Abstract
Purpose
Polyphosphazenes are synthetic polymers that contain phosphate-nitrogen molecules. Polyphosphazene’s inorganic main structure is capable of hydrolytic degradation and modulated by choosing the relevant side group. Poly[di(carboxylatophenoxy)-phosphazene (PCPP) is a polymer that is preferred due to its macromolecular structure, excellent film-forming, and microcapsule forming ability. It has biological activity when used with antigenic proteins of bacteria and viruses as microcapsule forms. Sodium chloride is added to the solution of the PCPP polymer to create coacervate microdroplets. These microdroplets are stabilized by cross-linking by adding calcium ions. PCPP is known as a biocompatible polymer; however, there are no studies on the cytotoxicity of the microparticle or water-soluble form of the PCPP.
Methods
To show the compatibility of PCPP on cell culture, different concentrations of PCPP’s cytotoxic effect were investigated. The microparticle form of PCPP polymer was obtained by the coacervation method. The size of these microcapsules was shown by the dynamic light scattering. Also, the cytotoxicity of PCPP microparticles and PCPP polymer in different concentrations were examined using mouse fibroblast cell lines by XTT assay. The number of cells undergoing apoptosis was determined by the DAPI staining assay. Concentration-dependent cytotoxicity was shown by this study.
Results
PCPP microparticles and soluble form of this polymer have shown no toxic effects in studied concentrations. The average size range of the PCPP microparticles was found to be 2828 µm. This study also showed that, when ISO standards are referenced, both soluble and microparticle form of the PCPPdid not demonstrate any toxic effect on the L929 cell line.
Conclusions
PCPP is not toxic at the concentrations studied in in vitro cell culture and supports the literature regarding its biocompatibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyphosphazenes are synthetic polymers that contain phosphate-nitrogen molecules. It was first synthesized by Liebig and Wohler in 1834 by the reaction between ammonium chloride and phosphopentachloride. Polyphosphazene’s inorganic main structure is flexible. It can be degraded hydrolytically and the structure can be modified by choosing the appropriate side group [1].
Poly[di(carboxylatophenoxy)-phosphazene] (PCPP) is a multifunctional polymer. It has advantageous properties such as injectability, water-solubility, biodegradability, and controllable hydrolytic degradation rates [2]. Its main structure allows the formation of microcapsule form by various methods. One of them is coacervation. In the coacervation method, drugs and antigens are active substances and the polymer acts as a shell material for them [3, 4]. These coacervated microcapsules could be used for pharmacological formulations. For example, antigen encapsulated microcapsules were benefited in vaccine studies against bacterial, protozoal, and viral agents [5] because of their adjuvant properties. PCPP formulated with antigenic molecules, acts as an adjuvant by enhancing immune response, and released immune molecules (antigens) slowly [6]. This is critical in providing a continuous release of antigen during weeks to months so that the humoral immune response is stimulated repeatedly by slow-release formulations from polymer particles [7, 8].
Polyphosphazenes are generally known as biocompatible polymers. In vitro and in vivo biocompatibility of some polyphosphazenes were shown by previous studies [9]. Laurencin et al. showed poly[(ethyl glycinate) phosphazene] (PNEG) did not affect cell growth negatively [10]. They also showed that ethyl glycinate/methyl phenoxy substituted polyphosphazenes have no adverse effect on cell adhesion and proliferation [11]. Cytotoxicity of poly[bis(ethyl-4-aminobutyro) phosphazene] has been studied by Gumusderelioglu and Gur (2002) [12], where they show that extracts of the polymeric film had not significantly decreased the cell viability [9].
Andrianov et al. (2009) stated in their studies that the PCPP polymer is biocompatible and non-toxic [2, 13]. However, there are no studies on the cytotoxicity of the water-soluble form of the PCPP compared to the microparticle form depending on concentration on cell culture. The aim of this study is to produce PCPP microparticles and investigate the toxic effect of different concentrations of PCPP polymer and microparticles in vitro cell culture by XTT assay and DAPI staining methods.
Material and Method
Preparation of Polymer Solution
PCPP polymer is commercially available from Sigma (catalog no: 652717). The dried PCPP polymer was dissolved in PBS (130 mM NaCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4 Sigma) with the concentration of 0.2% (w/v). The solution was stirred at room temperature for 120 h [14].
Microencapsulation of PCPP
A 1.9 mL 6.2% (w/v) NaCl was added dropwise into the 1 mL of PCPP polymer (0.2% w/v) solution. It was stirred at room temperature for 20 min. The mixture was then added to 200 mL of 8.8% (w/v) CaCl2 solution. It was stirred at room temperature for 10 min. The final mixture was centrifuged at 3456 rpm (1340 g) for 10 min. Supernatant was discarded, and the pellet was washed by 10 ml of dH20 [15].
Size Analysis of Microcapsules by Dynamic Light Scattering
One milliliter of solutions containing PCPP microparticles were analyzed in terms of size and potential by Zeta-Sizer (Malvern Zetasizer MPT-2) device.
L929 Cell Culture
The L929 mouse fibroblast cell line was widely used in toxicity studies [16]. L929 cells were transferred to DMEM-F12 medium containing 10% FBS, penicillin–streptomycin, and centrifuged at 1000 rpm for 5 min. The supernatant was discarded, and 5 mL of DMEM-F12 (10% FBS) was added to the pellet and transferred to the sterile cell culture flask. Cells were incubated at 37 °C with 5% CO2. When cells were 80–90% confluent, they were detached using Trypsin–EDTA and were transferred to Falcon tubes containing 5 mL of medium. They were centrifuged at 1000 rpm for 5 min. The pellet was resuspended and diluted in the Trypan Blue solution for cell counting.
Cytotoxicity Experiments: XTT Assay
L929 cells were seeded in a sterile, flat bottom, 96-well plate, with the concentration of 1 × 104 cells/well. Cells were incubated for 24 h at 37 °C with 5% CO2. PCPP microparticles and soluble PCPP polymer at concentrations of 1 µg/mL, 2 µg/mL, 4 µg/mL, 6 µg/mL, 8 µg/mL, 10 µg/mL, 20 µg/mL, 30 µg/mL, 40 µg/mL, 50 µg/mL were added to the cells and PBS was added to the control group. Cells were incubated at 37 °C with 5% CO2 for 24 h. The medium in the plate was removed, and 100 µL of XTT solution (4 mg tetrazolium salt had dissolved in 10 ml cell culture medium with 10 µL PMS) was added to each well. Cells were incubated for 4 h, and absorbance was measured by multiple readers (Labline) at 450 nm [17].
DAPI Staining
The DAPI staining method was used to determine the nuclear morphologies of cells. L929 cells (3 × 105) were seeded into 24-well plates and incubated for 24 h. Cells were treated with PCPP microparticles and soluble PCPP polymer at concentrations of 1 µg/mL and 50 µg/mL for 24 h. Following treatment, cells were stained with DAPI solution. The plate was incubated at 37 °C for 15 min in a dark environment. All the liquid in the plate was aspirated and washed with PBS. After washing, the cells were examined using a fluorescent microscope [18].
Results
Size Analysis Results of PCPP Microparticles with DLS
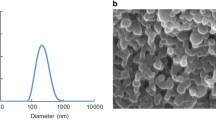
As seen in Fig. 1, the size range of PCPP microparticles was found to be 2.237 µm, and the PDI value was 0.513. Measurements were repeated, and the PCPP size range was found to be 2.239 µm with a 0.533 PDI value. In another measurement, the PCPP size range had been found 2.434 µm with a 0.632 PDI value. Regarding these three measurements, the average size range and PDI value of PCPP microparticles was found as 2828 µm and 0.559 respectively.
In the measurements taken, a single peak was observed from the microparticles. The obtained peaks appear to have a monodisperse structure. According to previous studies, the size range should be 1 and 10 µm of coacervate microcapsules [13]. The measurements show that the PCPP microparticles are the expected size.
Results of XTT Cytotoxicity Assay
The effects of PCPP microparticle and soluble form on viability on the L929 (fibroblast) cell line were tested at various concentrations. Cell viability was expressed as percent viability by the control, and results of different concentrations were shown in Fig. 2. The viability of cells was 147.9% in a lower concentration of polymer (1 µg/mL). The toxicity profile of PCPP polymer was similar to microparticles, and they have the least toxic effect at a concentration of 1 µg/mL. It has been seen that the low concentration of both PCPP polymer and microparticles have a viability value above 100%.
Cell viability after PCPP microparticles and polymer treatments decreased at higher concentrations, but cell viability at these concentrations was still higher than 80%. According to ISO standards, results over 80% are considered non-toxic [19]. Our results also showed that, according to ISO standards, both PCPP polymer and microparticles have no toxic effect on studied concentrations.
Results of DAPI Staining Assay
DAPI (4′, 6-diamidino-2-phenylindole dihydrochloride) is a fluorescent substance and widely used to mark the apoptotic cell [20]. L929 cells, exposed to microparticle and soluble form of PCPP polymer at high (50 µg/mL) and low (1 µg/mL) concentrations for 24 h, were stained with DAPI dye. The number of apoptotic cells which was exposed with PCPP microparticles and PCPP polymer is shown in Table 1.
According to the DAPI results, the images of apoptotic cells with high and low concentrations in the polymer and microparticles had shown in Fig. 3 and Fig. 4.
When the DAPI and XTT results had been compared, the viability values corresponding to the concentrations found in both showed consistency. The apoptotic effects of the polymer and microparticles are not high when compared to the control. The apoptotic effect of the polymer in cells is less than in the form of particles. It also appears to cause less cell apoptosis at low concentrations.
Discussion and Conclusion
In the present study, we demonstrated that the microparticle and soluble form of this polymer had no significant toxic effect in examined concentrations. PCPP has a high potential as a vaccine carrier and adjuvant against a wide range of bacterial and viral pathogens [21], and it is often used in microparticle form. The microparticle form of this immunostimulant polymer is releases the active substance in a controlled manner [5].
In the previous study of A. K. Andrianov et al. (1998), PCPP microparticle size was found to be between 1 and 10 µm [13]. In our present study, we found the average size range of PCPP microparticles as 2828 µm. The size range of microparticles produced in this study is compatible with the A. K. Andrianov et al. (1998). Moreover, the microparticles have a monodisperse structure as a result of the DLS method.
Furthermore, this study and relevant studies stated that soluble and microparticle forms of PCPP are biocompatible [1, 13, 15, 22]. However, there is no comparative study to investigate the cytotoxic effect of PCPP in cell culture. Therefore, in this study, the biocompatibility of microparticles and soluble form of PCPP were compared. The toxic effect and viability percentages of different concentrations of PCPP polymer and PCPP microparticles on L929 cells had been investigated by XTT and DAPI methods. According to ISO standards, results over 80% are considered non-toxic for toxicity values of substances [19]. This study also showed that when ISO standards-referenced, both soluble and microparticle forms of PCPP in studied concentrations have no toxic effect.
In addition to the XTT assay to determine cell viability, the DAPI staining method demonstrated its apoptotic effects. When these cells had compared with the control group, it was seen that the apoptotic effect is not significant. The results thus obtained are consistent with the XTT results.
According to the data obtained, the PCPP polymer is not toxic at the concentrations studied in vitro cell culture and supports the biocompatibility results in the previous studies.
References
Andrianov AK, DeCollibus DP, Gillis HA, Henry HK, Marin A, Prausnitz MR, Mutwiri G. Poly [di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci. 2009;106(45):18936–41.
Andrianov AK (Ed.). Polyphosphazenes for biomedical applications. John Wiley & Sons. 2009.
Sawant S, Shegokar R. Bone scaffolds: what’s new in nanoparticle drug delivery research?. In Nanobiomaterials in Hard Tissue Engineering (pp. 155–187). William Andrew Publishing. 2016.
Ghosh SK. Functional coatings and microencapsulation: a general perspective. Functional coatings. 2006;1–28.
Shim DH, Ko HJ, Volker G, Potter AA, Mutwiri G, Babiuk LA, Kweon MN. Efficacy of poly [di (sodium carboxylatophenoxy) phosphazene](PCPP) as mucosal adjuvant to induce protective immunity against respiratory pathogens. Vaccine. 2010;28(11):2311–7.
Andrianov AK, Marin A, Chen J. Synthesis, properties, and biological activity of poly [di (sodium carboxylatoethylphenoxy) phosphazene]. Biomacromol. 2006;7(1):394–9.
Andrianov AK, Chen J. Polyphosphazene microspheres: preparation by ionic complexation of phosphazene polyacids with spermine. J Appl Polym Sci. 2006;101(1):414–9.
Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–90.
Baillargeon AL, Mequanint K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. BioMed research intern. 2014.
Laurencin CT, Morris CD, Pierres-Jacques H, Schwartz ER, Keaton AR, Zou L. The development of bone bioerodible polymer composites for skeletal tissue regeneration: studies of initial cell attachment and spread. Polym Adv Technol. 1992;3:369–364.
Laurencin CT, Norman ME, Elgendy HM, El-Amin SF, Allcock HR, Pucher SR, Ambrosio AA. Use of polyphosphazenes for skeletal tissue regeneration. J Biomed Mater Res. 1993;27(7):963–73.
Gümüşderelioǧlu M, Gür A. Synthesis, characterization, in vitro degradation and cytotoxicity of poly [bis (ethyl 4-aminobutyro) phosphazene]. React Funct Polym. 2002;52(2):71–80.
Andrianov AK, Chen J, Payne LG. Preparation of hydrogel microspheres by coacervation of aqueous polyphosphazene solutions. Biomaterials. 1998;19(1–3):109–15.
Andrianov AK, Svirkin YY, LeGolvan MP. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromol. 2004;5(5):1999–2006.
Garlapati S, Eng NF, Wilson HL, Buchanan R, Mutwiri GK, Babiuk LA, Gerdts V. PCPP (poly [di (carboxylatophenoxy)-phosphazene]) microparticles co-encapsulating ovalbumin and CpG oligo-deoxynucleotides are potent enhancers of antigen specific Th1 immune responses in mice. Vaccine. 2010;28(52):8306–14.
Braakhuis HM, Park MV, Gosens I, De Jong WH, Cassee FR. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part Fibre Toxicol. 2014;11(1):18.
Cakir-Koc R, Budama-Kilinc Y, Kokcu Y, Kecel-Gunduz S. Molecular docking of immunogenic peptide of Toxoplasma gondii and encapsulation with polymer as vaccine candidate. Artificial cells, nanomedicine, and biotechnology. 2018;46(sup2):744–54.
Pajaniradje S, Mohankumar K, Pamidimukkala R, Subramanian S, Rajagopalan R. Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. BioMed research intern. 2014:2014.
I. S. O. (ISO), Iso 10993–5: 2009. biological evaluation of medical devices–part 5: tests for in vitro cytotoxicity, 2009.
Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora 1. Limnol Oceanogr. 1980;25(5):943–8.
Temel M. Bazı diamin ve dihidroksi türevli, ağ yapılı polifosfazen mikrokürelerin sentezi ve yapısal özelliklerinin incelenmesi (Master's thesis, Bilecik Şeyh Edebali Üniversitesi, Fen Bilimleri Enstitüsü). 2015.
Rothemund S, Teasdale I. Preparation of polyphosphazenes: a tutorial review. Chem Soc Rev. 2016;45(19):5200–15.
Funding
This work was supported by the TUBITAK- 3001—Startup Research and Development Projects Support Program (Grant Number: 118S721).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ustun-Karatop, E., Cakır-Koc, R. Biocompatibility of Poly[di(carboxylatophenoxy)-phosphazene] Polymer: In Vitro Cytotoxicity in Cell Culture. J Pharm Innov 17, 1199–1204 (2022). https://doi.org/10.1007/s12247-021-09598-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09598-7