Abstract
Purpose
This work evaluates the probability and impact of using high-temperature accelerated aging for determining shelf life based on reaction mechanism changes.
Method
Simulations are made to calculate the probability of a significant error in determining shelf life based on high-temperature stability data. Published distributions of activation energies and varied transition temperatures (Tx, the temperature where both processes are equally involved) to calculate shelf impacts from mechanism changes using high temperature data to assign long-term shelf life.
Results
High-temperature mechanism changes with respect to individual degradation products rarely occur when using accelerated stability studies. Even in the uncommon scenario of a mechanism change with temperature, the probability of there being a practical error in a shelf life determination from using these data is calculated to be less than 25%. High temperature modeling does bring a prediction risk when there is either a phase change or secondary degradation.
Conclusion
High temperature data can reliably be used to determine long-term shelf life in most cases. Changes of mechanism with temperature rarely occur and when they do, most often they will not result in a longer shelf life assigned than will ultimately be observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A US Food and Drug Administration (FDA) guidance [1] asserts, “We do not believe it is reasonable to perform accelerated testing at very high temperatures for a very short time and expect to extrapolate results to a very long expiration dating period since the actual mechanism of degradation at high temperature may be different than at room temperature.” While no definitions were provided in the guidance for what constitutes very high temperatures, this has often been assumed to be any temperatures greater than the International Conference on Harmonisation (ICH) accelerated condition for room temperature, or 40 °C. However, recent predictive models based on accelerated aging have proven reliable even up to 90 °C for drug substances [2], small-molecule drug products (see, for example, [3,4,5,6]), and biological drug products (see, for example, [7,8,9,10,11,12]). The increased use and accuracy of such studies call into question the scientific validity of the concerns expressed in the FDA guidance. While the FDA statement can be viewed as only a guidance, these kinds of statements indicate significant concern to health authorities and applicants, often requiring detailed specific explanations for any use of predictive models. The present paper delves into the likelihood of such mechanism changes, when skepticism for use of high-temperature predictive stability is warranted based on known pitfalls and how, in most cases, such data can be used reliably even when there is in fact a mechanism change.
Mechanism Changes with Temperature
At a given temperature, molecules possess a distribution of energy values. Reaction rates increase with increased temperature as the percentage of molecules within this distribution with adequate energy to overcome a reaction barrier increases. The change in energy distribution with temperature, in general, follows the Arrhenius equation (Eq. 1), where k is a rate constant for a reaction, A is the pre-exponential factor (the rate limit when every molecule has sufficient energy to go over the reaction barrier), Ea is the activation energy or barrier height for the reaction, R is the gas constant, and T is the temperature in Kelvin.
For most drug products, especially in the solid state, the overall rate constant is difficult to determine since it represents a composite of separate rate constants from the different physical states (e.g., crystalline bulk, surface sites, amorphous sites, excipient-drug interfacial sites). One practical solution to this challenge that still enables the use of the Arrhenius equation invokes isoconversion principles wherein the rate is only determined for a specific amount of degradation [5]. In pharmaceutical applications, this has meant using the time to reach a specification limit to assign an effective isoconversion rate constant. It should be noted that this is not a true rate constant when the process is not linear. Using isoconversion links the timescale of the testing to the accelerated temperatures: the shorter the time for the testing, the higher the temperatures must be. Although it is not the focus of this paper, a term has been added to account for moisture’s impact on solid-state reactivity [13], as reflected in Eq. 2 (B is a humidity sensitive parameter and ERH is the equilibrium relative humidity).
Importantly, the equation indicates that there is no interaction term between temperature and humidity except to the extent that temperature impacts the moisture permeability of packaging which can be handled explicitly. This enables us to focus on high-temperature effects for the present discussion without regard to humidity effects on stability. For most small-molecule pharmaceutical products, shelf life is gated by the formation of specific degradation products related to the drug substance based on tight safety (toxicity) specification limits rather than loss of potency or other stability-indicating parameters. To be clear, drug-related impurities may not actually be hazardous; however, health authorities set tight specification limits for these degradants absent specific data or justifications supporting their safety levels. Given shelf life limited by growth of related substances, for there to be an issue with use of high temperature data based on a mechanism change, the transformation of the drug to a specific degradant must proceed by at least two competing mechanisms that have differing activation energies. For example, one of the most common degradation processes is hydrolysis [14]. It is theoretically possible that a specific hydrolytic process could proceed by both catalyzed (lower activation energy) and uncatalyzed (higher activation energy) mechanisms. If the dominant mechanism changes with temperature and causes an error in the prediction of the long-term stability, the temperature at which the change occurs and the magnitude of the difference between the two activation energies determine the degree of error induced.
A critical factor dictating the degree of error introduced by using high-temperature data to project shelf life is the temperature where both mechanisms show equivalent rates of degradant formation, Tx, as described in Eq. 3 (assuming no humidity dependence).
Here, ∆Ea is the difference in activation energies between two mechanisms to the same product, R is the gas constant, and ∆lnA is the difference in collision frequencies between the two mechanisms. Values of Tx fit into three categories with respect to the high temperatures used for accelerated stability modeling: (1) Tx is at a lower temperature than the long-term storage conditions, (2) Tx is at a greater temperature than the highest accelerated condition used to predict stability, or (3) Tx is within the range of the storage and accelerated conditions. In the first two cases, the impact of any mechanism change will be less significant.
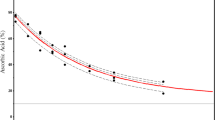
The degree to which a high-temperature change in mechanism results in a change in the projected shelf life also depends on the relative activation energies of the different mechanisms. For a shelf life to be overestimated from accelerated data, the activation energy of the predominant reaction mechanism at higher temperatures must be greater than that of the predominant reaction mechanism at lower temperatures. The opposite case, where the predominant reaction mechanism at higher temperatures has a lower activation energy, results in an underprediction of shelf life and a more conservative estimate of stability. The distribution of activation energies for decomposition of drug-like molecules in solution has been reported to be 99 ± 29 kJ/mol [15]. This value and distribution are derived experimentally for a wide range of degradation pathways; however, it represents a lower mean and wider distribution than observed for actual drug products since many of the lower end activation energy molecules are too unstable to isolate. Nonetheless, we can take this distribution as a conservative extreme. Based on this assumed distribution, there is approximately a 25% probability that for those cases where two mechanisms can produce the same degradation product, the activation energy of the higher temperature mechanism is equal or greater than 29 kJ/mol more than the lower temperature mechanism (calculated assuming a normal distribution with a 29 kJ/mol standard deviation using a Monte Carlo simulation for potential outcomes). Assuming a high-temperature change in mechanism with this 29 kJ/mol difference in activation energies and a Tx within the temperature range (56 °C), Fig. 1 shows a calculated Arrhenius plot of stability data, using exact rates at 50, 60, 70, and 80 °C to make a linear projection to 25 °C. As can be seen in this example calculation, the mean predicted 25 °C rate (23.2 ppm/week, or a shelf life of 4.14 years with a specification limit of 0.5%) is below the actual rate (29.5 ppm/week; shelf life of 3.26 years) and would suggest a longer shelf life than reality. However, Fig. 1 also shows that if one assumes a reasonable experimental error bar for the rate data (10% RSD assumed), the actual shelf life falls within the 95% confidence interval typically used to assign shelf life from accelerated data (29.6 ppm/week; 3.25 years). This means that even in the unlikely event that there is a change in mechanism with the higher temperature mechanism having the greater activation energy and where the Tx is in a range that has a relatively high impact, it is unlikely that the difference in activation energies between the mechanisms will be sufficient to result in a longer shelf life assignment based on the practice of using the 95% confidence value propagated from the experimental errors.
Theoretical Arrhenius curve when there is a change in mechanism from low to high temperatures using four accelerated conditions (50, 60, 70, 80 °C) to predict the ambient (25 °C) shelf life. Calculations assume a difference in activation energies between high and low temperatures of 29 kJ/mol and a crossover temperature (Tx) of 56 °C (1/T of 0.00304 K−1). Calculations were carried out assuming a 10% relative standard deviation (RSD) in the rates (arbitrary units of ppm/week) measured at each temperature with errors propagated using a Monte Carlo simulation
Given that changes in mechanism at higher temperatures rarely cause a statistically significant error in prediction, it is worth reevaluating the caveat imbedded in the FDA guidance. Degradants are most often detected chromatographically using stability-indicating methods. In the scenario where two distinct drug degradation products are formed via a single mechanistic process each, if the reactions resulting in these two degradation products have different activation energies, the ratio of the two degradants will change as a function of temperature. Using high-temperature aging will accurately predict the behavior for each specific degradant even though the chromatogram will change appearance with temperature. It is even possible that a degradant with a high activation energy will only be evident at higher temperatures and not appear above noise levels at low temperatures. Nonetheless, the high temperature data will accurately predict the long-term stability behavior. In general, chromatograms containing multiple degradants will appear very different as a function of temperature, which is most likely the origin of the FDA guidance assertion of mechanism change: it is easy to confuse a change in degradation product distribution with a change in the underlying mechanism of reaction.
Non-Predictive Changes with Temperature
There exist temperature-dependent kinetic changes that represent more significant concerns for predictive modeling from accelerated testing than mechanistic switches. These can generally be anticipated from product knowledge or detected from inspection of the data. First, for high-temperature accelerated stability studies to accurately predict lower-temperature storage stability, the drug’s physical form generally must remain the same at accelerated conditions as it is under ambient storage. Phase changes that potentially impact stability behavior include, among other factors, drug crystallization (for amorphous drug forms), formation of solid solutions with excipients that melt, and aggregation and other higher order structural changes in solution. Another common discontinuity is the melting of frozen solutions. In each of these cases, the mobility of the drug molecules changes in a discontinuous fashion above the phase transition which can result in non-predictive accelerated stability behavior. For this reason, it is important to characterize physical form changes that occur with temperature or relative humidity for a drug product. Limitations on the temperature and relative humidity design space used for accelerated aging experiments more commonly arise from a physical form change than a mechanism change. If a phase change is crossed during the experiment, the discontinuous change in kinetics can usually be detected through data analysis and the affected conditions excluded.
Another potential issue where high-temperature results can be misleading is in the case of sequential reactions, described in Eq. 4, where the intermediate “B” is a primary degradant that limits shelf life.
In this case, B is formed from the drug “A” at a rate corresponding to k1 and lost with a rate corresponding to k2. Classically, the behavior of B as a function of time is characterized in terms of steady-state behavior; i.e., after an initial growth phase, the rates of formation and degradation of B become equal, and the concentration of B will remain constant until the level of A decreases sufficiently to decrease the rate of formation of B. In heterogeneous systems, such as solid dosage forms, there may be a limited amount of drug in a reactive state such that depletion of the active can occur at low total conversions. Depending on the relative activation energies for the formation and loss processes, the steady-state concentration of B can vary significantly. For example, when the activation energy for the loss process (k2) is higher than that for the formation process (k1), B may not be observed at all at high temperatures but may accumulate significantly enough at lower temperatures to limit shelf life. While this scenario can lead to deviations in the high-temperature-predicted extrapolations to low temperatures if undetected, it is often clear from the overall kinetic behavior observed at high temperatures that there is secondary degradation occurring. With sufficient data, accurate stability models for long-term conditions that account for secondary degradation can be generated.
Conclusions
Even though the product distribution as a function of temperature can change significantly with temperature, specific degradant formation rarely involves a change in mechanism at high temperatures. Even for cases where there is indeed a mechanism change, the impact is sufficiently minor in most cases that it will not result in an erroneous assignment of too long a shelf life. Using high temperatures for modeling long-term shelf life brings greater risk when there is discontinuous behavior due to phase changes than it does from mechanism changes. Another potential risk involves secondary degradation processes. These risks can be minimized by careful experimental design and examination of the accelerated stability data.
Data Availability
The theoretical datasets and Excel code used as part of the current study are available from the corresponding author on reasonable request.
References
“Expiration dating and stability testing for human drug products” [https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/expiration-dating-and-stability-testing-human-drug-products], 2014.
Williams HE, Bright J, Roddy E, Poulton A, Cosgrove SD, Turner F, Harrison P, Brookes A, MacDougall E, Abbott A, Gordon C. A comparison of drug substance predicted chemical stability with ICH compliant stability studies. Drug Dev Ind Pharm. 2019;45(3):379–86. https://doi.org/10.1080/03639045.2018.1542707.
Qiu F, Scrivens G. eds. Accelerated predictive stability (APS): fundamentals and pharmaceutical industry practices. Academic Press 2018.
McMahon ME, Abbott A, Babayan Y, Carhart J, Chen C, Debie E, Fu M, Hoaglund-Hyzer C, Lennard A, Li H, Mazzeo T, McCaig L, Pischel S, Qiu F, Stephens D, Timpano R, Webb D, Wolfe C, Woodlief K, Wu Y. Considerations for updates to ICH Q1 and Q5C stability guidelines: embracing current technology and risk assessment strategies. AAPS J. 2021;23:1–9. https://doi.org/10.1208/s12248-021-00641-6.
Waterman KC, Swanson JT, Lippold BL. A scientific and statistical analysis of accelerated aging for pharmaceuticals. Part 1: accuracy of fitting methods. J Pharm Sci. 2014;103(10):3000–3006; https://doi.org/10.1002/jps.24075.
González-González O, Ramirez IO, Ramirez BI, O’Connell P, Ballesteros MP, Torrado JJ, Serrano DR. Drug stability: ICH versus accelerated predictive stability studies. Pharmaceutics. 2022;14(11):2324. https://doi.org/10.3390/pharmaceutics14112324.
Rauk AP, Guo K, Hu Y, Cahya S, Weiss WF IV. Arrhenius time-scaled least squares: a simple, robust approach to accelerated stability data analysis for bioproducts. J Pharm Sci. 2014;103(8):2278–86. https://doi.org/10.1002/jps.24063.
Waterman R, Lewis J, Waterman KC. Accelerated stability modeling for peptides: a case study with bacitracin. AAPS PharmSciTech. 2017;18(5):1692–8. https://doi.org/10.1208/s12249-016-0635-7.
Kuzman D, Bunc M, Ravnik M, Reiter F, Žagar L, Bončina M. Long-term stability predictions of therapeutic monoclonal antibodies in solution using Arrhenius-based kinetics. Sci Rep. 2021;11(1):20534. https://doi.org/10.1038/s41598-021-99875-9.
Huelsmeyer M, Kuzman D, Bončina M, Martinez J, Steinbrugger C, Weusten J, Calero-Rubio C, Roche W, Niederhaus B, VanHaelst Y, Hrynyk M, Ballesta P, Achard H, Augusto S, Guillois M, Pszczolinski C, Gerasimov M, Neyra C, Ponduri D, Ramesh S, Clénet D. A universal tool for stability predictions of biotherapeutics, vaccines and in vitro diagnostic products. Sci Rep. 2023;13:10077. https://doi.org/10.1038/s41598-023-35870-6.
Shalaev E, Ohtake S, Moussa EM, Searles J, Nail S, Roberts CJ. Accelerated storage for shelf-life prediction of lyophiles: temperature dependence of degradation of amorphous small molecular weight drugs and proteins. J Pharm Sci. 2023;112(6):1509–22. https://doi.org/10.1016/j.xphs.2023.02.008.
Evers A, Clénet D, Pfeiffer-Marek S. Long-term stability prediction for developability assessment of biopharmaceutics using advanced kinetic modeling. Pharmaceutics. 2022;14(2):375. https://doi.org/10.3390/pharmaceutics14020375.
Waterman KC, MacDonald BC. Package selection for moisture protection for solid, oral drug products. J Pharm Sci. 2010;99(11):4437–52. https://doi.org/10.1002/jps.22161.
Waterman KC, Adami RC, Alsante KM, Antipas AS, Arenson DR, Carrier R, Hong J, Landis MS, Lombardo F, Shah JC, Shalaev E. Hydrolysis in pharmaceutical formulations. Pharm Dev Tech. 2002;7(2):113–46. https://doi.org/10.1081/PDT-120003494.
MacFaul PA, Ruston L, Wood JM. Activation energies for the decomposition of pharmaceuticals and their application to predicting hydrolytic stability in drug discovery. MedChemComm. 2011;2(2):140–2. https://doi.org/10.1039/C0MD00214C.
Acknowledgments
Extremely helpful suggestions and edits were provided by Drs. Maria Krisch and Kristina Flavier.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Waterman, K.C. Mechanism Changes with High Temperature: Significance for Use of Accelerated Aging Modeling of Pharmaceuticals. J Pharm Innov 18, 2459–2463 (2023). https://doi.org/10.1007/s12247-023-09791-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09791-w