Abstract
The purpose of this study is to investigate perineural invasion (PNI) as a prognostic factor in gastric cancer patients. 455 patients submitted to extended (D2 or more) lymphadenectomy (median number of 39 retrieved lymph nodes, range: 15–140) between 1995 and 2012 were retrospectively studied. Patients were categorized in two groups according to the PNI status, and PNI positivity was assessed in presence of cancer cells in the perinerium or the neural fascicles using hematoxylin and eosin staining. Median follow-up for surviving patients was 80.3 months. Survival analysis was performed by univariate and multivariate analysis, using a Cox proportional hazards model. 162 patients (33.9%) had positive PNI; this was strongly associated with advanced stages of disease, residual tumor, lymphovascular invasion, Lauren diffuse-mixed histotype and tumor size. Five-year cancer-related survival was 65,7% and 20,6% in PNI negative vs. positive groups, respectively (p < 0.001). The prognostic impact of PNI at univariate analysis was particularly evident in patients submitted to R0 surgery, early as well as advanced stage, advanced nodal stage and T status. At multivariate analysis, PNI did not result statistically significant in the overall series, but emerged as an independent prognostic factor in the group of patients with Lauren intestinal histotype (p = 0.005, hazard ratio: 1.99, 95% confidence interval 1.24–3.19). PNI is related to advanced stage and poor long-term survival in gastric cancer, and may serve as an adjunctive prognostic factor in the intestinal histotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma (GC) is one of the leading causes of death for cancer worldwide, and it is the second most common neoplasm of the digestive tract after colo-rectal cancer [1]. Its incidence has been decreasing during the last decades, but long-term survival remains dismal above all in Western Countries, with no significant improvements in recent years [2]. Radical surgery (R0 gastrectomy) is still the mainstay of GC treatment, and D2 lymphadenectomy is nowadays accepted as the standard approach in most treatment guidelines all-over the world [3,4,5]. However, due to the poor prognosis of advanced forms, neo-adjuvant/adjuvant chemotherapy or chemo-radiotherapy have been proposed, in order to increase the chance of cure [6, 7]. The identification of clinical-pathological prognostic factors is essential, because it can allow a better selection of the patients suitable to tailored treatments. Traditionally, several studies have described histology, lymphovascular infiltration, depth of invasion, nodal status, and tumor site as significant prognostic factors for early and advanced disease [8, 9]. However, for a more accurate definition of prognosis, the finding of new clinical-pathological factors could be of help. Perineural invasion (PNI) is a pathologic parameter strictly related to a poor prognosis in head and neck cancer [10], urinary tract [11] and gastrointestinal neoplasms especially in pancreatic cancer [12]. Although in many reports PNI appears as an independent prognostic factor, its prognostic value in GC is still debated. The aim of this retrospective study is to evaluate the impact of PNI in the long-term survival of GC patients undergoing extended lymphadenectomy.
Materials & Methods
In this retrospective cohort study, 455 consecutive patients with GC surgically treated in our Unit of General Surgery and Surgical Oncology, University of Siena, Italy, from January 1995 to December 2012 were analyzed. Preoperative staging was assessed by CT-scan, oesophago-gastro-duodenoscopy and, when required, endoscopic ultrasound. The endoscopic biopsies for an appropriate histological evaluation and definition of the neoplasm were always obtained before the surgical procedure. Patients submitted to neo-adjuvant treatments and esophago-gastric junction Siewert type I tumors were excluded. The surgical procedures, total or partial gastrectomy, were performed according to the preoperative staging and tumor location. An extended (D2 or more) lymphadenectomy was performed in all cases, as reported previously [13, 14], and only patients with at least 15 nodes retrieved at pathologic examination were included in the study. Extended surgery, considered as multivisceral resections for locally advanced GC, was also performed when needed (e.g., direct infiltration of spleen, gallbladder, liver, colon, pancreas).
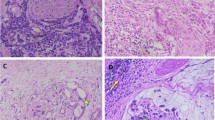
Tumor stage was defined according to the 7th pTMN system classification proposed by the International Union Against Cancer [15]. All cases treated before 2010 were revised and restaged according to the last TNM edition, as previously reported [16]. Pathological classification was performed by an experienced pathologist dedicated to GC diagnosis and staging (C.V.). Clinical and individual characteristics, surgical procedures and histological findings were recorded in a specific database. Patients were submitted to regular outpatient follow-up examinations, according to a standard protocol [9]. All cases were divided in two groups according to the perineural invasion (PNI) status. PNI positivity was assessed by the presence of cancer cells in the perinerium or in the neural fascicles using hematoxylin and eosin staining (Fig. 1 ). Informed consent for the data management for research studies was obtained for all included patients.
The statistical analysis was performed with the X2 test or Fisher exact test to compare categorical variables. The Mann–Whitney U test was used to compare continuous variables not normally distributed. Cumulative survival was calculated by the life table method of Kaplan and Meier, and the log-rank test was used to assess significant differences. Multivariate analysis was performed using a Cox proportional hazards model by considering the following risk factors: sex (male vs. female), age (continuous variable), tumor location (upper, middle, others vs. lower), Lauren histotype (diffuse-mixed vs. intestinal) depth of tumor invasion (pT2, pT3 or pT4 vs. pT1), lymph node involvement (pN1, pN2, pN3a or pN3b vs. pN0), M stage (M1 vs. M0), R category (R+ vs. R0), vascular invasion (present vs. absent), lymphatic invasion (present vs. absent), and PNI status (PNI+ vs. PNI-). Significant variables were selected with a forward step procedure. Statistical significance was determined at p value <0.05. The entire statistical analysis was performed with SPSS statistical program (Version 17.0) and reviewed by an expert statistician in gastric cancer surgical research (D.M.).
Results
PNI positivity was detected in 162 out of the 455 GC patients (33.9%). Median age was 69 (inter-quartile range, IQR: 60–75) and 67 (IQR: 60–75) years for PNI- and PNI+ groups, respectively. A total of 271 patients were male: 174 in the group PNI- and 97 in the group PNI+. Subtotal gastrectomy was performed in 292 cases, and total gastrectomy in 163. A median number of 39 lymph nodes (range: 15–140) was retrieved, without statistical difference between the two groups (p = 0.137). The correlations between PNI status and clinical-pathological factors are shown in Table 1. PNI positivity was strongly associated with depth of invasion, lymph-node metastasis, tumor grading and stage (p < 0.001), and it was also related to lymphovascular invasion, Lauren diffuse-mixed histotype, large tumor size, linitis plastica and upper third location. A large proportion (48.8%) of patients with PNI positivity was submitted to R+ resections. No association between PNI positivity, gender (p = 0.919) and age (p = 0.761) was found.
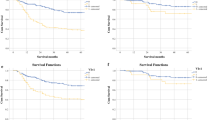
Complete follow-up data were available for 392 patients (median follow-up for surviving patients: 80.3 months, IQR: 38–122). The 5-year cancer-related survival (CRS) was 65.7% in PNI-negative patients vs. 20.6% in PNI-positive group (p < 0.001) (Fig. 2 ). The potential impact of PNI status on long-term survival was evaluated stratifying our series according to different pathological factors (Table 2 ). This analysis revealed that PNI status was strongly associated with worse prognosis in patients with different tumor locations, histotype, surgical radicality and in advanced as well as early stages of diseases (Fig. 3 ). However, the highest prognostic effect was identified in distal tumors, more advanced nodal status, M0 cases, and patients submitted to R0 resection. When analyzing CRS according to Lauren hystotype, both intestinal and diffuse PNI-positive patients had worse prognosis compared to negative cases (Fig. 4 ). Multivariate analysis identified pN status (p < 0.001), pT status (p < 0.001), radicality (p < 0.001), age (p = 0.010), sex (p = 0.010), and vascular invasion (p = 0.013) as independent prognostic factors, whereas PNI status was not confirmed as a significant factor (Table 3). However, when PNI status was evaluated in patients with different Lauren hystotypes, it was identified as an independent prognostic factor in the intestinal type subgroup (p = 0.005, HR 1.99, 95% C.I. 1.24–3.19), whereas it did not results as statistically significant in the diffuse-mixed cases (p = 0.273).
Discussion
PNI is defined as the presence of cancer cells along the sides of nerves and/or inside the epineurial, perineurial and endoneurial spaces of the neuronal sheath [17].
This feature, investigated by several authors, is a marker of poor outcome and it has been associated with worse survival in many malignancies, like pancreatic, colorectal, prostate head and neck cancers [18]. Fouquet et al. [19] analyzed the impact of PNI on early tumor recurrence in patient submitted to pancreaticoduodenectomy for adenocarcinoma of the head of the pancreas, showing PNI as the most important factor associated to 1-year recurrence and poor disease-free and overall survival. Moreover, in prostate cancer, PNI positivity is associated with higher mortality rates, increased risk of recurrence and positive surgical margins [20,21,22].
In GC, PNI was investigated in some studies, but its role as an independent prognostic factor remains still debated.
In the present analysis, conducted in a consecutive series of patients submitted to extended lymphadenectomy, with a high number of median removed lymph nodes, PNI positivity was found in 33.9% of the patients. These results are in line with the published literature: in a recent meta-analysis, the median percentage of PNI positivity is 40.9% (range 6.8%–75.6%) [18].
As described in other papers [23, 24], there is a strong association between PNI and stage of GC. PNI positivity notably increases with depth of invasion and number of metastatic lymphnodes; it was observed in 3/72 (4.2%) of T1 vs. 126/224 (56.3%) of T4 tumors, as reported by others [23, 24]. Regarding the N status, PNI positivity was detected only in the 8.6% of N0 patients vs. 57.2% of N3 patients. Bilici et al. [25] observed PNI positivity in 41 out of 45 (91.1%) pN3 patients. Despite we did not found any difference between two groups in the number of harvested lymph nodes, the median number of metastatic lymph nodes in PNI+ group is notably higher than PNI-negative group (11 vs. 1; p < 0.001), which confirms the very high propensity of PNI-positive cases to lymph nodal spread. According to our data, PNI was also linked to lymphovascular invasion, and similar results are reported in different papers [26, 27]. This behavior could be linked to the infiltration of the vasa nervorum and the lymphatic network around the nerves [26].
In our series PNI positivity was more often found in the upper third lesions. This finding could be justified by the anatomic features of the stomach. In the upper third of the stomach there are relatively large autonomic nerves with larger perineural space, so the tumor cells could easily spread through the gap between the nerves and tissue [26]. Linitis plastica is a very aggressive disease, with a propensity to the massive infiltration of the gastric wall, and the higher frequency of PNI fit well with the behavior of this particular form of GC.
We found statistically significant correlation between PNI positivity and diffuse-mixed Lauren histotype, as also reported by Zhou et al. [28]. These results are not confirmed by other authors [25, 27].
In our cohort, we observed a notable difference in terms of long-term survival between PNI+ and PNI- patients (p < 0.001). Even when stratifying according to UICC stages, we identified a strong correlation between PNI positivity and a poor survival, in advanced (III-IV) as well as early stages (I-II). Even if the present retrospective study is not able to identify potential impact of adjuvant therapies in early stages of GC, cases with PNI positivity could benefit from adjuvant treatments, which should be confirmed in specifically designed prospective studies.
Several studies have evaluated the prognostic value of PNI in GC. Bilici et al. in a cohort of 238 patients submitted to curative surgery, identified PNI as an independent prognostic factor at multivariate analysis [25]. Similar results are reported by several Authors [24, 29]. Scartozzi et al. [30] evaluated 734 gastric cancer patients surgically treated with curative intent. Cohort was divided in two groups according to the presence or absence of lymphovascular invasion (LV) and PNI status, and LV/PNI positivity was strongly related to poor survival at multivariate analysis.
One of the largest studies (7757 patients enrolled) was performed by Kim et al. [31]. The prognostic value of PNI at univariate analysis was statistically significant; however, multivariate analysis for the evaluation of independent factors affecting both overall and disease-free survival was not performed. These authors also focused on the evaluation of risk factors of recurrence demonstrating that PNI presence is related to higher risk of GC recurrence.
On the other hand, in other papers PNI positivity did not result as an independent prognostic factor [23, 28]. The results of our study are in line with the papers above mentioned. We observed that PNI-positive tumors had a poor survival compared to PNI-negative tumors, but at multivariate analysis PNI was not an independent prognostic factor, not providing any additional information to the other prognostic parameters already known. This may be due to the very high correlation between PNI status and advanced stages, which limited its prognostic value when controlling for stronger prognostic variables, above all in well-staged tumors with a high number of removed and analyzed lymphnodes.
In a further analysis of our series, we stratified the prognostic value of PNI according to Lauren histotype, and PNI positivity emerged as independent prognostic factor in the intestinal subgroup of patients. This is a new finding in literature, and could require more potential explanations. Several studies reported different impact of conventional or biological prognostic factors in different Lauren histotypes [32, 33]. Besides histomorphometrical characteristics, the intestinal and the diffuse histotypes show evident differences in epidemiological, clinical and molecular features [34]. The intestinal type is more common in males and older patients, whereas the diffuse type usually affects younger patients with a lower male-female ratio. A characteristic of the diffuse type is also the greater biological aggressiveness, in particular the higher propensity to peritoneal dissemination and lymph nodal spread. Differences in clinical and molecular characteristics may be also at the basis of the variability in the prognostic weight of pathological factors as PNI status.
The correlation between Lauren histotype and the prognostic value of PNI could justify the different results in current literature. Indeed, when analyzing different series, we noted that PNI status emerged as a strong prognostic factor in series with a higher percentage of intestinal histotypes [25, 27].
Probably the presence of PNI in GC could be considered as the clinical-pathological phenotypic expression of distinct molecular features. Cristescu et al. [35] identified four GC molecular subtypes linked to different patterns of genomic alterations: microsatellite stable epithelial-to-mesenchymal transition (MSS/EMT), microsatellite instability (MSI), microsatellite stability with intact TP53 activity (MSS/TP53+) and microsatellite stability with TP53 functional loss (MSS/TP53-). These molecular subtypes are related not only to specific genomic features but also to clinical characteristics, recurrence pattern and prognosis. They observed that tumors in MSS/EMT subtype are generally diffuse histotype, diagnosed at advanced stage and with the worst prognosis. Interesting, PNI positivity is significantly associated with MSS/EMT subtype. PNI in GC deserves further investigations in the future regarding the molecular characteristics and potential indications to targeted therapies.
Conclusion
The analysis of a large series of GC patients treated by extended lymphadenectomy confirmed the strong association between PNI status and more advanced stage, which involved a poor prognosis of PNI-positive group. Survival multivariate analysis in different subgroups identified PNI status as an independent prognostic factor in the intestinal type group, but not in the diffuse-mixed histotypes. This new finding may shed more light on the real clinical value of PNI status in GC patients.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403. doi:10.1016/j.ejca.2012.12.027
Marrelli D, Pedrazzani C, Morgagni P, de Manzoni G, Pacelli F, Coniglio A, Marchet A, Saragoni L, Giacopuzzi S, Roviello F, Italian Research Group for Gastric Cancer (2011) Changing clinical and pathological features of gastric cancer over time. Br J Surg 98:1273–1283. doi:10.1002/bjs.7528
Japanese Gastric Cancer Association (2016) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer, doi:10.1007/s10120-016-0622-4
De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, Tomezzoli A, Berselli M, Catalano F, Di Leo A, Framarini M, Giacopuzzi S, Graziosi L, Marchet A, Marini M, Milandri C, Mura G, Orsenigo E, Quagliuolo V, Rausei S, Ricci R, Rosa F, Roviello G, Sansonetti A, Sgroi G, Tiberio GA, Verlato G, Vindigni C, Rosati R, Roviello F (2017) The Italian research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. doi:10.1007/s10120-016-0615-3
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, European Society for Medical Oncology (ESMO), European Society of Surgical Oncology (ESSO), European Society of Radiotherapy and Oncology (ESTRO) (2014) Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 40:584–591. doi:10.1016/j.ejso.2013.09.020
MacDonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J med 345:725–730. doi:10.1056/NEJMoa010187
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Trial Participants MAGIC (2006) Perioperative chemotherapy versus surgery alone for Resectable gastroesophageal cancer. N Engl J med 355:11–20. doi:10.1056/NEJMoa055531
Verlato G, Marrelli D, Accordini S, Bencivenga M, Di Leo A, Marchet A, Petrioli R, Zoppini G, Muggeo M, Roviello F, de Manzoni G (2015) Short-term and long-term risk factors in gastric cancer. World J Gastroenterol 21:6434–6443. doi:10.3748/wjg.v21.i21.6434
Marrelli D, Morgagni P, de Manzoni G, Marchet A, Baiocchi GL, Giacopuzzi S, Coniglio A, Mocellin S, Saragoni L, Roviello F, Italian Research Group for Gastric Cancer (2015) External validation of a score predictive of recurrence after radical surgery for non-cardia gastric cancer: results of a follow-up study. J am Coll Surg 221:280–290. doi:10.1016/j.jamcollsurg.2015.03.042
Clayman GL, Lee JJ, HolsingerFC ZX, Duvic M, El-Naggar AK, Prieto VG, Altamirano E, Tucker SL, Strom SS, Kripke ML, Lippman SM (2005) Mortality risk from squamous cell skin cancer. J Clin Oncol 23:759–765. doi:10.1200/JCO.2005.02.155
Kang M, Oh JJ, Lee S, Hong SK, Lee SE, Byun SS (2016) Perineural invasion and Lymphovascular invasion are associated with increased risk of biochemical recurrence in patients undergoing radical prostatectomy. Ann Surg Oncol 23:2699–2706. doi:10.1245/s10434-016-5153-z
Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N (2011) Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas 40:464–468. doi:10.1097/MPA.0b013e31820b5d37
Marrelli D, Pedrazzani C, Neri A, Corso G, DeStefano A, Pinto E, Roviello F (2007) Complications after extended (D2) and superextended (D3) lymphadenectomy for gastric cancer: analysis of potential risk factors. Ann Surg Oncol 14:25–33. doi:10.1245/s10434-006-9063-3
Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, Corso G, de Manzoni G (2010) Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol 36:439–446. doi:10.1016/j.ejso.2010.03.008
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) Stomach. In: American joint committee on cancer staging manual, 7th edn. Springer, New York, pp 117–126
Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F, Italian Research Group for Gastric Cancer (IRGGC) (2012) Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized western centers. Ann Surg 255:486–491. doi:10.1097/SLA.0b013e3182389b1a
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115:3379–3391. doi:10.1002/cncr.24396
Deng J, You Q, Gao Y, Yu Q, Zhao P, Zheng Y, Fang W, Xu N, Teng L (2014) Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS One 9:e88907. doi:10.1371/journal.pone.0088907
Fouquet T, Germain A, Brunaud L, Bresler L, Ayav A (2014) Is perineural invasion more accurate than other factors to predict early recurrence after pancreatoduodenectomy for pancreatic head adenocarcinoma? World J Surg 38:2132–2137. doi:10.1007/s00268-014-2465-7
Saeter T, Bogaard M, Vlatkovic L, Waaler G, Servoll E, Nesland JM, Axcrona K, Axcrona U (2016) The relationship between perineural invasion, tumor grade, reactive stroma and prostate cancer-specific mortality: a clinicopathologic study on a population-based cohort. Prostate 76:207–214. doi:10.1002/pros.23112
Sertkaya Z, Öztürk Mİ, Koca O, Güneş M, Karaman Mİ (2014) Predictive values for extracapsular extension in prostate cancer patients with PSA values below 10 ng/mL. Turk J Urol 40:130–133. doi:10.5152/tud.2014.00086
Ozcan F (2001) Correlation of perineural invasion on radical prostatectomy specimens with other pathologic prognostic factors and PSA failure. Eur Urol 40:308–312
Duraker N, Sişman S, Can G (2003) The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surg Today 33:95–10
Tanaka A, Watanabe T, Okuno K, Yasutomi M (1994) Perineural invasion as a predictor of recurrence of gastric cancer. Cancer 73:550–555
Bilici A, Seker M, Ustaalioglu BB, Kefeli U, Yildirim E, Yavuzer D, Aydin FM, Salepci T, Oncel M, Gumus M (2010) Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol 17:2037–2044. doi:10.1245/s10434-010-1027-y
Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H (2014) Incorporation of perineural invasion of gastric carcinoma into the 7th edition tumor-node-metastasis staging system. Tumour Biol 35:9429–9436. doi:10.1007/s13277-014-2258-5
Selçukbiricik F, Tural D, Büyükünal E, Serdengeçti S (2012) Perineural invasion independent prognostic factors in patients with gastric cancer undergoing curative resection. Asian Pac J Cancer Prev 13:3149–3152
Zhou ZH, Xu GF, Zhang WJ, Zhao HB, Wu YY (2014) Reevaluating significance of perineural invasion in gastric cancer based on double immunohistochemical staining. Arch Pathol lab med 138:229–234. doi:10.5858/arpa.2012-0669-OA
Tianhang L, Guoen F, Jianwei B, Liye M (2008) The effect of perineural invasion on overall survival in patients with gastric carcinoma. J Gastrointest Surg 12:1263–1267. doi:10.1007/s11605-008-0529-4
Scartozzi M, Galizia E, Verdecchia L, Berardi R, Graziano F, Catalano V, Giordani P, Mari D, Silva RR, Marmorale C, Zingaretti C, Cascinu S (2006) Lymphatic, blood vessel and perineural invasion identifies early-stage high-risk radically resected gastric cancer patients. Br J Cancer 95:445–449
Kim DH, Kim SM, Hyun JK, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S (2013) Changes in postoperative recurrence and prognostic risk factors for patients with gastric cancer who underwent curative gastric resection during different time periods. Ann Surg Oncol 20:2317–2327. doi:10.1245/s10434-012-2700-0
Marrelli D, Polom K, Pascale V, Vindigni C, Piagnerelli R, De Franco L, Ferrara F, Roviello G, Garosi L, Petrioli R, Roviello F (2016) Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol 23:943–950. doi:10.1245/s10434-015-4931-3
Roviello F, Marrelli D, Vindigni C, De Stefano A, Spina D, Pinto E (1999) P53 accumulation is a prognostic factor in intestinal-type gastric carcinoma but not in the diffuse type. Ann Surg Oncol 6:739–745. doi:10.1007/s10434-999-0739-3
Marrelli D, Polom K, de Manzoni G, Morgagni P, Baiocchi GL, Roviello F (2015) Multimodal treatment of gastric cancer in the west: where are we going? World J Gastroenterol 21:7954–7969. doi:10.3748/wjg.v21.i26.7954
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat med 21:449–456. doi:10.1038/nm.3850
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional Review Board Statement
The study was reviewed and approved by the University of Siena Institutional Review Board.
Conflict of Interest
The authors have no conflict of interest to report.
Informed Consent
All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Rights and permissions
About this article
Cite this article
De Franco, L., Marrelli, D., Voglino, C. et al. Prognostic Value of Perineural Invasion in Resected Gastric Cancer Patients According to Lauren Histotype. Pathol. Oncol. Res. 24, 393–400 (2018). https://doi.org/10.1007/s12253-017-0257-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0257-8