Abstract
Purpose
The purpose of the present work was to develop levofloxacin-flurbiprofen coloaded PLGA (LEV-FLU-PLGA) nanoparticles with surface modification using chitosan to attain mucoadhesion for the treatment of bacterial conjunctivitis.
Method
Polymeric nanoparticles were prepared by nanoprecipitation method and evaluated for parameters like particle size, PDI, zeta potential, entrapment efficiency (%), in vitro drug release, ex vivo permeation studies, microbial assay against Staphylococcus aureus and ocular tolerance using Hen’s egg test-chorioallantoic membrane (HET-CAM). Furthermore, surface of optimized PLGA nanoparticle formulation was modified by coating with chitosan.
Results
LEV-FLU-PLGA nanoparticles demonstrated particle size of 166.1 nm with PDI of 0.137 and zeta potential of − 16.8 mV. The entrapment efficiency was found to be 39.37% for levofloxacin (LEV) and 48.33% for flurbiprofen (FLU), whereas for surface-modified nanoparticles, it was found to be 42.05% for LEV and 45.26% for FLU. LEV-FLU chitosan-coated PLGA nanoparticles showed an increase in particle size, i.e., 333.6 nm with PDI of 0.319 and an inversion of zeta potential to 37.67 mV. The developed nanosystems showed sustained release and improved eye permeability. Microbiological studies showed equivalent zone of inhibition to that of marketed formulation. HET-CAM assay revealed the non-irritant nature of drug-loaded PLGA nanoparticles; however, chitosan-coated PLGA nanoparticles were found to be moderately irritating owing to the acidic nature of formulation.
Conclusion
The nanoparticulate system provides prolonged drug release making it a promising alternative to conventional dosage forms. It reduces systemic effects of locally acting drugs, improving therapeutic efficacy and patient compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection or Inflammation of the conjunctiva is known as conjunctivitis and is characterized by dilatation of the conjunctival vessels, resulting in hyperemia and edema of the conjunctiva, typically with associated discharge. Conjunctivitis can be divided into infectious and non-infectious type where viruses and bacteria are the most common causes of infection. Non-infectious conjunctivitis includes allergic, toxic, and cicatricial conjunctivitis, as well as inflammation secondary to immune-mediated diseases and neoplastic processes. Bacterial conjunctivitis is usually classified into acute, hyperacute, and chronic according to the mode of onset and the severity of the clinical response. Acute bacterial conjunctivitis is the most prevalent infectious condition of the eye that is commonly caused by micro-organisms like Streptococcus pneumonia, Hameophilus influenza and Neisseria gonorrhea. Hyper-acute bacterial conjunctivitis is characterized by abrupt onset, profuse, thick, yellow-green purulent secretion, mixed ocular injection and chemosis, and sometimes the formation of an inflammatory membrane. Chronic bacterial conjunctivitis generally lasts more than 4 weeks and relapses frequently; the most common causes include Staphylococcus aureus, Moraxella lacunata and enteric bacteria [1]. Treatment of bacterial conjunctivitis includes application of ocular antibiotics ultimately to kill the root cause of infection, i.e., pathogens. Newer generation fluoroquinolone antibiotics have been the most commonly recommended treatment regimen, as they act quickly against a broad spectrum of pathogens, are bactericidal in action and are well tolerated [2]. NSAIDs are topically applied to the ocular tissues to prevent meiosis during ophthalmic surgeries, diminish postoperative inflammations and manage cystoids macular edema and seasonal allergic conjunctivitis. Recently, their effectiveness in reducing bacterial colonization of contact lenses and inhibiting bacterial adhesion to cornea has been reported [3]. If an anti-inflammatory agent is co-administered along with anti-bacterial agent, it may aid the healing process thereby reducing patient discomfort and spreadability of disease. Literature reports the codelivery of small molecules and macromolecules in treatment of cancer and HIV in nanoparticulate systems [4, 5].
Levofloxacin (LEV), an antibiotic of the fluoroquinolone family, which inhibits the bacterial enzymes DNA gyrase and topoisomerase IV along with flurbiprofen (FLU), a non-steroidal anti-inflammatory agent that inhibits cyclooxygenase enzyme, can be used in combination. In the present work, the synergistic combination of levofloxacin and flurbiprofen-coloaded nanoparticles are explored with the rationality of achieving prolonged ocular delivery and reduced frequency of dosing, which will offer better patient compliance.
Ophthalmic drug delivery system is one of the most challenging delivery systems, as the eye is protected by its unique anatomy and physiology that makes entry of drugs within the eye difficult. Hence, various efforts are made by pharmaceutical scientists to enhance the pre-corneal residence time, thereby increasing the drug penetration into the eye. Amongst all the developed systems, nanoparticles come out to be the most promising application in ocular drug delivery, as nano-sized systems protect the ocular-instilled drugs from metabolism by tear fluid enzymes and increase their permeation through corneal membrane [6]. Furthermore, controlling nanoparticle surface properties such as charge and degree of lipophilicity could reduce the unfavourable chemical properties of the free molecule. Biodegradable polymeric NPs may control the drug level at the infection site, which is expected to enhance the drug efficacy, to decrease the number of doses administered and to reduce the side effects [7]. Poly(lactic-co-glycolic acid) (PLGA) nanoparticles have been used to control the delivery of antibiotics in several ways. The major challenge in using PLGA nanoparticles in the treatment of bacterial conjunctivitis is to remain in the anterior chamber of eye without being flushed by lachrymal fluid. Thus, there is need to modify the surface of PLGA nanoparticles to improve their effectiveness by enhancing their retention in the anterior chamber of the eye [8].
Mucoadhesiveness in ocular drug delivery is a robust approach that prolongs drug retention time and increases the membrane permeability and intracellular uptake of drugs. Chitosan being cationic hydrophilic offers strong binding with negatively charged cellular surface together with bioadhesion to the mucin layer of conjunctiva and corneal surface. Its inherent anti-microbial activity makes the chitosan suitable mucoadhesive polymer for surface modification of PLGA nanoparticles [9]. The present study was aimed at design and development of ocular mucoadhesive nanoparticulate system containing levofloxacin hemihydrate salt and flurbiprofen and to demonstrate its anti-microbial efficacy. Furthermore, the enhancement of the mucoadhesive properties of these nanoparticles to attain prolonged mucin binding was one of the major aims. The physical properties like particle size, entrapment efficiency and surface charge in relation to formulation variables were evaluated. Finally, in vitro release, ex vivo transcorneal permeation and ocular irritation potential were investigated for suitability of topical ocular delivery of developed mucin binding mucoadhesive formulation.
Material and Methods
Material
Levofloxacin hemihydrate and flurbiprofen were kindly gifted by Sun Pharmaceuticals Industries Ltd., Mumbai, India and Macleods Pharmaceuticals Pvt. Ltd. Mumbai, India, respectively. PLGA Resomer RG 504 and chitosan (molecular weight 400 KD) were obtained as gift sample from Evonik Industries, Mumbai, India and Sangam Laboratories, Mumbai, India, respectively. Other chemicals were of analytical grade and purchased from S.D. Fine-Chem Limited, Mumbai.
Methods
Preparation of PLGA Nanoparticles
Levofloxacin-flurbiprofen PLGA nanoparticles were prepared with slight modification of previously reported nanoprecipitation technique. In brief, different ratios of LEV: FLU (1:1) mixture and PLGA were dissolved in acetone (2 ml) at room temperature (Table 1).
Drug mixture to polymer ratio was varied from 1:2 to 1:10 to achieve maximum entrapment efficiency for both the drugs (keeping both the drugs constant at 4 mg each and varying polymer concentrations). This prepared organic phase was then added dropwise at a rate of 1 ml/min using a 21-gauge syringe to 10 ml aqueous solution of poloxamer 188 (1.5% w/v) solution under vortex mixing using cyclomixer (Remi). The mixture was vortexed for additional 5 min. Excess of acetone was evaporated overnight under mild stirring using a magnetic stirrer at room temperature. Nanosuspension obtained was centrifuged using Optima max, Beckman coulter, USA ultracentrifuge at 50,000 rpm for 30 min at 4 °C. The supernatant was discarded; nanoparticles were washed with distilled water. Lyophilization of nanoparticles was accomplished using mannitol as cryoprotectant at the ratio of 1:5. The chitosan-coated PLGA nanoparticles were prepared using optimized drug mixture: polymer ratio (effect of drug mixture: polymer ratio on particle size, polydispersity index, zeta potential, and percent entrapment efficiency data is represented in Table 1). Lev-Flu PLGA nanoparticle formulation F-3 was optimized and selected for further study on the basis of its lower particle size, higher entrapment efficiency, and drug loading.
Preparation of Chitosan-coated PLGA Nanoparticles
Chitosan-PLGA nanoparticles (also referred to as Nanoplex in several research articles) were prepared by modification of ionotropic gelation technique similar to previously described method by Jain et al. [10]. Chitosan concentration was finalized through the preliminary studies. In brief, either 4 mg LEV-FLU (1:1) mixture or individual drug 2 mg along with PLGA (0.2% w/v) was dissolved in 2 ml of acetone. Chitosan was dissolved in acetate buffer pH 4.6 at room temperature to obtain concentration of 3 mg/ml followed the addition of poloxamer 188 (1.5% w/v). The organic phase was drop-wise added at the rate of 1 ml/min in 10 ml of chitosan solution containing poloxamer 188. The formed dispersion was stirred continuously for 6 h at room temperature to evaporate acetone. Nanosuspension obtained was centrifuged using Optima max, Beckman coulter, USA ultracentrifuge at 50,000 rpm for 30 min at 4 °C. The supernatant was discarded, and nanoparticles were washed and reconstituted with distilled water. Lyophilization of nanoparticles was accomplished using mannitol as cryoprotectant at the ratio of 1:5.
Characterization of PLGA and Chitosan-coated-PLGA Nanoparticles
Particle Size and ζ Potential
Aqueous nanosuspensions were diluted ten-fold with filtered distilled water to ensure light scattering intensity was within instrument’s sensitivity range. Particle size and polydispersity index were determined by dynamic light scattering using zetasizer (Nanoseries, NanoZS, Malvern, UK). The zeta potential of nanoparticles was measured by Laser Doppler velocimetry using zetasizer (Nanoseries, NanoZS, Malvern, UK). All measurements were performed in triplicate at 25 °C, and the values are reported as mean ± standard deviation.
Percent Entrapment Efficiency
Entrapment efficiency of PLGA and chitosan-PLGA nanoparticles was determined by ultracentrifugation and was indirectly estimated by UV spectrophotometric analysis of supernatant. One milliliter of formulation was centrifuged at 4 °C for 30 min using Ultracentrifuge (Optima Max XP ultracentrifuge, Beckman Coulter, USA). Appropriate volume of supernatant was diluted with simulated tear fluid (STF) and analyzed on a UV spectrophotometer. Entrapment efficiency and drug loading of both the drugs was calculated using the following equation
As the developed formulation consists of two drugs, simultaneous equation method was developed that allowed co-determination of both the drugs accurately. Supernatant was appropriately diluted with STF and was analyzed using a UV spectrophotometer at two wavelengths, i.e., 248 nm (absorbance maxima of FLU) and 288 nm (absorbance maxima of LEV) and concentration of un-entrapped drugs was calculated using the following formula
where, A248 and A288 are the absorbance of the diluted sample at 248 and 288 nm, respectively.
In Vitro Release Study
In vitro release study of marketed eye drops of LEV and FLU, LEV-FLU-PLGA nanoparticles, and LEV- FLU chitosan-PLGA nanoparticles were performed in simulated tear fluid (STF), pH 7.4 using dialysis bags (molecular weight cut off 12,000–14,000 Da) at 37 °C under magnetic stirring in triplicates. Briefly, nanoparticle suspension containing drugs to be investigated was carefully tied within a dialysis sac, and a bag was suspended in a 100-ml beaker containing 50 ml of STF that was magnetically stirred at 37 °C. Samples of volume of 1 ml were withdrawn at predetermined time intervals and replaced with equal quantity of fresh STF. These samples were further suitably diluted and analyzed using UV spectroscopy. In vitro release profiles of formulated nanoparticles coloaded with LUV and FLU were compared with marketed drug formulations.
Release Kinetics of Nanoparticles
Data obtained from in vitro dissolution study was further investigated for release kinetics using the DDSolver software program. DDSolver computer program was used to shorten the calculation time, eliminate calculation errors, and determine the correct release profile. After obtaining the release profiles, data were transferred to the DDSolver program to determine the five most important and widespread criteria: coefficient of determination (R2), adjusted coefficient of determination (\({R}_{adjusted}^{2}\)), Akaike Information Criterion (AIC) and Model Selection Criterion (MSC). The highest R2, \({R}_{adjusted}^{2}\), and MSC values and the lowest AIC values were used for evaluating Higuchi Peppas-Sahlin and Weibull models [11].
Ex vivo Transcorneal Permeation Study
The transcorneal permeability of developed formulations was studied on excised goat corneas and compared with marketed formulation. Fresh whole eyeballs obtained from local butcher’s shop were dissected carefully to remove intact cornea with attached scleral tissue that was used as permeability barrier. It was equilibrated with STF, pH 7.4 for 2–3 h at 37 °C. The study was conducted using Franz diffusion cell, where the upper chamber served as a donor compartment in which 1 ml of formulation under investigation was placed. The lower chamber served as a receiver compartment that contained STF. Excised goat cornea was fixed between clamped donor and receptor compartments ensuring that the epithelial side of the cornea faced the donor compartment. The entire system was maintained at 37 °C. At predetermined time intervals, 1 ml of aliquot was withdrawn from receptor compartment and replaced with fresh STF. This aliquot was then suitably diluted and analyzed using a UV spectrophotometer. The apparent corneal permeability coefficient (Papp, cm/s) was determined using the expression [12].
where \(\Delta Q/\Delta t\) indicates the flux across the corneal tissue (\(\mathrm{\mu g}\cdot {\mathrm{min}}^{-1}\)) obtained from the slope of the linear portion of the permeation plot for the amount of drug in the receiving chamber (Q) versus time (t); 60 is the unit conversion from minutes to seconds; A is the corneal area available for penetration (1.32 \({\mathrm{cm}}^{2}\)), and \({C}_{0}\) is the initial concentration of drug in the donor compartment.
Mucoadhesion Study
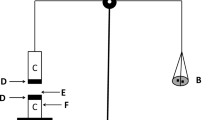
Mucoadhesion of LEV and FLU-loaded chitosan–PLGA nanoparticles were evaluated in vitro by a method described by Ansari et al., 2018 [13] with slight modifications. Mucoadhesion was measured by a modified two-pan balance fabricated in our laboratory.
Fabrication of Balance
The two pans of physical balance were removed, and the right pan was replaced with a lighter pan C, containing a plastic container (K), whereas on the left side a teflon cylinder (E) of 1.5-cm diameter and 3-cm height was hung using thread. The height of this total set was adjusted to accommodate a glass container (F) of 4.2-cm diameter and 4.2-cm height; below it is having a headspace of 0.5 cm in between. A block G, 3.8-cm in diameter and 2-cm in height was fabricated and kept inside the glass container that was placed on the left-hand setup of balance. Coverslips were attached to the Teflon blocks for the purpose of placing mucin as well as formulation. The sides were then balanced in such a way that the right-hand side was 5 g heavier than the left. Mucin film was prepared by placing a drop of 3% w/v porcine mucin prepared in distilled water, on both the coverslips, spreading it and allowing to air dry. After preparation mucin film on the coverslips, a drop of formulation under investigation was placed on the lower coverslip. Formulation was allowed to adhere to the mucin film by removing the 5 g weight and kept in position for 5 min. Water was then added into the plastic container until the two coverslips detached from each other. Weight in grams of water required to separate the two surfaces was determined, and mucoadhesive force was calculated using the formula,
where F is t.ce in dynes/cm2; i is the minimum weight in grams required to break the mucoadhesive bond; and g is the acceleration due to gravity (cm/s2).
Microbiological Studies
The microbiological assay is based upon a comparison of the growth inhibition of bacteria by measured concentrations of antibiotics to be examined with that produced by known concentrations of a standard preparation of the antibiotic having a known activity. The main objective of this study was to evaluate the anti-microbial efficacy of the optimized formulations using Staphylococcus aureus (ATCC 27697) and compare with marketed formulation (cylinder-plate method). Approximately 20 ml of sterile nutrient agar seeded with 0.25 ml of test micro-organism was poured in sterile petri-plates and was allowed to set at room temperature. Cups were made with the help of a sterile borer on the solidified agar layer. A total of 100-μl samples of 0.9% w/v saline, blank PLGA formulation, FLU- and LEV-loaded PLGA formulation, blank chitosan-PLGA formulation, and FLU and LEV-loaded chitosan-PLGA formulation were added in the bored cups. The plates were then incubated at 37 °C for 24 h. The diameter of zone of inhibition was measured, and readings were taken in triplicate.
Determination of Residual Solvent
Acetone used in the preparation of nanoparticles belongs to class 3 solvents that have low toxic potential to human and has a limit of less than 5000 ppm per day. The level of residual organic solvent acetone in freeze-dried LEV-FLU PLGA nanoparticle and LEV-FLU chitosan-PLGA NP was determined by gas chromatography (Scion 436, Bruker) with a set of standard acetone solutions.
Ocular Tolerance Test by HET-CAM
Eye irritation can be caused by numerous factors, one of which includes the irritation caused by the various formulation components. In vitro techniques such as The Bovine Corneal Opacity and Permeability (BCOP) assay, the Hen’s egg test-chorioallantoic Membrane (HET-CAM) assay, The isolated chicken eye (ICE) assay and isolated rabbit eye (IRE) assay have been developed and validated for evaluation of ocular tolerability of developed formulations. In this study, modified hen’s egg chorioallantoic membrane (CAM) test was performed. The objective of the study was to evaluate and compare the eye irritation potential of blank and drug-loaded formulations. The potential irritancy of compounds may be detected in the CAM of the egg after exposure to test compounds by observation for changes like hemorrhage (bleeding from blood vessels), lysis (disappearance of small Cam blood vessels), and coagulation (either thrombosis in blood vessels or visual evidence of denaturation of proteins of extravascular membrane [14, 15].
Briefly, fresh fertile White Leghorn chicken eggs (50–60 g) were obtained from Central Poultry Development Organization (Mumbai). Eggs were candled prior to use and non-viable or defective eggs were discarded. Three eggs were taken for each formulation. Sodium chloride solution (0.9% w/v) in distilled water served as negative control, whereas 0.1 N sodium hydroxide solution in distilled water served as positive control. Eggs were placed in an incubator at 37 ± 0.2 °C and 40 ± 2% relative humidity and were hand rotated daily until day 8. On day 9, eggs were removed from the incubator for use in the assay. Air cell of the egg was marked, cut, and pared off. Care was taken while removing the eggshell to ensure that the inner membrane is not injured. Eggs were then placed back into the incubator for 30 min. After 30 min, eggs were removed from the incubator, and 0.3 ml of the test solutions was placed directly onto the CAM and observed for 300 s for sign of hemorrhage or lysis reactions. Images of endpoints were taken using stereomicroscope. A numerical score depending on the extent of hemorrhage, lysis, and coagulation was assigned to each CAM. The time of appearance of each irritant’s effect was recorded in seconds. Index describing the irritancy potential was calculated using the following formula [16]:
where H = time taken in seconds of hemorrhage reactions on CAM; L = time taken in seconds of vessel lysis on CAM; and C = time taken in seconds of coagulation formation on CAM.
After the treatment, the main reaction was scored within 300 s of time either (hemorrhage or lysis or coagulation) according to the following scheme:
0 = no reaction; 1 = slight reaction; 2 = moderate reaction; 3 = severe reaction; and mean irritation score was determined.
Results
Characterization of PLGA Nanoparticles
Particle size distribution and polydispersity index of LEV-FLU-loaded PLGA nanoparticles varied from 186.7 to 159.3 nm and 0.110 to 0.140, respectively which is suitable for ophthalmic preparation. Drug-loaded nanoparticles showed negative zeta potential similar to their blank PLGA counterparts, ranging from − 15.96 to − 17.86 mV as drug mixture:polymer ratio was varied. The percent entrapment efficiency of drug-loaded PLGA nanoparticles was found to be ranging from 29.45 to 39.37 ± 0.26 for LEV, whereas for FLU it was from 48.33 ± 1.19 to 29.28 ± 3.3 as the drug mixture:polymer ratio was varied. Drug mixture:polymer ratio 1:5 showed maximum entrapment for both the drugs, i.e., levofloxacin and flurbiprofen. All the results are shown in Table 1.
For the purpose of comparison between drug-loaded PLGA nanoparticle and drug-loaded chitosan-PLGA nanoparticle, it was necessary to keep the excipient profile as similar as possible. Hence, 0.2% w/v PLGA, 1.5% w/v poloxamer 188, and drug mixture: polymer ratio of 1:5 was kept similar for drug-loaded chitosan-PLGA nanoparticle as optimized drug-loaded PLGA nanoparticles.
In addition, chitosan-coated PLGA nanoparticles were prepared by loading the individual drugs in separate formulations. Table 2 reveals the results of drug-loaded chitosan-coated PLGA nanoparticles.
The chitosan-coated LEV-FLU-PLGA nanoparticles led to an increase in particle size and polydispersity index from 166.1 ± 3.7 nm with PDI 0.137 to 333.6 ± 5.13 nm particle size with PDI of 0.319 in comparison to the uncoated LEV-FLU-PLGA nanoparticles. Zeta potential of chitosan-coated nanoparticles was found to be 37.67 mV, wherein the drug entrapment was found to be 42.05 ± 1.87% for LEV and 45.26 ± 3.42% for FLU. The drug loading in nanoparticles was found to be in the range from 8.78 ± 3.58% to 10.1 ± 2.67% for LEV, and for FLU, it was 10 ± 2.46 to 11.6 ± 1.53%. The results of particles size, polydispersity index, zeta potential, entrapment efficiency, and drug loading of chitosan-coated LEV-PLGA nanoparticles and chitosan coated-FLU-PLGA nanoparticles are also indicated in Table 2.
In Vitro Release Study
The in vitro release profiles of LEV and FLU from PLGA nanoparticles and chitosan-PLGA nanoparticles are shown in Fig. 1 (a) and (b), respectively. LEV-FLU-PLGA and chitosan-coated LEV-FLU-PLGA nanoparticles released 87.44 ± 3.91% and 80.15 ± 0.87% of LEV, respectively after 24 h. LEV eye drop released 98.99 ± 0.58% drug in 4 h, whereas LEV-PLGA nanoparticles released 76.54 ± 4.01% of LEV in 24 h.
Similarly, LEV-FLU-PLGA and chitosan-coated LEV-FLU-PLGA nanoparticles released 96.24 ± 0.74 and 81.75 ± 2.45% of FLU, respectively, after 24 h. FLU eye drop released 88.75 ± 0.98% drug in 4 h, whereas FLU-PLGA nanoparticles released 76.85 ± 3.35% of FLU in 24 h.
Release Kinetics of Nanoparticles
Correlations of LEV and FLU release data for nanoparticles are explained by a Higuchi Peppas-Sahlin and Weibull models with regression coefficients, MSC, and AIC, and values are indicated in Table 3.
Ex Vivo Transcorneal Permeation Study
The transcorneal permeation profile of LEV as well as FLU is shown in Fig. 1 (c) and (d). Within 8 h of study, 41.19% of FLU and 31.14% of LEV permeated from their respective drug solutions. The percent drug permeation from LEV-FLU-PLGA and chitosan-coated LEV-FLU-PLGA nanoparticles was found to be 34.79 ± 1.81% and 38.09 ± 2.93% for LEV; whereas for FLU, release was found to be 45.04 ± 2.88% and 46.7 ± 4.11%, respectively. The values of apparent permeability of LEV and FLU-marketed preparations and nanoparticles are indicated in Table 4.
Mucoadhesion Study
Most methodologies found in the literature are based on the evaluation of mucoadhesive strength, that is, the peak detachment force required to break the bond between the model mucosal membrane and the mucoadhesive formulation. This method was used in the present study to ascertain the mucoadhesive strength of LEV-FLU chitosan PLGA nanoparticles. Chitosan-PLGA nanoparticles were found to be mucoadhesive with mucoadhesive strength and force of mucoadhesion of 10.08 ± 0.251 g (mean ± SD, n = 3) and 4370.97 ± 109.04 dynes/cm2 (mean ± SD, n = 3), respectively. Chitosan-PLGA nanoparticles showed mucoadhesiveness due to the presence of chitosan. Chitosan is a linear polyamine containing free amine groups that are readily available for crosslinking and shows strong mucoadhesion due to its cationic nature which allows chitosan to combine with negatively charged sialic acid residues present in mucus or with multivalent anions. Thus, by coating non-mucoadhesive PLGA nanoparticles with a cationic mucoadhesive polymer like chitosan may improve corneal retention and hence show improved ocular bioavailability.
Microbiological Studies
The results of zone of inhibition by cup plate method are shown in Table 5 as mean values.
The values of zone of inhibition produced by LEV solution, LEV-FLU PLGA nanoparticles, LEV-FLU chitosan-PLGA nanoparticles, and blank chitosan-PLGA nanoparticles were found to be 35.66 ± 1.21 mm, 36.66 ± 1.21 mm, 37.5 ± 1.64 mm and 20.5 ± 1.04 mm, respectively (Fig. 2).
Determination of Residual Solvent
Acetone used for the preparation of nanoparticles is considered moderately toxic and is known as an eye irritant. Acetone is classified as class 3 solvent and specifies a limit of less than 5000 ppm per day. The level of residual acetone was analyzed using static headspace gas chromatography, and chromatogram is shown in Fig. 3. Acetone was not detected in both the developed lyophilized nanoparticles, indicating their effective removal while processing.
Ocular Tolerance Test by HET-CAM
Irritation scores of blank PLGA nanoparticle, LEV-FLU PLGA nanoparticle, blank chitosan-PLGA nanoparticle, and LEV-FLU chitosan PLGA nanoparticle were found to be 0.068, 0.068, 3.39, and 3.095, respectively, as shown in Table 6, and vascular responses are shown in Fig. 4.
Discussion
Nanoprecipitation (solvent displacement) is the most widely explored method for the fabrication of PLGA nanoparticles, as it is a straightforward technique, easy to perform, and does not require extended shearing/stirring rates, sonication, or very high temperatures. Furthermore, all the ingredients were processed by membrane filtration followed by formulation in aseptic condition. The filtration of drug and polymer solution was done using sterile hydrophilic filters, i.e., nylon 6,6 membrane filter. The properties of hydrophilic filters are that it does not adsorb the hydrophobic materials that are passed through it. In our study, it is of hydrophobic nature, thus adsorption of polymer is negligible. Moreover, the adsorptive losses of both the drugs were also calculated through UV–visible spectrophotometry. A drug solution of levofloxacin and flurbiprofen (10 ppm) was prepared (n = 3) and passed through a nylon 6,6 membrane filter. The 10-ppm solution of both the drugs was analyzed by UV–visible spectrophotometry before and after filtration at 288 nm and 248 nm, respectively. The absorbance of both the drug solution (LEV: 0.726 ± 0.0045 and Flu: 0.867 ± 0.0036) was similar before and after filtration with a relative standard deviation (%) of less than 2. Thus, it confirmed no drug adsorption or adsorptive losses on the membrane filter.
For the topical ocular drug delivery systems, the particle size is an important parameter. If the particle induces increased tear flow, a rapid drainage of the instilled dose would occur and could reduce the precorneal residence time and thus reduce the bioavailability [6]. The observed narrow polydispersity index (0.110 to 0.140) is the indication of monodispersed nanoparticles. It was observed that the presence of coloaded LEV and FLU affected the particle size distribution as can be seen by comparing with the blank PLGA nanoparticles. Drug incorporation led to an increase in particle size, and as the drug mixture:polymer ratio was increased, there was not much change in particle size and its distribution. The similar results were reported for PLGA nanoparticles of levofloxacin by Gupta et al. [2], for flurbiprofen by Öztürk et al. [11], and for paclitaxel-flurbiprofen-coloaded PLGA nanoparticles by Şahin et al. [4]. The negative value of zeta potential of PLGA nanoparticles promotes particle stability because the repulsive forces prevent aggregation with ageing. It has been previously reported that the negative charge on PLGA nanoparticles is due to the ionization of carboxylic end groups of PLGA. The results presented here are in accordance with those obtained for similar systems [11].
Percent encapsulation efficiency results are shown in Table 2. EE(%) is one of the most important physicochemical characteristics of nanoparticles which governs the effective dose in clinical use. Nanoparticles with higher encapsulation of drug will be required in less quantity for the effective dose in clinical use. When the drug concentration is high, and polymer as well as volume of organic solvent is kept constant, the solubilization capacity of polymer reduces; hence, the drug does not solubilize in polymer, leading to decrease in entrapment of drug. As the drug concentration decreases there is relatively more polymer available for drug entrapment, leading to increased physical entrapment of the drug. However, after a certain ratio, it is observed that there is a decrease or no change in entrapment. Reported studies indicate similar results in terms of entrapment efficiency [2, 17]. In the present study, it was seen that entrapment of both the drugs increased when drug mixture:polymer ratio was increased from 1:2 to 1:5. Beyond the ratio of 1:5, entrapment decreased; hence the ratio of 1:5 was selected to prepare surface-modified nanoparticles. FLU showed comparatively better entrapment efficiency as it is a hydrophobic drug and has more affinity for hydrophobic polymer PLGA when compared to hydrophilic drug LEV. It is reported that when the drugs were individually loaded in PLGA nanoparticles by similar method, LEV showed a maximum entrapment efficiency of 86.13% and FLU was that of 94.60% [2, 18]. The decrease of the drug entrapment efficiencies of PLGA and chitosan-coated PLGA nanoparticles could be attributed to the fact that in the present study, both the drugs were concomitantly loaded and since both of them are competing for the same polymer system, there was a decrease in individual drug entrapment.
The in vitro release profiles of LEV and FLU from PLGA nanoparticles and chitosan-PLGA nanoparticles are plotted in the Fig. 1 (a) and (b), respectively. Drug-loaded PLGA nanoparticles as well as chitosan-PLGA nanoparticles followed biphasic patterns of release consisting of an immediate release (burst effect), followed by a slower release profile. The initial burst release for both the drugs (initial 1 h) can be attributed to the un-entrapped drug present in the formulation and also the fraction of drug which is adsorbed or weakly bound to the surface of nanoparticles [19]. The results revealed that the drug release from chitosan-coated PLGA nanoparticles was more sustained than PLGA nanoparticles. This could be attributed to the presence of chitosan coating, which further retards the release of drugs [17, 20]. The sustained release was due to the slow diffusion of the entrapped drug from the nanoparticles. The fraction of drug distributed within the matrix shows release either by drug diffusion or erosion of matrix. The low molecular weight of both the drugs, i.e., FLU is 244.26 and that of LEV is 370.4 which improve the diffusion mechanism. At the end of 24 h, 76% of FLU and of 80% of LEV were released from the chitosan-PLGA nanoparticles and 96% of FLU and 87% of LEV were released from the PLGA nanoparticles [18].
Kinetic modeling of LEV and FLU release from nanoparticles is shown in Table 3. After obtaining the release profiles, data was transferred to the DDSolver program to determine the four important criteria: coefficient of determination (Rsqr, \({R}^{2}\), or COD), adjusted coefficient of determination (Rsqr_adj or \({R}_{adjusted}^{2}\)), Akaike Information Criterion (AIC), Model Selection Criterion (MSC). The highest \({R}^{2}\), \({R}_{adjusted}^{2}\) and \(MSC\) values and the lowest \(AIC\) values were used for evaluating Higuchi, Peppas-Sahlin, and Weibull model. Generally, MSC value of more than 2 to 3 indicates good fit [21].
In this study, the burst release effect was observed in initial 1 h of the in vitro dissolution testing, followed by the sustained drug release for remaining hours. Burst release leads to a high level of drug delivery in initial hours which is important for the delivery system to reach the therapeutic drug concentration for effective treatment [22]. When the kinetics results were examined, the values of \({R}^{2}\), \({R}_{adjusted}^{2}\), MSC, and AIC were very similar for the Peppas-Sahlin and Weibull models. In other words, a higher correlation was observed in the Peppas-Sahlin and Weibull models. Therefore, the results of this study indicate that the release of both LEV and FLU from nanoparticles (PLGA and chitosan-coated PLGA nanoparticles) is not predominantly driven by a solo mechanism, but a combined mechanism of Fickian (pure diffusion phenomenon) and non-Fickian release (due to the relaxation of the polymer chain between networks). Literature supports this kind of release behavior for nanoparticulate drug delivery [5].
The ability of drug molecules to diffuse through epithelial barriers depends on the chemical nature, size and conformation, lipid/water partition coefficient, and the degree of ionization of the drug molecules. Drug diffusion through cornea occurs through three primary layers, i.e., epithelium, stroma, and endothelium. Depending on physicochemical properties of drug diffusional resistance offered by individual layers differs greatly. Lipidic nature of epithelium offers the greatest resistance to the diffusion of ionic or relatively hydrophilic agents. The aqueous stroma is a major rate limiting barrier to the diffusion of hydrophobic agents. In the case of the developed surface-modified NPs coloaded with FLU and LEV, FLU (BCS Class II drug) showed better permeation due to its hydrophobic nature and, hence, can easily traverse transcellularly through the rate limiting hydrophobic corneal epithelial membrane. Permeability of both the drugs from PLGA nanoparticles showed an increase in percent of drug permeated through the excised cornea, wherein FLU showed 45.04% and LEV showed 34.79% drug diffusion. This increased permeation through nanoparticles may be attributed to the agglomeration of nanoparticles, as depot near the cornea from which drug is slowly delivered to the precorneal area [2]. Apparent permeability values indicates that co-inclusion of LEV and FLU in the nanoparticles considerably increased the penetration rate of the drug across the cornea. Chitosan-coated PLGA nanoparticles showed a significantly higher drug permeation capability compared with the commercial eye drops. This favorable penetration of LEV as well as FLU across the cornea could be attributed to the agglomeration of the nanoparticles in the conjunctival sac, thus forming a depot from which the drug is slowly delivered to the precorneal area [18]. Additionally, chitosan-PLGA nanoparticles showed mucoadhesiveness due to the presence of chitosan. Chitosan is a linear polyamine containing free amine groups that are readily available for crosslinking and shows strong mucoadhesion due to its cationic nature which allows chitosan to combine with negatively charged sialic acid residues present in mucus or with multivalent anions. Thus, by coating non-mucoadhesive PLGA nanoparticles with a cationic mucoadhesive polymer like chitosan may improve corneal retention and, hence, show improved ocular bioavailability.
In anti-microbial testing, a distinct zone of inhibition was observed in the petri-plate containing standard solution of LEV, drug-loaded nanoparticles, and blank chitosan-PLGA nanoparticles. Petri-plates with 0.9% saline and blank PLGA nanoparticles did not show any zone of inhibition. Blank chitosan-PLGA nanoparticles showed a distinct zone of inhibition. This is due to the presence of chitosan which has been proved to show anti-bacterial activity in general and also against S. aureus.
As is evident from Fig. 4, PLGA nanoparticles (both blank and drug-loaded) and saline did not show any reaction on CAM and, hence, was found to be non-irritant to the eye. Positive control was markedly red due to hemorrhage and lysis of blood vessels in contact with 0.1 N NaOH. However, chitosan-PLGA nanoparticles (both blank and drug-loaded) were found to be mildly irritant. This could be probably due to the acidic nature of formulation, i.e., pH 5 which was essential for the solubilization of chitosan. The present investigation was undertaken to evaluate the potential of chitosan-coated nanoparticle for ocular use; however, based on the results observed, use of chitosan glutamate is recommended instead of chitosan because it is water soluble and can be used at neutral pH.
To conclude, polymeric nanoparticles have been extensively investigated in ocular applications; not many studies have been conducted on nanoparticles as a drug delivery system for the combination delivery of an antibiotic and NSAIDS for bacterial conjunctivitis. In this study, we have successfully encapsulated both LEV and FLU in PLGA nanoparticles by nanoprecipitation method, which were then modified by coating with chitosan. Our results demonstrated that LEV-FLU-loaded chitosan-coated PLGA nanoparticles had optimal particle size with narrow PDI and positive zeta potential value which was suitable for topical ocular delivery. Sustained release with better tolerability at corneal site and comparable anti-microbial activity of developed nanoparticle was observed. Hence, within the scope of experimental design, we conclude that the developed formulation is suitable for sustained co-delivery of levofloxacin and flurbiprofen in treatment of bacterial conjunctivitis which would reduce dosing frequency and improve patient compliance. However, it warrants clinical evaluation and application.
Data Availability
The data that support the findings of this study are available from the corresponding author, Dr. Ujwala Shinde, upon reasonable request.
References
Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5–17.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J Drug Target. 2011;19:409–17.
El-Sayed MM, Hussein AK, Sarhan HA, Mansour HF. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev Ind Pharm [Internet]. Taylor & Francis. 2017;43:902–10. Available from: https://doi.org/10.1080/03639045.2016.1272120
Sahin A, Caban-Toktas S, Tonbul H, Yerlikaya F, Aktas Y, Capan Y. Development of paclitaxel and flurbiprofen Co-loaded PLGA nanoparticles: understanding critical formulation and process parameters using Plackett–Burman design. Istanbul J Pharm. 2019.
Yang H, Li J, Patel SK, Palmer KE, Devlin B, Rohan LC. Design of poly(lactic-co-glycolic acid) (plga) nanoparticles for vaginal co-delivery of griffthsin and dapivirine and their synergistic effect for hiv prophylaxis. Pharmaceutics. 2019;11:1–21.
Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64.
Al-Nemrawi NK, Alshraiedeh NH, Zayed AL, Altaani BM. Low molecular weight chitosan-coated PLGA nanoparticles for pulmonary delivery of tobramycin for cystic fibrosis. Pharmaceuticals. 2018;11.
Gadad A, Dandagi P, Mastiholimath V. Moxifloxacin loaded polymeric nanoparticles for sustained ocular drug delivery. Int J Pharm Sci Nanotech. 2012;5:1727–34.
Arafa MG, Girgis GNS, El-Dahan MS. Chitosan-coated PLGA nanoparticles for enhanced ocular anti-inflammatory efficacy of atorvastatin calcium. Int J Nanomedicine. 2020;15:1335–47.
Jain GK, Pathan SA, Akhter S, Jayabalan N, Talegaonkar S, Khar RK, et al. Microscopic and spectroscopic evaluation of novel PLGA-chitosan nanoplexes as an ocular delivery system. Colloids Surfaces B Biointerfaces [Internet]. Elsevier B.V. 2011;82:397–403. Available from: https://doi.org/10.1016/j.colsurfb.2010.09.010
Öztürk AA, Yenilmez E, Şenel B, Kıyan HT, Güven UM. Effect of different molecular weight PLGA on flurbiprofen nanoparticles: formulation, characterization, cytotoxicity, and in vivo anti-inflammatory effect by using HET-CAM assay. Drug Dev Ind Pharm [Internet]. Taylor & Francis. 2020;46:682–95. Available from: https://doi.org/10.1080/03639045.2020.1755304
Mahboobian MM, Seyfoddin A, Aboofazeli R, Foroutan SM, Rupenthal ID. Brinzolamide–loaded nanoemulsions: ex vivo transcorneal permeation, cell viability and ocular irritation tests. Pharm Dev Technol. 2019;24:600–6.
Ansari M, Sadarani B, Majumdar A. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. J Drug Deliv Sci Technol [Internet]. Elsevier; 2018;44:278–88. Available from: https://doi.org/10.1016/j.jddst.2017.12.007
Cazedey ECL, Carvalho FC, Fiorentino FAM, Gremião MPD, Salgado HRN. Corrositex®, BCOP and HET-CAM as alternative methods to animal experimentation. Brazilian J Pharm Sci. 2009;45:759–66.
Luepke NP. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem Toxicol. 1985;23:287–91.
Rangel KC, Villela LZ, Pereira K de C, Colepicolo P, Debonsi HM, Gaspar LR. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res [Internet]. Elsevier; 2020;50:101984. Available from: https://doi.org/10.1016/j.algal.2020.101984
Mohee R, Mudhoo A. Analysis of the physical properties of an in-vessel composting matrix. Powder Technol. 2005;155:92–9.
Veemvoemg ML. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J Pharm Sci. 2006;95:2393–405.
Shinde UA, Joshi PN, Jain DD, Singh K. Preparation and evaluation of N-trimethyl chitosan nanoparticles of flurbiprofen for ocular delivery. Curr Eye Res [Internet]. Taylor & Francis; 2019;44:575–82. Available from: https://doi.org/10.1080/02713683.2019.1567793
Pandit J, Sultana Y, Aqil M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif Cells, Nanomedicine Biotechnol [Internet]. Informa UK Limited, trading as Taylor 8 Francis Group. 2017;45:1397–407. Available from: https://doi.org/10.1080/21691401.2016.1243545
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–71.
Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–36.
Acknowledgements
We would like to thank the research group of Dr. Renuka Munshi Kulkarni of Nair Hospital, Mumbai for their valuable technical assistance with HET CAM studies.
Author information
Authors and Affiliations
Contributions
Ujwala A. Shinde: conceptualization, resources, supervision and project administration. Yusra Bharkat: methodology and validation. Kavita Singh: writing, review and editing.
Corresponding author
Ethics declarations
Ethics Approval
None.
Competing Interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Financial Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shinde, U., Barkat, Y. & Singh, K. Coloaded Surface–Modified PLGA Nanoparticles for Sustained Ocular Delivery of Levofloxacin and Flurbiprofen. J Pharm Innov 18, 2348–2361 (2023). https://doi.org/10.1007/s12247-023-09796-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09796-5