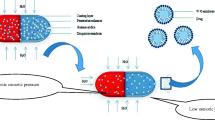
Abstract
Objective
The aim of this work was to prepare push-pull osmotic pump capsules using crosslinked hard gelatin capsules as a structural assembly for delivery of four model drugs with different water solubilities including diltiazem hydrochloride, propranolol hydrochloride, ambroxol hydrochloride, and paracetamol.
Methods
A hard gelatin capsule was crosslinked in formaldehyde vapor for 12 h. Then, a push-pull osmotic pump capsule was prepared, and formulation factors were investigated, i.e., the amount and solubility of model drugs, the amount of polyethylene oxide in pull layer, and size of the capsule. Drug release was evaluated to clarify the release characteristic in several release mediums.
Results
Results showed that drug release was independent of drug solubility, drug amount, and capsule size. Almost all of the drug release approached Higuchi’s release model. However, ambroxol hydrochloride could not deliver via this device because of its rather high-density drug particle. Reduction of the polyethylene oxide amount resulted in less drug release. Increasing osmolality of the medium reduced drug release. Drug release studies using a medium with digestive enzymes did not alter drug release compared to medium without enzymes. Push-pull osmotic pump capsules prepared from stored crosslinked hard gelatin capsule shells provided reproducible drug release characteristic.
Conclusion
This developed push-pull osmotic pump capsule is an alternative osmotic pump device for delivery of drugs with different water solubilities.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral drug administration is the most widely used drug administration route nowadays, due to its convenient way compared to other routes. However, the rate and amount of drug absorption from conventional preparations may vary and be unpredictable, depending on physicochemical properties of the drug, the presence of pharmaceutical excipients, food consumption, pH of gastrointestinal (GI) tract, GI motility, etc. Subtherapeutic and supratherapeutic plasma drug levels are usually found, causing treatment failure and side effects in some patients, respectively. Ideal oral drug delivery should deliver a measurable and reproducible amount of the drug to the target site over an extended period of time. A controlled release dosage form provides a uniform extent of the drug at the absorption site. It can maintain plasma drug concentrations within a therapeutic range. A controlled release dosage form minimizes side effects and also decreases the dosing frequency. An osmotic pump is one type of controlled release dosage forms which produces a measurable and reproducible amount of the drug over an extended period [1]. In general, osmotic pump system has advantages over other types of controlled release system in terms of independent of drug release from pH of GI tract, GI motility, and concomitant food intake [1].
The elementary osmotic pump (EOP) is the first oral osmotic pump that can be used in humans. However, the EOP is unsuitable for delivery of water-insoluble drugs [2]. The push-pull osmotic pump (PPOP) was developed by Alza Corporation. PPOP is a two compartments device; the first compartment is a push layer which contains a swellable polymer with an osmogen. The other compartment is a drug layer or a pull layer containing the active drug and entraining agent. The tablet core is coated with a semipermeable membrane. Finally, the tablet is drilled to produce the delivery orifice on the drug layer side [3]. During the laser drilling process, side identification is needed; thus, inorganic pigment is usually added into the push layer. During the operation, water is drawn into both compartments simultaneously. Following imbibition of water into the system, the drug would be in a dissolved state or as suspended particles depending on its solubility properties. As the polymer in the push layer expands, it pumps the drug solution or suspension out via the delivery orifice [4, 5]. This allows both water-soluble and water-insoluble drugs to be deliverable using PPOP.
Capsule-based osmotic pumps have been introduced using a cellulose acetate (CA) capsule shell for controlling water permeation into the osmotic pump. Waterman et al. produced a CA capsule using a molding method with several steps. Firstly, Tween® 80 solution was sprayed onto the high-density polyethylene mold. Then, a cellulose acetate solution was coated onto the mold and then dried. The CA capsule shell was trimmed using a blade. The capsule shell was removed from the mold using positive air pressure. Then, the capsule shell was drilled to produce the delivery orifice at the end of capsule cap. Finally, push and pull tablets were filled into this capsule shell to obtain the universal capsule-based PPOP [6]. Liu et al. prepare CA capsules using a Coni-Snap® hard gelatin capsule as a mold. A coating solution containing CA and plasticizer was filled into a Coni-Snap® hard gelatin capsule. Then, the filled Coni-Snap® capsule was dried to obtain a CA capsule shell inside the molding capsule. Finally, the shell was withdrawn from the Coni-Snap® capsule shell, and the obtained capsule is used in the further step of osmotic pump preparation [7]. Jin et al. prepared the colon-specific osmotic pump capsule shells by the dipping technique. Stainless steel molds were dipped into a coating solution containing CA, Eudragit® S100, and plasticizer. They were slowly withdrawn from the coating solution at a constant speed, followed by horizontal rotation. The above step was done three times, then dried. They were withdrawn from the molds by capsule pliers and cut into the desired size. The tip of capsule cap was laser-drilled to produce the delivery orifice. Finally, the delivery orifice was sealed with Eudragit® S100 solution [8]. Sun et al. prepared an enteric positioning osmotic pump capsule by dipping method. Stainless steel molds were dipped into coating solution containing cellulose acetate and hydroxypropyl methylcellulose phthalate HP50 three times, then, dried. The obtained enteric positioning osmotic pump capsules were removed from the mold by capsule pliers and cut into sizes. They were laser drilled to produce the delivery orifice followed by sealing the hole with hydroxypropyl methylcellulose phthalate HP50 [9]. However, CA capsule shell preparation is a complex procedure and requires a specific design of the equipment at industrial levels compared to using a hard gelatin capsule shell which is commonly available.
Osmotic pump drug delivery systems are usually prepared as tablet-based products, and a number of products on the market are based on this technology platform. For the past few years, the authors have introduced the crosslinked hard gelatin capsules (HGCs) coated with CA to prepare an EOP for delivery of the drugs, and it was found that the crosslinked HGCs were another promising structural assembly for an EOP. The authors succeeded in using the crosslinked HGCs as a structural assembly of EOP for delivery of diltiazem hydrochloride and propranolol hydrochloride, which are freely water-soluble and water-soluble model drugs, respectively [10]. Another study also characterized the application of HGCs to prepare an EOP system of a sparingly water-soluble drug and the physicochemical property as well as mechanical property of crosslinked HGCs. It was found that the EOP capsules were more suitable for delivery of a freely water-soluble drug than a sparingly water-soluble drug [11]. We thus expected that these crosslinked HGCs can also be applicable as structural assemblies of another type of osmotic pump such as PPOP. To the best of our knowledge, application of crosslinked HGCs as a structural assembly for a PPOP system has not previously been reported. The aim of this work was, therefore, to prepare a PPOP capsule using crosslinked HGCs as a structural assembly for delivery of model drugs with different water solubilities including diltiazem hydrochloride, propranolol hydrochloride, ambroxol hydrochloride, and paracetamol. Formulation factors were investigated i.e., the amount and solubility of a model drug, the amount of polyethylene oxide, and the size of the capsule. Drug release was also evaluated to clarify the release characteristic in various release mediums.
Materials and Methods
Materials
Diltiazem hydrochloride (DIL HCl) was obtained from Siam Pharmaceutical Co., Ltd., Thailand. Propranolol hydrochloride (PRO HCl) was purchased from Changzhou Yabang Pharmaceutical Co., Ltd., China. Ambroxol hydrochloride (AMB HCl) was obtained from Biolab Co., Ltd., Thailand. Paracetamol (PAR) was purchased from Srichand United Dispensary Co., Ltd., Thailand. Formaldehyde (40%v/v) and sodium chloride (NaCl) were purchased from Carlo Erba, France. Colorless and transparent hard gelatin capsule no. 1 was a gift from Capsule Products Co., Ltd, Thailand. Cellulose acetate (Opadry® CA, composed of cellulose acetate 398-10 and polyethylene glycol 3350) and polyethylene oxide (PEO) (Polyox™ N-80 and Polyox™ Coagulant with approximate molecular weights (Mw) of 200 K and 5000 K, respectively) were gifts from Colorcon Inc., USA. Hydroxypropyl methylcellulose (grade E5) and polyethylene glycol 4000 were obtained from Onimax Co., Ltd., Thailand. Spray dried lactose was purchased from Molkerei Meggle Wasserburg GmbH & Co., Germany. Pigment (Adilake Carmoisine®) was purchased from Adinop Co., Ltd., Thailand. Pepsin and pancreatin were purchased from Sigma-Aldrich, USA. Methanol (HPLC grade) was purchased from Honeywell-Burdick & Jackson, USA. Other chemicals and organic solvents were analytical grades.
Evaluation of Drug Properties
Solubility
The 0.5 mL solvent—water, isoosmolality adjusted hydrochloric acid (HCl) pH 1.2, isoosmolality adjusted phosphate buffer solution (PBS) pH 6.8, simulated gastric fluid (SGF), simulated intestinal fluid (SIF), 0.45% NaCl, 0.9% NaCl, or 3% NaCl—was added to a microcentrifuge tube (n = 3). The excess amount of each model drug was then added. The mixture was shaken at 37 °C for 24 h in water bath. The obtained mixture was filtered using a syringe filter with pore size of 0.45 µm. The supernatant was diluted into proper concentration and analyzed for drug content by high performance liquid chromatography (HPLC) (Agilent 1260 infinity, Agilent, USA).
Apparent Density
The apparent density of drug powder was determined using the helium gas displacement technique. Drug powder was passed through a 60-mesh sieve to prevent drug agglomeration. The drug powder was accurately weighed and transferred to the microcell with a lid cover. The determination was performed in five replicates using a gas pycnometer (Ultrapyc 1200e, Quantachrome Instruments, USA). The means and SD of the apparent density of drug powder were reported.
Particle Size and Shape
The drug powder was passed through a 60-mesh sieve to break up powder agglomeration. Each model drug powder was analyzed for particle size using a laser diffraction particle size analyzer (Mastersizer 2000, Malvern Instruments Ltd., UK). The mean diameter; 10, 50, and 90 vol. % less than or equal to diameter (D10, D50, D90, respectively); and span were reported. In addition, a scanning electron microscope (SEM) was used to define approximate particle size and shape of drug powder.
Crosslinking of Hard Gelatin Capsules
Body and cap of HGCs no. 1 were separated, spread on Petri dish, and placed in desiccator that was pre-equilibrated with formaldehyde vapor. HGCs were exposed to formaldehyde vapor for 12 h. Then, the crosslinked capsules were removed from the desiccator and dried overnight in a hot air oven at 40 °C.
Preparation of Push-Pull Osmotic Pump Capsules
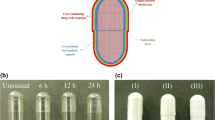
All ingredients of the pull and push layers were individually mixed by the geometric dilution technique. The composition of pull and push layers is shown in Table 1. The 90 mg of push layer mixture was added into the body of crosslinked HGCs. The 180 mg of pull layer mixture was then added. A capsule body was snapped with a capsule cap. The obtained capsule was subcoated and coated using manual dipping method. The capsule once dipped in the subcoating polymer solution (3%w/w hydroxypropyl methylcellulose E5 and 2%w/w polyethylene glycol 4000 was dissolved in a mixture of 95% ethanol and water, 1:1 volume ratio) and dried. Then, the subcoated capsule was dipped in a semipermeable membrane polymer solution (6%w/w cellulose acetate dissolved in the mixture of acetone and water, 9:1 volume ratio) and dried. Cellulose acetate coating process was repeated till eight coating layers were obtained. Then, the coated capsule was dried overnight in a hot air oven at 40 °C. Finally, the capsule was drilled with a 0.6-mm diameter needle at the top of capsule cap to make the delivery orifice. The schematic structure of the developed PPOP capsule is shown in Fig. 1a. Formulation factors were varied from the standard composition as presented in Table 1, i.e., the amount (10, 30, and 50 mg) and type of model drugs (DIL HCl, PRO HCl, AMB HCl, and PAR), the amount of PEO Mw 200 K (90, 130, 170 mg), and size of capsule (No. 1 vs. No. 2) in order to observe drug release characteristics. Moreover, the crosslinked HGCs stored for 12 months were used to prepare PPOP capsules to investigate the reproducibility of drug release.
Drug Release Study
Dissolution apparatus 2 (Model: 72-600-400, Hanson Research Corp., USA) was employed for drug release testing. The paddle speed was controlled at 100 ± 1 rpm. The release medium was 900 mL of water, HCl pH 1.2 (isoosmolality), PBS pH 6.8 (isoosmolality), 0.45% NaCl, 0.9% NaCl, 3% NaCl, SGF, or SIF. The medium temperature was controlled at 37 ± 0.5 °C. The PPOP capsules (n = 3) were sunk at the bottom of the vessel using a sinker. The release medium of 3 mL was withdrawn at various time intervals for 12 h. After sampling, the same volume of fresh medium was replenished. The withdrawn medium was filtered and injected into the HPLC instrument. Amount of drug release was calculated from the calibration curve of each model drug in each medium; then, drug release profiles were constructed. DDSolver, an add-in program was used for modeling of drug release [12]. The release rate and lag time of drug release were calculated based on the best-fitted release kinetic model i.e., zero-order, first-order, or Higuchi’s release model.
Drug Assays
The drug analyses were performed using HPLC instrument (Agilent 1260 infinity, Agilent, USA) equipped with a photodiode array detector. A Luna C18 column (250 × 4.6 mm i.d., 5 µm) with a temperature of 25 °C was used for separation. Methanol and acetate buffer pH 4.5 were used as a mobile phase. The isocratic system with a flow rate of 1 mL/min was used. The injection volume was set at 10 µL. The volume ratio of methanol and acetate buffer pH 4.5, quantitation wavelength, and total analysis time are shown in Table 2.
Results and Discussion
Drug Properties
DIL HCl, PRO HCl, AMB HCl, and PAR were chosen as model drugs to prepare PPOP capsules. Water solubility determinations (at 37 °C) of DIL HCl, PRO HCl, AMB HCl, and PAR were found to be 516.14 ± 22.19, 217.64 ± 9.78, 29.47 ± 4.06, and 20.82 ± 0.69 mg/mL, respectively (Table 3). This result indicated that DIL HCl and PRO HCl were freely water-soluble while AMB HCl and PAR were sparingly water-soluble. In addition, solubility of model drugs in other media is also shown in Table 3. DIL HCl was freely soluble in all media. PRO HCl was freely soluble in all media, but to be sparingly soluble in 3% NaCl. AMB HCl was sparingly soluble in all media, but slightly soluble in 3% NaCl. Finally, PAR was sparingly soluble in all media. In addition, with increase in ionic strength of the medium, the solubility of all model drugs decreased (Table 3). Sink condition refers to the excess solubilizing capacity of the release medium. Most sources recommended at least three times (3 ×) greater than the volume needed to completely solubilize the drug. Some sources recommended 5 × and even 10 × [13]. This present work used 10 to 50 mg of drug substances, and the volume of release medium was 900 mL. So, the volume of release medium ranged from approximately 100 to 50,000 times greater than the minimum volume to solubilize the drug. It could be confirmed that the conditions performed in release study reached the sink condition.
Apparent density of each model drug was determined using gas displacement technique (Table 4). Of all four drugs, AMB HCl and PAR had the highest and the lowest apparent densities, respectively. Intact AMB HCl and pulverized AMB HCl had similar apparent densities.
Particle size of each model drug is presented in Table 4. PAR and AMB HCl had the largest and the smallest particle sizes, respectively. PAR also had wider particle size distribution, which was due to the agglomeration of the drug particles due to electrostatic charging [14]. Particle size of AMB HCl became smaller after pulverization, but it had similar apparent density. SEM photomicrograph of four model drugs is shown in Fig. 2. DIL HCl particle was rod-shaped. AMB HCl particle had rhombus shape with some irregularities, while both PRO HCl and PAR particles had irregular shapes.
Push-Pull Osmotic Pump Capsules
Formaldehyde is a well-known chemical agent that can induce crosslinking of formulations containing gelatin [15, 16]. The previous work revealed that crosslinked HGCs could be used as a structural assembly for EOP. HGCs crosslinked in formaldehyde vapor for 12 h were suitable for use in an osmotic pump capsule delivery system due to its insoluble characteristic, low formaldehyde residue, and stable drug release property [11]. This work also used formaldehyde as a crosslinking agent to make crosslinked hard gelatin capsules for use as a structural assembly of PPOP system. Previous studies reported that the PPOP system in tablet form was appropriate for delivery of low water-soluble drugs while EOP was suitable for highly water-soluble drugs [4]. In this work, PPOP capsules were also tasked for delivery of both high and low water-soluble drugs in order to investigate how the system behaves in terms of release characteristics in comparison with the other osmotic systems. The physical appearance of developing PPOP capsules is shown in Fig. 1b, HPMC E5 was used as a subcoating polymer to improve coating efficiency of the cellulose acetate layer. A shiny surface was found when the capsule was subcoated with HPMC while a matted surface resulted when the capsule was coated with CA.
Effect of Amount and Water Solubility of Drug
Drug loading was an important factor affecting the drug release from the PPOP system especially poorly water-soluble drug. Malaterre et al. evaluated tablet core factors influencing isradipine release kinetics and loadability of PPOP system of which loading dose was a factor studied in their work. They found that over 90% isradipine was released within 14 h. The drug was loaded up to 20%, but this was not achieved for 30% drug loading [17]. Our study showed that drug doses varied from 10 to 50 mg or 3.70 to 18.52% of powder mixture filled into the PPOP capsule had a similar drug release pattern. It was observed that drug release was independent of loading doses (Fig. 3) and drug substances (Fig. 4) for all three model drugs except AMB HCl. Drug release of model drugs from PPOP capsules approached Higuchi’s release model rather than zero-order and first-order release model. Release rate of high water-soluble drugs—DIL HCl and PRO HCl—was independent of loading dose. In the case of PAR, a low water-soluble drug, release rate was apparently decreased while loading dose increased (Fig. 5a). Lag time of drug release seemed to be stable and independent of loading dose (Fig. 5b). However, lag times determined using DDSolver were apparently longer than, by direct observation from release graphs, which were approximately between 2 and 4 h. A few publications showed that drug release from PPOP system was independent of drug loading, which is similar to our findings. Waterman et al. developed universal PPOP cellulose acetate capsules for delivery of several drugs. They found that drug delivery from universal PPOP capsules did not depend on the drug dose [6]. Furthermore, Missaghi et al. investigated the critical core formulation of PPOP tablet by varying dose and water solubility properties of four model drugs including glipizide, theophylline, PAR, and verapamil HCl, a practically insoluble, slightly water-soluble, sparingly water-soluble, and water-soluble drug, respectively. Delivery of low dose of glipizide, theophylline, and PAR provided similar drug release, but drug releases were different when a medium dose was used. According to the variation of theophylline dose, drug release was comparable when low and medium doses of theophylline were used, while drug release of high dose theophylline was dramatically low. The effect of loading dose of theophylline was similar to those of verapamil HCl [18].
AMB HCl has low water solubility. The apparent density of AMB HCl is higher than other model drugs, so the PEO Mw 200 K could not suspend the drug particles during the operation. Finally, drug suspensions could not be pumped out through the delivery orifice of PPOP capsule. In addition, pulverized AMB HCl was also used in PPOP capsule formulation. The particle size of AMB HCl was decreased from 39.43 ± 0.25 to 27.16 ± 0.16 µm after pulverization; however, the apparent density of pulverized AMB HCl was the same as of the intact AMB HCl (Table 4). Both intact AMB HCl and pulverized AMB HCl could not be delivered using this developed PPOP capsule due to its high density; thus, AMB HCl PPOP capsule was not further investigated in the other topics. It was indicated that the apparent density had a major effect rather than particle size on drug delivery through orifice. In contrast, PAR PPOP capsule exhibited better release profiles, though PAR has low water solubility and larger particle size. This should be attributed to lower particle density of PAR compared to AMB HCl (Table 4).
Effect of Amount of PEO Mw 200 K
PEO is a water-soluble polymer that can be used to generate a high osmotic pressure. Uniformity of swelling rate of PEO ensured that the release rate of the drug from the delivery device was relatively constant. Owing to its inherent properties, PEO is one of the most popular materials for the osmotic pump [19, 20]. The increment of PEO amount in the pull layer of PPOP increased the viscosity of the drug suspension or drug solution within the system, which increased its stability by inhibiting the aggregation of precipitation of low water-soluble drugs [21]. Nie et al. varied the PEO Mw 100 K in pull layer from 200 to 300 mg to which the total weight of pull layer was 452 mg. The author mentioned that usage of PEO for 200 and 250 mg had no influence on drug release, while 300 mg had a lower drug release. However, the similarity factor when compared every two groups was higher than 50, indicating that all three groups had a similar drug release pattern [21]. There were other studies that reported the same results. The increase of PEO Mw 100 K in the pull layer could reduce the drug release rate [22, 23].
This present work is contrary to the previous works. The amount of PEO Mw 200 K in the pull layer of PPOP capsule was varied between 90 and 170 mg. Usage of 90 mg PEO Mw 200 K reduced the drug release dramatically (Fig. 6). Increasing the amount of PEO Mw 200 K increased the drug release rate (Fig. 5c) and decreased lag time of drug release (Fig. 5d). The result was similar to the study of Li et al. [24], when they varied the amount of PEO Mw 200 K in the pull layer of a tri-layer ascending osmotic pump tablet. It was described that a small amount of PEO could not entirely suspend the drug. Furthermore, we proposed that the optimal osmotic pressure difference between pull and push layers was an important factor affecting drug release. The larger difference of osmotic pressure due to low content of PEO Mw 200 K in pull layer could provide lower drug release from the osmotic device. According to our results, the highly water-soluble drug was unnecessary to suspend within the device because of it rapidly dissolving when in contact with water. However, the amount of PEO Mw 200 K had a similar effect for low water-soluble drugs. The result indicated that the amount of PEO Mw 200 K contained in the pull layer provided the same influencing effect on drug release of each drug type in our developed PPOP capsule.
Effect of Capsule Size
Capsule sizes, nos. 1 and 2, were another factor to be investigated. However, both capsule sizes were drilled to make the same 0.6-mm delivery orifice. The powder mixture was loaded in crosslinked HGC nos. 1 and 2 as proportion; thus, drug content in capsule no. 2 (8.33 mg/capsule) was slightly lower than capsule no. 1 (10 mg/capsule). Figure 7 displays the similarity in drug release from both capsule nos. 1 and 2 PPOP for all model drugs. Thus, it is unlikely that the size of capsule had an effect on drug release.
Effect of Osmolality of Release Medium
Osmolality plays an important role in drug release from the osmotic pump system. According to Poiseuille’s law of laminar flow, the pressure difference between the device and release medium affected the drug release rate [23, 25,26,27]. An increase in NaCl concentration (or osmolality) of release medium provided a slower drug release rate. Hill et al. investigated the osmolality differences between formulation and medium. The results showed that the higher difference of osmolality provided a higher drug release rate [28]. It was found that 0.45% NaCl, 0.9% NaCl, and 3% NaCl had osmolality values of 143.67 ± 0.58, 286.00 ± 2.00, and 948.00 ± 3.61 mOsm/kg, respectively. The variation of osmolality of release medium was used in order to mimic the effect of osmolality variation in the GI tract that might affect drug release from the PPOP capsule. Drug release of all three model drugs was decreased when osmolality of release medium increased (Fig. 8). Figure 5e, f show drug release rate and lag time of drug release in media with different osmolalities, respectively. The results indicated that the increment of NaCl concentration (or osmolality value) caused a lower drug release rate. A lower rate and a lower amount of drug release were found in 3% NaCl medium. It could be concluded that drug release from PPOP capsules in media with different osmolalities was independent of drug type but dependent of osmolality of release medium.
Effect of pH of Release Medium With and Without Digestive Enzyme
The other factor investigated in this work was the pH of release media. Previous works reported the effect of pH of release medium on drug release. The medium pH 1.0, 6.8, and 7.4 did not affect allopurinol release from PPOP tablets [21]. Liu and Xu prepared PPOP tablets containing nifedipine and evaluated drug release in different media. The results showed no significant difference in drug release in different media i.e., water, SIF, and SGF [29]. In addition, acetaminophen release from tablet-filled PPOP capsules in 0.01 N HCl and SIF were also similar [6]. These previous works were similar to the report of Liu et al. [30] that micronized nimodipine loaded PPOP tablets in three different media. All of above results indicated that drug release from an osmotic pump system was independent of pH of release medium. According to our work, DIL HCl and PAR released from PPOP capsule were similar for all four media: isoosmolality adjusted HCl pH 1.2, isoosmolality adjusted PBS pH 6.8, SGF, and SIF. But, DIL HCl release in SGF was fairly lower than in other media (Fig. 9).
Previous reports indicated the reversibility of crosslinking of HGCs by an enzyme in the GI tract [16, 31]. However, we mentioned that enzymes in the release medium did not alter drug release from PPOP capsules using crosslinked HGCs with a high crosslinking degree as shown in Fig. 9. Our result was comparable to the previous report of Jain and Naik that prepared crosslinked HGCs to make GI tract-resistant capsules. The crosslinked HGCs exhibited in vitro resistance in both SGF and SIF as well as in a human study [32].
Effect of Storage Time of Crosslinked HGCs
Crosslinked HGC shells were stored for 12 months, and after that, they were used to freshly prepare of PPOP capsules every 3 months to evaluate the effect of storage time on drug release. Drug release of both DIL HCl and PAR from PPOP capsules had similar release patterns (Fig. 10). However, variation of drug release of some formulations could be found. The highest variation was observed in drug release of DIL HCl. Delivery of DIL HCl using PPOP capsule might not appropriate for freely water-soluble drugs. DIL HCl dissolves well in water by its nature, so drug release could be more affected by its solubilization property besides the PPOP system.
Figure 5g, h display the relationship between storage time of crosslinked HGC shells and Higuchi’s release rate and lag time of PPOP capsules, respectively. Variation of drug release rates from PPOP capsules prepared from storage crosslinked HGC shells was in the range of 10%/h1/2. The lag time of drug release varied approximately in the range of 1 h. It follows that storage time of crosslinked HGC shells did not affect release rate and lag time of drug release from PPOP capsule.
Conclusions
This work proposed a PPOP system using crosslinked HGCs as a structural assembly for the delivery of model drugs with different water solubilities. Cumulative drug release was independent of different drug substances, loading doses, and capsule sizes. Drug release rate was increased when the amount of the pull layer (PEO Mw 200 K) increased, while lag time was decreased. The osmolality of release medium affected the drug release from PPOP capsules. Increasing osmolality reduced drug release rate, while lag time was increased. Dramatically low drug release was found in hyperosmolality medium. A drug release study using a medium with enzymes (pepsin or pancreatin) did not alter drug release, compared to medium without enzymes. Moreover, storage time of crosslinked HGCs did not affect drug release of the model drugs. The developed PPOP capsule was an alternative device for osmotic drug delivery systems and is applicable for delivery of high and low water-soluble drugs. However, all are employed as model drug substances regardless of therapeutic dose at this stage of the work. Further investigation is required to demonstrate an in vivo performance of this delivery device to prove its ability to maintain the therapeutic concentration of a drug in systemic circulation.
References
Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev Ind Pharm. 2000;26(7):695–708.
Liu L, Ku J, Khang G, Lee B, Rhee JM, Lee HB. Nifedipine controlled delivery by sandwiched osmotic tablet system. J Control Release. 2000;68(2):145–56.
Wong PSL, Gupta SK, Stewart BE. Osmotically controlled tablets. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified-release drug delivery technology, vol. 2. 2nd ed. Informa Healthcare: New York; 2003. p. 101–14.
Srikonda S, Kotamraj P, Barclay B. Osmotic controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. McGraw-Hill: New York; 2006. p. 203–29.
Allen LV, Popovich NG, Ansel HC, editors. Ansel’s pharmaceutical dosage forms and drug delivery systems. 9th ed. China: Lippincott Williams & Wilkins; 2010.
Waterman KC, Goeken GS, Konagurthu S, Likar MD, MacDonald BC, Mahajan N, Swaminathan V. Osmotic capsules: a universal oral, controlled-release drug delivery dosage form. J Control Release. 2011;152(2):264–9.
Liu D, Yu S, Zhu Z, Lyu C, Bai C, Ge H, Yang X, Pan W. Controlled delivery of carvedilol nanosuspension from osmotic pump capsule: in vitro and in vivo evaluation. Int J Pharm. 2014;475(1–2):496–503.
Jin D, Wang B, Hu R, Su D, Chen J, Zhou H, Lu W, Guo Y, Fang W, Gao S. A novel colon-specific osmotic pump capsule of Panax notoginseng saponins (PNS): formulation, optimization, and in vitro-in vivo evaluation. AAPS PharmSciTech. 2018;19(5):2322–9.
Sun Y, Zhu S, Lu W, Chen J, Sun C, Guo Y, Wang B, Gao S, Fang W, Hu R. A novel enteric positioning osmotic pump capsule-based controlled release system of sinomenine hydrochloride: in vitro and in vivo evaluation. J Drug Deliv Sci Technol. 2019;49:188–94.
Wichianprasit N, Kulvanich P. Osmotically controlled drug delivery system using a crosslinked and non-crosslinked hard gelatin capsule. In: Asian Federation of Pharmaceutical Sciences, Fukuoka, Japan, October 2009. p 193.
Monton C, Kulvanich P. Characterization of crosslinked hard gelatin capsules for a structural assembly of elementary osmotic pump delivery system. J Pharm Invest. 2019;49:655–65.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71.
Rohrs BR. Dissolution method development for poorly soluble compounds. Dissolution Technol. 2001;8(3):6–12.
Jallo LJ, Dave RN. Explaining electrostatic charging and flow of surface-modified acetaminophen powders as a function of relative humidity through surface energetics. J Pharm Sci. 2015;104(7):2225–32.
Gold TB, Buice RG Jr, Lodder RA, Digenis GA. Detection of formaldehyde-induced crosslinking in soft elastic gelatin capsules using near-infrared spectrophotometry. Pharm Dev Technol. 1998;3(2):209–14.
Marchais H, Cayzeele G, Legendre JY, Skiba M, Arnaud P. Cross-linking of hard gelatin carbamazepine capsules: effect of dissolution conditions on in vitro drug release. Eur J Pharm Sci. 2003;19(2–3):129–32.
Malaterre V, Ogorka J, Loggia N, Gurny R. Evaluation of the tablet core factors influencing the release kinetics and the loadability of push–pull osmotic systems. Drug Dev Ind Pharm. 2009;35(4):433–9.
Missaghi S, Patel P, Farrell T, Huatan H, Rajabi-Siahboomi A. Investigation of critical core formulation and process parameters for osmotic pump oral drug delivery. AAPS PharmSciTech. 2014;15(1):149–60.
Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Control Release. 2002;79(1–3):7–27.
Ma L, Deng L, Chen J. Applications of poly(ethylene oxide) in controlled release tablet systems: a review. Drug Dev Ind Pharm. 2014;40(7):845–51.
Nie Sf, Li W, Luan L, Pan W, Wang X. Studies on bi-layer osmotic pump tablets of water-insoluble allopurinol with large dose: in vitro and in vivo. Drug Dev Ind Pharm. 2007;33(9):1024–9.
Wu C, Zhao Z, Zhao Y, Hao Y, Liu Y, Liu C. Preparation of a push–pull osmotic pump of felodipine solubilized by mesoporous silica nanoparticles with a core–shell structure. Int J Pharm. 2014;475(1–2):298–305.
Zhang ZH, Li W, Nie SF, Tang X, Peng B, Tian L, Pan WS. Overcome side identification in PPOP by making orifices on both layers. Int J Pharm. 2009;371(1–2):1–7.
Li G, Wang Y, Chen H, Leng D, Ma P, Dong Y, Gao L, He Z. Can semipermeable membranes coating materials influence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: in vitro evaluation and in vivo pharmacokinetics study. Asian J Pharm Sci. 2015;10(2):128–37.
Lu EX, Jiang ZQ, Zhang QZ, Jiang XG. A water-insoluble drug monolithic osmotic tablet system utilizing gum arabic as an osmotic, suspending and expanding agent. J Control Release. 2003;92(3):375–82.
Malaterre V, Ogorka J, Loggia N, Gurny R. Approach to design push–pull osmotic pumps. Int J Pharm. 2009;376(1–2):56–62.
Xu H, Li Z, Pan H, Zhang Z, Liu D, Tian B, Ma S, Song S, Pan W. A novel bi-layer ascending release osmotic pump tablet: in vitro investigation and in vivo investigation in pharmacokinetic study and IVIVC evaluation. Int J Pharm. 2013;458(1):181–7.
Hill A, Geißler S, Weigandt M, Mäder K. Controlled delivery of nanosuspensions from osmotic pumps: zero order and non-zero order kinetics. J Control Release. 2012;158(3):403–12.
Liu L, Xu X. Preparation of bilayer-core osmotic pump tablet by coating the indented core tablet. Int J Pharm. 2008;352(1):225–30.
Liu X, Wang S, Chai L, Zhang D, Sun Y, Xu L, Sun J. A two-step strategy to design high bioavailable controlled-release nimodipine tablets: the push–pull osmotic pump in combination with the micronization/solid dispersion techniques. Int J Pharm. 2014;461(1–2):529–39.
Brown J, Madit N, Cole ET, Wilding IR, Cade D. The effect of cross-linking on the in vivo disintegration of hard gelatin capsules. Pharm Res. 1998;15(7):1026–30.
Jain NK, Naik SU. Design of a slow-release capsule using laser drilling. J Pharm Sci. 1984;73(12):1806–11.
Acknowledgements
We would like to acknowledge Biolab Co., Ltd., Thailand and Siam Pharmaceutical Co., Ltd., Thailand for drug substances, Capsules Products Co., Ltd., Thailand for hard gelatin capsules, Onimax Co., Ltd., Thailand, and Colorcon Inc., USA for pharmaceutical excipients used in this work. The authors acknowledge Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, for providing instrument and determination of osmolality of the release mediums. We greatly appreciate Mr. Joseph Eric Masterson and Ms. Gemma McIlwaine for their assistance in the English language in this paper.
Funding
This work was financially supported by the Graduate School and the College of Pharmacy, Rangsit University.
Author information
Authors and Affiliations
Contributions
Chaowalit Monton: investigation, formal analysis, methodology, writing—original draft. Poj Kulvanich: conceptualization, methodology, resources, writing—original draft, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monton, C., Kulvanich, P. Push-Pull Osmotic Pumps Using Crosslinked Hard Gelatin Capsule as a Structural Assembly for Delivery of Drugs with Different Water Solubilities. J Pharm Innov 17, 791–805 (2022). https://doi.org/10.1007/s12247-021-09562-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09562-5