Abstract
To investigate the effect of ultrasound combined with expression of Galectin-3, c-Met, HBME-1 and CK19 in differentiating malignant from benign thyroid nodules. Forty-six patients with thyroid nodules were studied with ultrasound and immunohistochemical staining of excised thyroid nodules. The data were classified and compared. The immunohistochemical staining revealed 8 benign and 41 malignant thyroid lesions. In ultrasound risk assessment, the malignancy risk was low in four nodules, medium in five and high in 37 with lymphatic metastasis in 26. A significant (P < 0.05) association existed in the expression of Galectin-3 with nodule boundary and lymphatic metastasis, in HBME-1 with nodule micro-calcification and in c-Met with nodule micro-calcification and lymphatic metastasis. CK19 expression was not significantly (P > 0.05) associated with any of ultrasound features of nodule. Galectin-3, c-Met, HBME-1 and CK19 were significantly (P < 0.05) different in malignant and benign thyroid lesions, with a significant (P < 0.01) tendency in all the molecular markers in predicting the malignant from benign lesions. The ultrasound characteristics could significantly (P < 0.001) predict malignant nodules with a significant (P < 0.05) prediction tendency. The scores of Galectin-3, c-Met and CK19 significantly (P < 0.05) increased with increase of ultrasound malignancy risk degree. In malignant and benign lesions differentiated by ultrasound, no significant (P > 0.05) difference existed in HBME-1 expression, however, with ultrasound malignancy risk increase, the score of HBME-1 expression increased significantly (P = 0.03). Galectin-3, c-Met, HBME-1 and CK19 have significantly greater expressions in thyroid malignant than benign lesions and their expression increases with increase of ultrasound malignancy risk. The combination of both ultrasound and molecular markers can be used to differentiate malignant and benign thyroid lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules have been increasingly detected over the past decades, and accurate diagnosis of benign or malignant nature of these nodules is crucial to decrease patient risk and reduce the dramatic heal care costs because most of these nodules are benign or behave indolently [1]. Some sonographic features of thyroid nodules have been regarded as suggestive of malignancy including intra-nodule flow, absence of a halo, hypo-echogenicity, solidity and taller-than-wide shape [1]. From these characteristics, a dedicated thyroid reporting system has been developed to classify thyroid nodules and stratify the malignancy [2]. However, this reporting system is not accurate in evaluating the malignancy of thyroid nodules because of imperfect sonographic criteria in identifying malignant echo patterns [3]. The use of immunohistochemical markers have been suggested to aid in the differential diagnosis of thyroid nodules, including Galectin-3, c-MET HBME-1 and CK19 [4, 5]. High expression of these molecular markers can be used to assist malignancy diagnosis of thyroid nodules. This study was to evaluate the effect of ultrasound combined with immunohistochemical markers in determining the malignancy of thyroid nodules.
Materials and Methods
This study was approved by the ethics committee of our hospital with the informed consent obtained from all patients. Forty-six patients who were suspected of thyroid nodules and had thyroid sonography and surgery at our hospital between December 2015 and September 2016 were enrolled, including 11 male and 35 female patients with an age range of 21–77 years (mean 47.1 ± 13.1). The thyroid tissue was obtained from these patients at thyroidectomy. Forty-six solid nodules were found in these patients and had immunohistochemical staining for evaluation of Galectin-3, c-Met, HBME-1 and CK19 using the Maxvision one-step method with reagents from Maixin Biotechnological Company (Maixin, Fuzhou, China). The thyroid tissue blocks were fixed with formalin and embedded with paraffin for immunohistochemical studies. Three pathologists who had no clinical knowledge of the patients reviewed and scored the slides. The positive staining for Galectin-3, HBME-1 and CK19 was scored as 0 if the percentage of positive cells was 0–25%, 1 for 26%–50%, 2 for 51%–75% and 3 for 76%–100%. For c-Met staining, the score was 0 if no positive cells were observed, 1 for positive cells <35%, 2 for 35%–75% and 3 for >75% [4].
For sonography scan, the Philips IU22 (Philips, Best, the Netherlands) and Siemens ACUSON 200 (Siemens Medical Solutions, Issaquah, WA, USA) color Doppler ultrasound systems were used for evaluation of the thyroid nodules by two physicians. The degree of the risk of malignancy was categorized into low, medium and high risk. Lymphatic metastasis represented high risk. The malignancy indexes of the nodule ultrasound features were marked hypoechogenicity, aspect ratio > 1 (taller-than-wide shape), unclear or lobulated boundary, irregular shape, echo attenuation behind the nodule, micro-calcification and central blood flow. The presentation of two or fewer features stood for low risk of malignancy and was marked as 0, three to four features for medium risk marked as 1, and five to seven features for high risk marked as 2. Suspected nodule lymphatic metastasis was all marked as 2. If disparity existed in the categorization of the risks, a third physician would be involved for reaching an agreement.
Statistical Analysis
Statistical analysis was performed with the SPSS19.0 software package (SPSS Inc., Chicago, IL, USA). Categorical data were presented as percentages, and Chi-square test or Fisher’s exact test was used for evaluation of risks and difference of malignant and benign nodules. The significance was set at P < 0.05.
Results
Forty-six nodules had immunohistochemical staining, and the range of the maximal diameter of the nodules ranged 0.3 cm–5.0 cm. There were 8 benign and 41 malignant nodules revealed by immunohistochemical staining. In ultrasound risk assessment, the degree of malignancy risk was low in four nodules, medium in five nodules and high in 37 nodules (Fig. 1). Among the 37 nodules with high risk of malignancy, 26 nodules had lymphatic metastasis.
A thyroid nodule had neck lymphatic metastasis found in ultrasound in a female patient aged 25 years. A thyroid cystic solid nodule (a-c) was demonstrated with a size of about 4.9 × 3.2 × 2.7 cm, irregular shape, unclear boundary (a and b), central blood flow (c) and micro-calcification (a, b and d). Suspicious metastatic lymph node was shown (arrow) with liquefied necrotic area (d). Pathological findings were thyroid papillary adenocarcinoma accompanied by lymph node metastasis
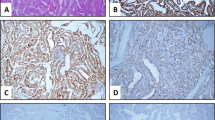
Galectin-3 and c-Met were mainly stained in the cytoplasm, HBME-1 was expressed primarily in the cell membrane and partly in the nuclei, and CK19 was expressed in the cytoplasm and nuclei (Fig. 2). For Galectin-3 expression, score 0 was found in one nodule, 1 in seven nodules, 2 in thirteen and 3 in twenty five (Table 1). For HBME-1, score 0 was found in fourteen nodules, 1 in five, 2 in ten and 3 in seventeen. For CK19, score 0 was found in none nodules, 1 in two, 2 in six and 3 in thirty eight. For c-Met, score 0 was found in four nodules, 1 in fourteen, 2 in eight and 3 in twenty.
Expression of cellular markers in a thyroid nodule in the same patient with thyroid papillary adenocarcinoma in Fig. 1. a and b. High magnification view (10 times, a) and (20 times, b) revealed strongly positive expression (+++) of Galectin-3 in the thyroid papillary adenocarcinoma. c and d. High magnification view (10 times) demonstrated strongly positive expression (+++) of HBME-1 (c) and CK19 (d) in the thyroid nodule. e and f. High magnification view (10 times) showed strongly positive expression (+++) of c-Met in the thyroid papillary adenocarcinoma
The expression of Galectin-3 was significantly (P < 0.05) associated with the nodule boundary and lymphatic metastasis, HBME-1 expression was significantly (P < 0.05) related to micro-calcification within the nodules, and c-Met expression was significantly (P < 0.05) associated with lymphatic metastasis and micro-calcification within the nodule. However, CK19 expression was not significantly (P > 0.05) associated with any of the ultrasound presentations of the nodules.
Among the 46 thyroid nodules, 17 nodules were greater than 2 cm and 29 were smaller than 2 cm. The maximal diameter of the nodule (> 2 cm or < 2 cm) was not significantly (P > 0.05) related to malignancy of the nodules.
The expression of Galectin-3, c-Met, HBME-1 and CK19 was significantly (P < 0.001, P < 0.001, P = 0.01 and P = 0.003, respectively) different in malignant and benign thyroid lesions, with a significant (P < 0.01) tendency in all the molecular markers in predicting the malignant from benign lesions. The ultrasound presentations could significantly (P < 0.001) predict the malignancy of the thyroid nodules with a significant (P < 0.05) prediction tendency.
The expression of Galectin-3, c-Met and CK19 significantly (P < 0.05) increased with increase of ultrasound malignancy risk. In malignant and benign lesions differentiated by ultrasound, no significant (P > 0.05) difference existed in the expression of HBME-1, however, with increase of ultrasound malignancy risk, the expression of HBME-1 also increased significantly (P = 0.03). The receiver operating characteristic curve (ROC) results (Fig. 3) revealed that Galectin-3 had the greatest value in diagnosing malignant nodules (Table 2). The sensitivity of combined diagnosis was performed in ultrasound combined with Galectin-3 or c-Met using the cut-off value of 1.5, and the sensitivity was proved to be the greatest in ultrasound combined with c-Met (Table 3).
Discussion
In evaluation of thyroid nodules, ultrasound description of these nodules should include location, shape, size, intra-nodule echo, component, calcification, halo, edge and intra-nodule flow [6]. However, not a single sonographic feature can be used to determine the benign or malignant nature of a thyroid nodule, and the combination of multiple features can greatly increase the sensitivity and specificity of diagnosis.
In assessing the malignancy risk of thyroid nodules, a model had been established but was too complex to be used clinically [7]. The TI-RADS classification system is based on different ultrasound presentations of type B and color Doppler ultrasound for determination of malignancy risk [6]. It has been pointed out that addition of the number of two-dimensional ultrasound malignancy features of the TI-RADS system can assist in diagnosing a thyroid nodule [2]. We believed that ultrasound features of height-width ratio > 1, irregular margin, unclear or lobulated edge, marked hypoechogenicity, posterior echo attenuation, micro-calculation and central flow within the nodule are closely associated with increase of malignancy risk [7, 8]. Marked hypoechogenicity indicates the intranodule echo lower than that of the anterior cervical band muscle [9]. It has been found that the ultrasound features of micro-calcification, marked hypoechogenicity and taller-than-wider shape are crucial to the diagnosis of malignancy of thyroid nodules [8]. Unclear or lobulated edge is an independent risk factor with high specificity but low sensitivity. Micro-calcification indicates fine strong echo foci equal to or less than 1 mm in diameter with or without acoustic shadow [10]. A definitive sign of comet tail would be considered to be colloidal deposition. Macro-calcification is defined as the echo foci greater than 1 mm, and macro and micro calcifications can be used to differentiate benign from malignant nodules.
The blood flow within the thyroid nodules can be classified into three types based on the Doppler presentations: type I, no blood flow; II, slight blood flow within the nodule and III, marked intranodule flow and slight peripheral flow. Central flow indicates that the blood vessels are mostly located in the central rather than the peripheral part of a nodule and is closely associated with malignancy risk increase [11]. Detection of metastatic lymph nodes by ultrasound is also an important index of malignancy. The ultrasound presentations of metastatic lymph nodes from thyroid cancer can be enlarged lymph nodes, abnormal structure of lymph nodes, abnormal blood flow signal, equal width and height in the lymph nodes, hyperechogenicity, liquidation and micro-calcification within the lymph nodes [12].
Galectin-3 is a member of growing family of ß-galacto-side-binding animal lectins and involves in regulation of cell-cell and cell-matrix interaction, neoplastic transformation, cell growth and apoptosis [13]. It has been basically suggested as a molecular marker of thyroid malignancy with high sensitivity and specificity, particularly in papillary carcinoma [5, 14, 15]. HBME-1 is a monoclonal antibody against an membrane antigen of mesothelial cells and has preferential reactivity with malignant thyroid tumors [14,15,16,17,18]. HBME-1 is highly expressed in differentiated thyroid carcinomas but may be partially or negatively expressed in undifferentiated thyroid carcinoma or in benign nodules. Differential expressions of cytokeratins have been evaluated in various thyroid tumors, and CK19 is one of these cytokeratins and is useful in diagnosis of papillary carcinoma which has diffuse and strong cytoplasmic staining of CK19 [14, 15, 19,20,21]. CK19 can be expressed in some focal areas in normal thyroid tissues but weakly expressed in some benign lesions. C-Met is the specific tyrosine-kinase receptor of hepatocyte growth factor (HGF), and the binding of these two molecules exerts mitogenic, motogenic, morphogenic and antiapoptotic activities in various cell types, including follicular thyroid cells [22, 23]. In thyroid tumors, C-Met and HGF are expressed at very high levels and frequently in papillary thyroid cancer (75%–100% of cases) while they are never expressed in follicular and anaplastic thyroid cancers but expressed at low levels and infrequently in benign lesions (20%–30%) [23, 24]. Immunohistochemistry for Galectin-3, CK19 and HBME-1 has been reported quite accurate in detecting or excluding malignancy in thyroid nodules [25]. Trimboli et al. [26] studied the immunohistochemistry of Galectin-3, CK19 and HBME-1 in determining uncertain thyroid nodules by core needle biopsy and found that these molecular markers can increase the accuracy of core needle biopsy in thyroid lesions with uncertain/non-diagnostic core needle biopsy report. Distinction between benign and malignant thyroid lesions is crucial for treatment of thyroid nodules, and the immunohistochemical staining for all the molecular markers of Galectin-3, HBME-1, CK19, high molecular weight cytokeratin, cyclin D1 and p27kip1 is significantly associated with differentiated thyroid carcinoma [5]. The sensitivity for diagnosis of differentiated thyroid carcinoma was 94.7% with Galectin-3, 91.3% with HBME-1 and 90.3% with CK19. The specificities of these markers were 95.5%, 69.7 and 83.1%, respectively. Co-expression of Galectin-3 and CK19 or Galectin-3 and HBME-1 was present in 93.2% of carcinomas but in none of the benign nodules. Combined use of HBME-1 and CK19 can increase the diagnostic accuracy of malignancy and the use of CK19 and high molecular weight cytokeratin can aid in differential diagnosis between thyroid papillary and follicular carcinomas [5].
In our study, none of the four molecular markers of Galectin-3, c-Met, CK19 and HBME-1 had direct correlation with the ultrasound imaging characteristics probably because the expression of these molecular markers could not affect the ultrasound imaging. The ultrasound malignancy risk increased with increase of the expression of Galectin-3, c-MET and CK19. No significant (P > 0.05) difference existed in the expression of HBME-1 in malignant and benign lesions differentiated by ultrasound, but the HBME-1 expression significantly (P < 0.05) increased with the increase of ultrasound malignancy risk. CK19 had low specificity in differentiating malignant from benign thyroid lesions. The expression of Galectin-3 and c-MET combined with ultrasound imaging could be used to increase the sensitivity of differentiating between malignant and benign lesions.
Genic mutations including BRAF, RET/PTC, PAX8/PPARγ and RAS have been frequently associated with different types of thyroid malignancies. These mutated genes are present in up to 70%–80% of thyroid carcinomas and may display aggressive behavior and serve as diagnosis markers in evaluating thyroid nodules [27]. BRAF mutation was present in most (72%) cytological-malignant thyroid nodules and more prevalent in conventional papillary thyroid carcinoma [28]. RAS mutation was detected in 10%–20% of papillary thyroid carcinomas, especially the follicular variant type, but it has also been increasingly found in benign thyroid adenomas [29]. PAX8/PPARγ is a growth inhibitor mutation and is responsible for cancerous growth, especially follicular carcinomas [30], and it has a comparatively higher prevalence in benign follicular thyroid adenomas, which precludes its use for a strict diagnosis of cancerous nodule. Such genic mutational analysis can increase the probability of pre-operative diagnosis of malignancy, especially in thyroid nodules, however, physicians are not recommended to integrate mutational analysis for risk stratification into routine clinical practice as not all genic mutation-positive tumors behave aggressively.
The immunophenotypical assay is for detection of biomarkers expressed by thyrocytes during normal or altered cell growth circumstances like cancers. A large number of immunohistochemical biomarkers have been identified including Galectin-3, c-Met, CD44, HBME-1 and CK19. CK19 has shown an elevated sensitivity for papillary thyroid carcinomas, but it is also expressed in some non-neoplastic cases like papillary thyroid hyperplasia, Hashimoto’s thyroiditis and benign adenomas [27]. HBME-1 had 100% sensitivity and 96.7% specificity for papillary thyroid carcinomas, and Galectin-3, which was believed to be expressed only by malignant cells, demonstrated moderate to severe immunohistochemical staining in 24/30 cases of papillary thyroid carcinoma and had the lowest expression in most cases of papillary hyperplasia (specificity 40% and sensitivity 100%) [27]. Although HBME1, CK19 and Galectin-3 are considered the diagnostic biomarkers of papillary thyroid carcinoma [15], the expression of these three markers is reduced in follicular thyroid carcinoma [31]. Moreover, the staining intensity of galectin-3 was weak while the HBME1 expression was occasionally distributed in tall cell thyroid carcinoma. Min et al. [31] studied the correlation of immunohistochemical biomarkers and BRAF mutation in histological variants of papillary thyroid carcinomas in the Korean population, and they found that all cases of tall cell variant of the papillary thyroid carcinoma harbored the BRAF V600E mutation, whereas the follicular variant had less BRAF V600E mutation. No definitive correlation existed between genic mutation and positive expression of immunohistochemical biomarkers in cancerous thyroid nodules. Although the diagnostic accuracy of individual markers is not yet definitive, a combination of these markers with characteristics of ultrasound or genic mutation can help reaching a correct diagnosis.
In this study, we combined preoperative ultrasound features with postoperative immunohistochemical parameters so as to establish a certain correlation of ultrasound features of malignant thyroid nodules with the immunohistochemical biomarkers for improving future diagnostic accuracy of ultrasound. In the future work, when some thyroid nodules do not have the typical features of malignancy on sonography, ultrasound-guided fine needle aspiration cytology can be further proposed for detection of the immunohistochemical markers mentioned above, and in this way, combined sonographic features with expression of immunohistochemical markers can be used to differentiate malignant from benign thyroid nodules so that the diagnostic surgery procedures can be decreased in number.
Some limitations existed in our study including a small cohort of samples with simple pathological classifications, which may result in non-significant difference in the expression of HBME-1 in malignant and benign lesions. Moreover, novel techniques like ultrasound angiography were not used in this study for quantitative evaluation of thyroid nodules.
In conclusion, Galectin-3, c-Met, HBME-1 and CK19 have significantly greater expressions in thyroid malignant than benign lesions and their expression increases with increase of the ultrasound malignancy risk. The combination of both ultrasound and molecular markers can be used to differentiate malignant and benign thyroid lesions.
References
Chi J, Walia E, Babyn P, Wang J, Groot G, Eramian M (2017) Thyroid nodule classification in ultrasound images by fine-tuning deep convolutional neural network. J Digit Imaging 30:477–486
Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK (2011) Thyroid imaging reporting and data system for us features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–899
Lingam RK, Qarib MH, Tolley NS (2013) Evaluating thyroid nodules: predicting and selecting malignant nodules for fine-needle aspiration (fna) cytology. Insights Imaging 4:617–624
Koo BS, Kim JM, Seo ST, Yoon YH, Kwon KR, Kim SH, Kwon HW, Bae WJ, Lim YC (2014) Upregulation of hgf and c-met is associated with subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol 21:2310–2317
Park YJ, Kwak SH, Kim DC, Kim H, Choe G, Park DJ, Jang HC, Park SH, Cho BY, Park SY (2007) Diagnostic value of galectin-3, hbme-1, cytokeratin 19, high molecular weight cytokeratin, cyclin d1 and p27(kip1) in the differential diagnosis of thyroid nodules. J Korean Med Sci 22:621–628
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P, AACE/AME/ETA Task Force on Thyroid Nodules (2010) American association of clinical endocrinologists, associazione medici endocrinologi, and european thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive summary of recommendations. Endocrine practice 16(3):468–475
Reading CC, Charboneau JW, Hay ID, Sebo TJ (2005) Sonography of thyroid nodules: a "classic pattern" diagnostic approach. Ultrasound Q 21:157–165
Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS (2002) New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 178:687–691
Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH, Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology (2008) Benign and malignant thyroid nodules: us differentiation--multicenter retrospective study. Radiology 247:762–770
Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS (2010) Positive predictive values of sonographic features of solid thyroid nodule. Clin Imaging 34:127–133
Rago T, Vitti P, Chiovato L, Mazzeo S, De Liperi A, Miccoli P et al (1998) Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in 'cold' thyroid nodules. Eur J Endocrinol 138:41–46
Sohn YM, Kwak JY, Kim EK, Moon HJ, Kim SJ, Kim MJ (2010) Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy. AJR Am J Roentgenol 194:38–43
Krzeslak A, Lipinska A (2004) Galectin-3 as a multifunctional protein. Cell Mol Biol Lett 9:305–328
de Matos PS, Ferreira AP, de Oliveira FF, Assumpcao LV, Metze K, Ward LS (2005) Usefulness of hbme-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology 47:391–401
Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT (2005) Galectin-3, fibronectin-1, cited-1, hbme1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Modern pathology 18(1):48–57
Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL (2001) Immunohistochemical diagnosis of papillary thyroid carcinoma. Modern pathology 14(4):338–342
Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y et al (2003) Hbme-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J 50:173–177
Sheibani K, Esteban JM, Bailey A, Battifora H, Weiss LM (1992) Immunopathologic and molecular studies as an aid to the diagnosis of malignant mesothelioma. Hum Pathol 23:107–116
Sahoo S, Hoda SA, Rosai J, DeLellis RA (2001) Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma: a note of caution. Am J Clin Pathol 116:696–702
Miettinen M, Kovatich AJ, Karkkainen P (1997) Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Archiv 431:407–413
Raphael SJ, McKeown-Eyssen G, Asa SL (1994) High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Modern pathology 7(3):295–300
Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson S (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251:802–804
Ruggeri RM, Vitarelli E, Barresi G, Trimarchi F, Benvenga S, Trovato M (2012) Hgf/c-met system pathways in benign and malignant histotypes of thyroid nodules: an immunohistochemical characterization. Histol Histopathol 27:113–121
Gentile A, Trusolino L, Comoglio PM (2008) The met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev 27:85–94
Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel SJ, Raab SS, Rosai J, Steward DL, Walsh PS, Wilde JI, Zeiger MA, Lanman RB, Haugen BR (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 367:705–715
Trimboli P, Guidobaldi L, Amendola S, Nasrollah N, Romanelli F, Attanasio D, Ramacciato G, Saggiorato E, Valabrega S, Crescenzi A (2016) Galectin-3 and hbme-1 improve the accuracy of core biopsy in indeterminate thyroid nodules. Endocrine 52:39–45
DA Bhatia P, Friedlander P, Aslam R, Kandil E (2015) Diagnostic potential of ancillary molecular testing in differentiation of benign and malignant thyroid nodules. Antiucancer Res 35:1237–1241
Cohen YRE, Clark DP, Zeiger MA, Umbricht CB, Tufano RP, Sidransky D, Westra WH (2004) Mutational analysis of braf in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res 10:2761–2765
Esapa CTJS, Kendall-Taylor P, Lennard TW, Harris PE (1999) Prevalence of ras mutations in thyroid neoplasia. Clin Endocrinol 50:529–535
Nikiforova MNLR, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE (2003) Ras point mutations and pax8-ppar gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 88:2318–2326
Min HSLC, Jung KC (2013) Correlation of immunohistochemical markers and braf mutation status with histological variants of papillary thyroid carcinoma in the korean population. J Korean Med Sci 28:534–541
Acknowledgements
This study was funded by Hebei Provincial Natural and Scientific Funding (no. H2015206405).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, RL., Wang, J., Zhang, FJ. et al. Ultrasound Risk Assessment Combined with Molecular Markers of Galectin-3, c-MET, HBME-1 and CK19 for Diagnosis of Malignant and Benign Thyroid Nodules. Pathol. Oncol. Res. 25, 1075–1081 (2019). https://doi.org/10.1007/s12253-018-0485-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0485-6