Abstract
Surgical resection is the most effective treatment for early stage lung adenocarcinoma. However, the rate of 5-year postoperative recurrence reaches 30%, Spread Through Air Spaces(STAS) is a recently described novel invasive pattern of lung cancer, According to the 2015 WHO classification. STAS is defined as“micropapillary clusters, solid nests, or single cells spreading within air spaces beyond the edge of the main tumor, However, the prognostic role of STAS in lung cancer is not known, The aim of the current study is to evaluate the association between STAS and clinical outcome of lung cancer patients after surgical resection through a meta-analysis. Systematic research was conducted using three online databases to search for studies published before August 1, 2018. The 5-year RFS and OS for non-small cell lung cancer patients after surgical resection with or without STAS were compared. The studies were selected according to rigorous inclusion and exclusion criteria. Meta-analysis was performed using hazard ratio (HR) and 95% confidence intervals (CIs) as effective measures. Included in our meta-analysis were 12 studies, published from 2015 to 2018, with a total of 3564 patients. Our results clearly depicted that the presence of STAS predicted a worse outcome for 5-year RFS with the combined HR of 1.84(95% CI: 1.59–2.12). Meta-analysis of these 8 studies showed that patients with the presence of STAS were associated with shorter 5-year OS (the pooled HR:1.78, 95% CI: 1.51–2.11). This meta-analysis illuminated that the presence of STAS might be a unfavorable prognostic factor for patients with NSCLC. it should be paid sufficient attention and recorded in pathologic reports, which can indicate treatment choice and prognosis of patients. In future, more studies with well-designed and large-scale are needed to confirm the conclusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the major cancer type for the estimated new cancer cases, lung cancer is the leading cause of cancer related death worldwide [1,2,3]. It’s prognosis still remains unsatisfactory; Although molecular-targeted therapy and immunotherapy have greatly improved the prognosis of patients with advanced lung cancer. Only surgical resection can provide a cure for lung cancer; specifically, for early-stage patients with pathological stage I non-small cell lung cancer (NSCLC).However, the 5-year postoperative recurrence rate reaches 30% [4]. Some prognostic indicators that negatively affect the prognosis after surgical resection have been identified, including solid tumour size, proportion of ground-glass opacity (GGO), pleural invasion, lymphovascular invasion, serum carcinoembryonic antigen (CEA) level and positron emission tomography (PET)/computed tomography (CT) findings [5]. Further prognostic indicators are sought to stratify patients with early-stage NSCLC more accurately and to determine optimum surgical procedures, such as lobectomy or limited resection, and adjuvant therapy.
An increasing number of studies have indicated that STAS is a new form of invasion in lung adenocarcinoma [5,6,7,8,9,10], First, Kadota et al. [6] reported that STAS was a significant risk factor of recurrence in small-sized adenocarcinomas treated with limited resection but not in those who underwent lobectomy. However, according to the reports by Uruga et aland Warth et al., STAS was significantly associated with a worse RFS and OS in patients with early-stage and stage I-IV adenocarcinoma that had been resected [7, 11]. According to their results, The prognostic significance of STAS in surgically resected lung cancer is still controversial. Therefore, it is necessary to perform a meta-analysis to comprehensively and systematically understand the prognostic value of STAS in lung cancer patients after surgical resection. In this study, we aimed to assess the prognostic significance of the presence of STAS in non-small cell lung cancer (NSCLC)。.
Material and Methods
Search Strategy
We performed a comprehensive literature search of articles through the following databases without date limitation: PubMed, The Cochrane Library and Web of Science databases. The search was updated to August 1, 2018. The main search terms included: “STAS” (e.g., “spread through air space,“and“spread through air spaces,”) and “lung cancer” [e.g., “lung neoplasm”, “lung carcinoma”,“non-small cell lung cancer (NSCLC),” “small cell lung cancer (SCLC)”].
The reference list was also checked for relevant articles.
Inclusion and Exclusion Criteria
Inclusion criteria for selecting the studies for this meta analysis were as follows criteria: (1) studied patients with NSCLC were pathological examination confirmed; (2) STAS was confirmed by pathological examination; (3) studied patients underwent primary curative surgical resection; (4) correlation of STAS with overall survival (OS) and/or Recurrence-free survival (RFS) and/or disease-free survival (DFS) was reported. Exclusion criteria were as follows: (1) abstracts, letters, case reports, reviews or nonclinical studies; (2) patients who underwent neoadjuvant chemotherapy and those with incomplete resection; (3) studies were not written in English; (4) studies with insufficient data for estimating hazard ratio (HR) and 95% confidence interval (CI); (5) patients were not non-small cell lung cancer (NSCLC).
Data Extraction and Quality Assessment
The following data were extracted by two independent investigators (Qifan Yin and Guang Yang); first author, publication year, nation, number of participants,participants characteristics (age, gender, the state of STAS, cancer type, stage, prognosis) and HRs with 95% CIs. Articles that could not be categorized based on title and abstract alone were retrieved for full-text review. If disagreement occurred, two investigators discussed and reached consensus with a third investigator (Peng Qie). The Newcastle-Ottawa Scale (NOS) was used to assess each of the included studies quality by two independent authors (Qifan Yin and Guang Yang). The NOS consists of three parts: selection(0-4points), comparability (0–2 points), and outcome assessment (0–3 points). NOS scores of≥6 were regarded as high-quality studies.
Statistical Analysis
All statistical analyses were conducted using Review Manager 5.3(The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 software (STATA, College Station, TX). We directly obtained HR and 95%CI from each literature or estimated these data, HR > 1 indicated a worse prognosis in lung cancer patients with the presence of STAS. Cochran’s Q test and Higgins I-squared statistic were undertaken to assess the heterogeneity of the included trials. Both fixed-effects (Mantel-Haenszel method) and random effects (DerSimonian - Laird method) models were used to calculate the pooled HRs and 95%CIs. A Pheterogeneity<0.10 or I2 > 50% suggested significant heterogeneity in the literature and a random-effect model was used. Otherwise, the fixed-effects model was adopted. Subgroup analysis was conducted to explore and explain the diversity (heterogeneity) among the results of different studies. Publication bias was assessed by Begg’s funnel plot and Egger’s linear regression test.19.All p-values were two-sided. The p < 0.05 was considered statistical significant.
Results
Study Characteristics
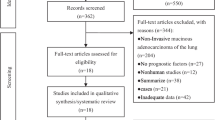
A total of 108 articles were collected in the initial search. After carefully inspection of these articles, 12 studies including 3564 patients published between 2015 and 2018 were finally enrolled in our meta-analysis.The detail processes of study selection were showed in the flow diagram (Fig. 1). Among them, nine studies were from Japan, one study was performed in The United States, China and German, respectively. HRs and 95%CIs were extracted directly in 12 studies, all of which calculated HRs by multivariate analysis.The proportion of the positive of STAS exceeded 30% in 9 studies. In our meta-analysis, 5 studies included only stage I disease, 4 studies included stage I-III disease, 2 studies involved all disease stages, 1 study included only the positive of lymph-node patients (N1/N2).In term of pathological pattern,8 studies were lung adenocarcinoma, 3 studies were lung squamous cell carcinoma, only one study was non-small cell lung cancer. All studies illuminated the association between STAS and OS, RFS. The characteristics of the enrolled studies were shown in Table 1:
STAS and OS,RFS in NSCLC
Eight studies presented the data to evaluated the association STAS and OS. Twelve studies presented the data to evaluated the relation between STAS and RFS. Considering the little heterogeneity (I2 = 36.7%, P = 0.082).Therefore, a fixed-effects model was applied.Our results clearly depicted that the presence of STAS predicted a worse outcome for 5-year RFS with the combined HR of 1.84(95% CI: 1.59–2.12; Fig. 2). Meta-analysis of these eight studies showed that patients with the presence of STAS were associated with shorter 5-year OS (HR obtained from fixed effects model: 1.78, 95% CI: 1.51–2.11; Fig. 2) with little heterogeneity (I2 = 15.8%, P = 0.302).
STAS and 5-Year RFS in Lobectomy
Only two studies were classified by surgical type, HR and 95%CI could be completely acquired.although other studies were categorized by surgical type,which did not provide complete HR and 95%CI.Therefore,only two studies presented the data to evaluated the association STAS and RFS in lobectomy. Meta-analysis of these two studies revealed that patients with the presence of STAS were not associated with 5-year RFS with combined HR:1.67, 95% CI: 0.93–2.68, Fig. 3) without heterogeneity (I2 = 0.0%, P = 0.642).
STAS and 5-Year RFS in Limited Resection
Three studies presented the data to evaluated the relation between STAS and 5-year RFSin limited resection with little heterogeneity (I2 = 30.4%,P = 0.237), So a fixed-effect model was applied.Our result clearly illuminated that patients with the presence of STAS were associated with shorter 5-year RFS in limited resection. (combined HR:4.05,95%CI:2.31–7.09, Fig. 4).
Publication Bias
Publication bias was evaluated by Begg’s funnel plot and Egger’s linear regression test, In OS group,Publication bias was not found in our study (P = 0.175 > 0.05 for Begg’s test).But in RFS group,there existed publication bias with P = 0.006 < 0.05 for Begg’s test. The picture of publication bias was shown in Fig. 5.
Discussion
Our meta-analysis included the prognosis of 3564 lung cancer patients from 12 individual studies, indicating that the presence of STAS significantly predicted poor 5-year RFS (HR:1.84, 95%CI: 1.59–2.12, Fig. 2) of NSCLC patients. There were 8 studies showing the data of association between STAS and OS in NSCLC patients. The pooled HR of 1.78 (95% CI: 1.51–2.11, Fig. 2) showed that patients with the positive of STAS were expected to have shorter 5-year OS after treatment. Taking all these in to consideration, The presence of STAS in surgically resected NSCLC patients may be have a significant risk factor for recurrence and a poor prognostic factor for overall survival.
In our current study, STAS was observed in 282 patients among 620 patients from two studies in the lobectomy group. The patients with STAS had a trend of shorter 5-year RFS than patients without STAS with pooled HR:1.67, 95% CI: 0.93–2.68, in the lobectomy groups, but no statistical significance was observed. As a matter of fact, The prognostic significance of STAS remains controversial in patients who underwent lobectomy, Kadota et al. reported that STAS was a crucial risk factor of recurrence in stage I adenocarcinomas treated with limited resection but not in those who underwent lobectomy [6]. From our outcome, the presence of STAS was inclined to increase the recurrence rate in the lobectomy group, but no statistical significance was observed. However, in Dai et al. study, 95% of patients underwent lobectomy, but the influence of STAS on recurrence and survival was still unfavorable [10].several studies also demonstrated the negative impact of STAS in patients who underwent lobectomy [5, 18, 19]. Shiono and Yanagawa also revealed that STAS is an independent risk factor for recurrence, even after excluding patients who underwent limited resection [14, 16]. Taken together, these data collectively suggest that STAS is an independent risk factor for recurrence, independent of type of surgery.
We revealed that STAS was a significant risk factor for recurrence in the limited resection group. The presence of STAS was associated with shorter 5-year RFS in limited resection. (combined HR:4.05,95%CI:2.31–7.09). Kadota et al. first reported that STAS was a significant risk factor for recurrence in patients with ≤2 cm stage I lung adenocarcinoma who underwent limited resection [6]. Shiono et al. also revealed that the patients with STAS undergoing limited resection had a higher rate of pulmonary metastasis than those with STAS undergoing lobectomy [16]. It suggested that the lung cancer with STAS might easily spread via spaces. Therefore, limited resection should be avoided for the patients with the presence of STAS, our conclusions need be confirmed by further studies.
As early as in 1995, aerogenous spread had been recognized as the presence of isolated clusters of tumor cells in the alveolar space, at that time aerogenous spread was neglected and had not been well studied. In 2015, Kadota et al. first reported that STAS was a significant risk factor for recurrence in patients with ≤2 cm stage I lung adenocarcinoma who underwent limited resection (including wedge resection, segmentectomy) but not in those who underwent lobectomy. According to the 2015 WHO classification, STAS is defined as“micropapillary clusters, solid nests, or single cells spreading within air spaces beyond the edge of the main tumor [6]. Dai et al. [10] showed that patients with stage IA adenocarcinomas with tumor larger than 2 to 3 cm showing STAS had an unfavorable prognosis comparable to stage IB patients with or without STAS, which suggested that STAS might be a factor to upgrade T stage. This result contributes to the accuracy of predicting the outcome of early-stage adenocarcinomas. More importantly, patients with ADC larger than 2-3cmwith STAS might benefit from postoperative chemotherapy.
STAS is also related to recurrence and survival in patients with squamous cell lung carcinoma [12, 20, 21]. Lu et al. [20] investigated 445 cases of surgically resected stages I to III squamous cell lung carcinoma and identified STAS in specimens from 30% of the patients. The patients with STAS had higher rates of recurrence and cancer-specific death than the patients without STAS, the difference in overall survival between patients with and without STAS was not significant. Yanagawa et al. [22] showed that the patients with STAS had a worse RFS and OS than the patients without STAS in the sub-lobar resection group. In the lobectomy group, the patients with STAS had a worse RFS than the patients without STAS. The patients with STAS had a trend of worse OS than patients with STAS in the lobectomy groups, but no statistical significance was observed. Therefore, the presence of STAS is a promising prognostic marker helpful for the clinical decision-making process regarding lung cancer treatment and prognosis in SQCC.
Some limitations of our meta-analysis should be addressed. First, the enrolled studies were retrospective, which contributed to that some bias were inevitable. Second,our meta-analysis only enrolled studies published in English language.So,it was more likely to appear publication bias. In RFS group, there existed publication bias with P = 0.006 < 0.05 for Begg’s test.the possible causes contributed to this problem might be that the majority of studies are from Japan, even part of studies are from same author, therefore, Publication bias is inevitable. Third, we just included NSCLC patients in our meta-analysis, future studies are needed to cover SCLC patients to confirm our conclusion.
Conclusion
In conclusion, this meta-analysis illuminated that the presence of STAS might be a unfavorable prognostic factor for patients with NSCLC. it should be paid sufficient attention and recorded in pathologic reports, which can indicate treatment choice and prognosis of patients. In future, more studies with well-designed and large-scale are needed to confirm the conclusion.
Abbreviations
- STAS:
-
Spread through air spaces
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- ADC:
-
Adenocarcinoma
- SQCC:
-
Squamous cell carcinoma
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- SCLC:
-
Small cell lung cancer
References
Brundage MD, Davies D, Mackillop WJ (2002) Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 122(3):1037–1057
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Mino-Kenudson M, Mark EJ (2011) Reflex testing for epidermal growth factor receptor mutation and anaplastic lymphoma kinase fluoresce nce in situ hybridization in non-small cell lung cancer. Arch Pathol Lab Med 135(5):655–664
Hung J-J, Jeng W-J, Hsu W-H, Chou T-Y, Huang B-S, Wu Y-C (2012) Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non–small-cell lung Cancer. J Thorac Oncol 7(7):1115–1123
Shiono S, Yanagawa N (2016) Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 23(4):567–572
Kadota K, Nitadori J-I, Sima CS et al (2015) Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 10(5):806–814
Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H, Weichert W (2015) Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 39(6):793–801
Lu S, Tan KS, Kadota K et al (2016) Spread through air spaces (STAS) is an independent predictor of recurrence in lung squamous cell carcinoma. Mod Pathol 29:475A-A
Morales-Oyarvide V, Mino-Kenudson M (2016) Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol Int 66(1):1–7
Dai C, Xie H, Su H, She Y, Zhu E, Fan Z, Zhou F, Ren Y, Xie D, Zheng H, Kadeer X, Chen D, Zhang L, Jiang G, Wu C, Chen C (2017) Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma > 2 to 3 cm. J Thorac Oncol 12(7):1052–1060
Uruga H, Fujii T, Fujimori S, Kohno T, Kishi K (2017) Semiquantitative assessment of tumor spread through air spaces (STAS) in early-stage lung adenocarcinomas. J Thorac Oncol 12(7):1046–1051
Kadota K, Kushida Y, Katsuki N, Ishikawa R, Ibuki E, Motoyama M, Nii K, Yokomise H, Bandoh S, Haba R (2017) Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol 41(8):1077–1086
Toyokawa G, Yamada Y, Tagawa T, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Oda Y, Maehara Y (2018) Significance of spread through air spaces in resected pathological stage I lung adenocarcinoma. Ann Thorac Surg 105(6):1655–1663
Yanagawa N, Shiono S, Endo M, Ogata S-Y (2018) Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer (Amsterdam, Netherlands) 120:14–21
Toyokawa G, Yamada Y, Tagawa T, Kinoshita F, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Hirai F, Oda Y, Maehara Y (2018) Significance of spread through air spaces in resected lung adenocarcinomas with lymph node metastasis. Clin Lung Cancer 19:395–400.e1
Shiono S, Endo M, Suzuki K, Yarimizu K, Hayasaka K, Yanagawa N (2018) Spread through air spaces is a prognostic factor in sublobar resection of non-small cell lung Cancer. Ann Thorac Surg 106(2):354–360
Toyokawa G, Yamada Y, Tagawa T, Oda Y (2018) Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited rese ction. Thorac Cancer 9(10):1255–1261
Lu S, Tan KS, Kadota K, Eguchi T, Bains S, Rekhtman N, Adusumilli PS, Travis WD (2017) Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J Thorac Oncol 12(2):223–234
Eguchi T, Kameda K, Lu S et al (2017) OA07.06 in early-stage lung adenocarcinomas, survival by tumor size (T) is further stratified by tumor spread through air spaces. J Thorac Oncol 12(1, Supplement):S270-S1
Lu S, Eguchi T, Tan KS, Bains S, Kadota K, Rekhtman N, Adusumilli P, Travis W (2017) Tumor spread through air spaces (STAS) in lung squamous cell cancer is an independent risk factor: a competing risk analysis. J Thorac Oncol 12(1):S412–S4S3
Yanagawa N, Shiono S, Abiko M, Suzuki K, Yarimizu K (2017) The clinical impact of spread through air spaces (STAS) in surgically resected pStage I lung squamous cell carcinoma. J Thorac Oncol 12(1):S1119–S1S20
Yanagawa N, Shiono S, Endo M, Ogata SY (2018) Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer (Amsterdam, Netherlands) 120:14–21
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
There was no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Yin, Q., Yang, G. et al. Prognostic Impact of Tumor Spread Through Air Spaces in Non-small Cell Lung Cancers: a Meta-Analysis Including 3564 Patients. Pathol. Oncol. Res. 25, 1303–1310 (2019). https://doi.org/10.1007/s12253-019-00616-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00616-1