell Int 17:59
Tsang CM, Cheung YC, Lui VW, Yip YL, Zhang G, Lin VW, Cheung KC, Feng Y, Tsao SW (2013) Berberine suppresses Tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting Stat3 activation induced by tumor associated fibroblasts. BMC Cancer 13:619
Zhou Y, Tao H, Li Y, Deng M, He B, Xia S, Zhang C, Liu S (2016) Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/Beta-catenin pathway. Eur J Pharmacol 789:109–118
Feng G, Zhang J, Feng X, Wu S, Huang D, Hu J, Zhu S, Song D (2016) Runx2 modified dental pulp stem cells (Dpscs) enhance new bone formation during rapid distraction osteogenesis (do). Differentiation 92:195–203
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Artigas N, Urena C, Rodriguez-Carballo E, Rosa JL, Ventura F (2014) Mitogen-activated protein kinase (Mapk)-regulated interactions between Osterix and Runx2 are critical for the transcriptional osteogenic program. J Biol Chem 289:27105–27117
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by Erk, Jnk, and P38 protein kinases. Science 298:1911–1912
Lee KS, Hong SH, Bae SC (2002) Both the Smad and P38 Mapk pathways play a crucial role in Runx2 expression following induction by transforming growth factor-Beta and Bone morphogenetic protein. Oncogene 21:7156–7163
Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2007) Mechanical stress-mediated Runx2 activation is dependent on Ras/Erk1/2 Mapk signaling in osteoblasts. J Cell Biochem 101:1266–1277
Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT (2009) Identification and functional characterization of Erk/Mapk phosphorylation sites in the Runx2 transcription factor. J Biol Chem 284:32533–32543
Huang RL, Yuan Y, Tu J, Zou GM, Li Q (2014) Opposing Tnf-alpha/Il-1beta- and bmp-2-activated Mapk signaling pathways converge on Runx2 to regulate bmp-2-induced osteoblastic differentiation. Cell Death Dis 5:e1187
Huang YF, Lin JJ, Lin CH, Su Y, Hung SC (2012) C-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by Bmp2 via phosphorylation of Runx2 at Ser104. J Bone Miner Res 27:1093–1105
Fujii Y, Kawase-Koga Y, Hojo H, Yano F, Sato M, Chung UI, Ohba S, Chikazu D (2018) Bone regeneration by human dental pulp stem cells using a Helioxanthin derivative and cell-sheet technology. Stem Cell Res Ther 9:24
Acknowledgments
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This work was supported by Qingdao Medical Science and Technology Guiding Project (No.2017-WJZD125), Qingdao Shinan District Science and Technology Development Fund (No.2016-3-042-YY) and Qingdao Stomatological Hospital Youth Fund Project (No.2018QN01, No.2019QNJJ02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Materials
Figure. S1
The adipogenic differentiation of hDPSCs. Oil red-O staining analysis for the lipid droplets formation in the hDPSCs after 14 days induction by special adipogenic differentiation induction medium (LM) containing 10% FBS, 1 mg/ml insulin, 1 mM dexamethasone, 0.5 mM isobutyl methylxanthine and 100 mM indomethacin. Cells cultured with routine medium (RM) was considered as control. (PNG 371 kb)
High resolution image
(TIF 974 kb)
Figure. S2
The effects of Berberine on the adipogenic differentiation of hDPSCs. hDPSCs were cultured with special adipogenic differentiation induction medium containing 1, 5 μM Berberine or 0.1% DMSO for 14 days, and Oil red-O staining was used to analyzed the lipid droplets formation. (PNG 542 kb)
High resolution image
(TIF 1445 kb)
Rights and permissions
About this article
Cite this article
Xin, BC., Wu, QS., Jin, S. et al. Berberine Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells Through Activating EGFR-MAPK-Runx2 Pathways. Pathol. Oncol. Res. 26, 1677–1685 (2020). https://doi.org/10.1007/s12253-019-00746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00746-6