Abstract
The present study was aimed to improve aqueous solubility and dissolution behavior of a poorly water-soluble antipsychotic drug, Paliperidone (PLDN), through the formation of inclusion complexes with beta-cyclodextrin (β-CD). The effect of water soluble polymer on the complexation efficiency and solubilizing ability of β-CD was investigated using Kollidon-30 (KDN) as a ternary component. The binary complex of PLDN: β-CD was prepared by co-precipitation method in 1:1 molar ratio and ternary complex was prepared analogously to the binary complex containing 0.3% of KDN. The solid state characterization of the complexes was carried out using DSC, FTIR, XRPD, and SEM studies. The phase solubility study carried out according to the Higuchi and Connors method revealed an increase in complexation efficiency of β-CD due to addition of KDN. Saturation solubility and in vitro dissolution studies of complexes showed that the aqueous solubility and dissolution rate of drug were improved substantially compared to drug alone. The ternary complex resulted more solubility enhancement and drug release compared to binary complexes. In conclusion, the addition of KDN to the binary system of PLDN:β-CD has a synergistic effect on the solubility enhancement ability of β-CD. Hence, ternary complexation can be used as a novel approach for solubility enhancement of PLDN.

Solubility enhancement by ternary complexation of Paliperidone
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the advent in the drug discovery, many drug molecules are being introduced into the market. But most of the newly introduced drugs are poorly water soluble. The solubility and dissolution of such drug molecules are rate limiting step to their absorption. Therefore, approaches that increase solubility of such compounds can improve their dissolution properties and thereby bioavailability. The literature cites numerous methods for solubility and bioavailability enhancement of poorly soluble drugs viz. particle size reduction, use of co-solvent(s)/ surfactant(s), solid dispersion with hydrophilic polymers, complexation with cyclodextrin, etc. The use of cyclodextrins (CDs) as a solubility enhancement tool has been well established for poorly water-soluble drugs [1–8].
CDs are oligosaccharides of α-d-glucopyranose with cyclic α-(1,4) linkage containing hydrophilic outer surface and somewhat lipophilic central cavity. The lipophilic drug molecule or lipophilic part of drug molecule can interact with interior cavity of cyclodextrin forming an inclusion complex. The hydroxy groups on the outer surface of the CDs are also able to form hydrogen bonds with other molecules to form non-inclusion complex. Thus, CDs can increase the aqueous solubility of poorly water-soluble guest molecules by forming water soluble complexes. Inclusion complexation of drug with CDs has numerous applications such as to reduce bitterness, improved stability, reduce gastrointestinal irritation, prevent drug-drug or drug-excipient interaction, control of volatility, and sublimation and controlled release of drug [9–13].
The natural cyclodextrins have a drawback of limited aqueous solubility. Therefore, the complexes resulting of poorly soluble drug and these CDs can be of limited solubility [9]. In addition, complexation efficiency of cyclodextrins is low, and therefore, relatively more amount of cyclodextrins may be required to prepare complexes of these CDs with drugs. Thus, the enhancement of the complexation efficiency and solubility enhancement ability of CDs is of great concern in cases where larger amounts of CDs are required to increase solubility of poorly soluble molecules [14].
It has been established that the addition of suitable auxiliary substance such as water soluble polymer to Drug-Cyclodextrin (CD) system increases the solubilization and complexation efficiency of CD. Polymers increase the complexation of CD with drug through the formation of ternary complexes or co-complexes [15–19]. The mechanism of increase in complexation efficiency of CDs in the presence of hydrophilic polymers is not yet understood completely [20]. Addition of water-soluble polymers improves the complexation efficiency and can reduce the amount of CDs required in formulation by up to 80 %. [21, 22].
Similar positive effects on CDs complexation efficiency by addition of ternary components such as hydroxyacids [23], triethanolamine [24, 25], amino acids [26, 27], and CD derivative [28] have also been reported in literature.
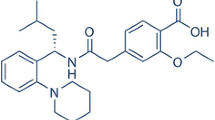
A BCS class II drug, paliperidone (PLDN), was selected as a model drug for the study. It is second generation atypical anti-psychotic drug indicated for treatment of schizophrenia in adults. It is the major active metabolite of risperidone. Its mechanism of action includes antagonism of central dopamine D2 receptors, serotonin 5-HT2A receptors, adrenergic receptors (α-1and α-2), and H1 histaminergic receptors. The PLDN is practically insoluble in water and possesses low absolute bioavailability with a half-life of 23 h. The lower bioavailability is further attributed to its reduced absorption in the colon [29–32]. The chemical structure of PLDN is given in Fig. 1.
Literature review reveals studies on complexation of PLDN with cyclodextrin [33], bioavailability enhancement using hydrophilic carriers and advanced technologies [34, 35]. In our recent study, an inclusion complexes of PLDN with HP-β-CD has shown a significant improvement in solubility and dissolution [36]. However, in present study, the influence of water soluble polymers on solubilizing and complexation abilities of beta-cyclodextrin (β-CD) was investigated through preparation of ternary complexes comprising of drug, β-CD, and hydrophilic polymer. It was hypothesized that the addition of hydrophilic polymer may increase the complexation efficacy and solubility enhancement ability of β-CD and therefore may results in an even better drug solubility/dissolution than a binary system.
Materials and Methods
Materials
PLDN and β-CD were obtained as a gift sample from Inventia Healthcare Pvt. Ltd., Thane, India and Gangwal Chemicals Pvt. Ltd., Mumbai, India, respectively. Hydroxypropyl methyl cellulose E-5 (HPMC) and polyethylene glycol (PEG) were purchased from S.D. Fine Chem Ltd., Mumbai, India. Kollidon-30 (KDN) and Lutrol F68 were obtained as a gift sample from Signet Chemical Corporation Pvt. Ltd., Mumbai, India. All other materials and solvents used in the study were of analytical reagent grade.
Methods
Determination of the Aqueous Solubility of PLDN
The solubility of PLDN in water was determined by saturation solubility method. PLDN was added in excess to the distilled water in vials. The vials were kept on the rotary shaker (Orbitek-Scigenics Biotech, India) at 25 °C and 100 rpm for 48 h. After equilibrium, the solutions were filtered using 0.45 μ membrane filter, diluted suitably and the amount of drug was determined using UV-Visible spectrophotometer (Shimadzu UV 1800, Japan) at 275 nm.
Screening of Polymer
The screening of polymers study was carried out to determine solubilization potential of hydrophilic polymers. Four different water soluble polymers, namely, Kollidon-30, Lutrol F68, HPMC, and PEG in various concentrations were tested for their effect on solubility of PLDN. The aqueous solutions of polymers were prepared in water in the concentration range of 0.1–0.4 % w/v. PLDN was added in excess to the aqueous solutions of polymer in vials. The vials were shaken using rotary shaker for 48 h at 25 °C and 100 rpm. After equilibrium, the solutions were filtered and the solubility of drug in different concentrations of aqueous solutions of polymers was determined spectrophotometrically.
Phase Solubility Study
Phase solubility study was carried out according to the Higuchi and Connors method [37]. For phase solubility study of a binary system, an excess amount of PLDN was added to aqueous solutions containing increasing concentrations of β-CD (3–15 mM). The solutions were kept on rotary shaker for 48 h at 25 °C. The solutions were allowed to equilibrate, filtered using 0.45 μ membrane filter, and diluted suitably. The amount of drug was determined spectrophotometrically at 275 nm. The phase solubility study for ternary system was performed similar to the binary system in presence of 0.3 % KDN (the concentration value obtained from the results of polymer screening study). The stability constant (Kc) was determined using the following equation
where S0 is the solubility of the PLDN in water in the absence of β-CD (binary system) or β-CD and KDN (Ternary system). It has been stated that for selection of complexation conditions it is more convenient to obtain complexation efficiency (CE) values for CDs [9]. Hence, the CE was also calculated using the following equation
Preparation of Physical Mixtures
The physical mixture (PM) of binary system was prepared containing 1:1 molar amounts of PLDN and β-CD. The weighed quantity of β-CD and PLDN was mixed gently and passed through 85# mesh with minimum abrasion. The ternary PM containing PLDN, β-CD, and KDN was prepared analogously to the binary physical mixture containing 0.3 % of KDN.
Preparation of Inclusion Complexes (ICs)
The inclusion complex (IC) of binary system was prepared by co-precipitation method containing 1:1 molar amount of PLDN and β-CD. The β-CD was dissolved in minimum quantity of distilled water. An equimolar amount of PLDN was added gradually to the β-CD solution. The solution was stirred continuously using magnetic stirrer (MLH 1, Remi Laboratory Instruments, India) for 24 h and solvent evaporated. The ternary complex was prepared analogously to the binary complex containing 0.3 % of KDN. The products were dried, pulverized, passed through 85# mesh, and stored in desiccator.
Characterization of ICs
Saturation Solubility of PLDN, PM and IC in Water
The aqueous solubility of PMs and ICs in distilled water was determined by saturation solubility method. The PMs and ICs in excess were added to the vials containing measured volume of distilled water. The vials were sealed and kept on the rotary shaker at 25 °C and 100 rpm. After 48 h, the solutions were filtered and the drug content was determined spectrophotometrically at 275 nm.
Powder X-ray Diffraction
Powder X-ray diffraction (PXRD) patterns of PLDN, β-CD, KDN, PMs, binary, and ternary ICs were obtained with a PANalytical X’pert Pro MPD Diffractometer (EA Almelo, The Netherlands). The process parameters were as below: Cu anode, 45 kV voltage, 40 mA current, scan step size 0.0170 (2θ), scan step time 20.0271 s, and measurement temperature 25 °C. To check the possible inclusion complex formation, the diffractograms of pure PLDN, β-CD and KDN were compared with PMs, binary, and ternary complexes with respect to peak intensity and the presence and/or absence of peaks.
Scanning Electron Microscopy
The scanning electron microscopy (SEM) study was performed on a scanning electron microscope EvoLS10 (Zeiss, Germany). The surface morphology of pure PLDN, β-CD, KDN, PMs, and ICs was examined.
Fourier Transform Infrared Spectroscopy
The spectra were obtained using a Fourier transform infrared (FT-IR) spectrometer (Perkin Elmer, Inc., USA) in the range of 4000–400 cm−1 using KBr disc method. The FT-IR spectras of PMs and ICs were compared with that of pure PLDN, β-CD, and KDN for intensity of peaks and modification and/or the absence of characteristic peaks.
Differential Scanning Calorimetry
The samples were examined on differential scanning calorimeter (DSC) (Mettler Toledo DSC 822) using Stare SW 10.00 software. An approximately 2.0 mg of each sample was scanned using aluminum pans in a nitrogen atmosphere with flow rate of 0.2 kg/m2 (cylinder secondary outlet pressure). The heating rate of 10 °C min−1 was maintained during the scanning over a temperature range of 50–300 °C.
In Vitro Dissolution Studies
In vitro dissolution tests for PLDN, PMs, and ICs (quantity equivalent to 12 mg of PLDN) were carried out using Tablet Dissolution Tester USP, TDT 06P (Electrolab, India) in 500 mL phosphate buffer, pH 6.8, at 37 °C with a paddle rotating at 50 rpm. The aliquot of samples was withdrawn at various intervals of time (10, 20, 30, 45, and 60 min), and the amount of PLDN dissolved was determined spectrophotometrically at 275 nm.
Results and Discussion
Screening of Polymer
As indicated in the Fig. 2, in the polymer screening study, it was found that among four water-soluble polymers used for the study, KDN has shown maximum solubility of PLDN. The solubility of PLDN increased linearly with increasing concentration of KDN up to 0.3 % concentration of KDN, beyond which no substantial increase in PLDN solubility was observed. Hence, 0.3 % KDN was used as a third component for phase solubility study of ternary system and preparation of ternary solid inclusion complexes.
Phase Solubility Studies
Polymers are known to interact with the outer surface of CDs and with drug-CD complexes, forming co-complexes or aggregates that show higher stability constants (Kc) values than those for the binary drug-CD system. The developed aggregates are capable of solubilizing drugs and other hydrophobic molecules [8, 19].
The phase solubility studies for both binary and ternary systems were carried out to investigate the effect of polymers on complexation efficiency of β-CD. The results of phase solubility studies are shown in Fig. 3. The phase solubility curve for both PLDN:β-CD and PLDN:β-CD:KDN systems showed AL type curve (among other curves like AP, AN, BS, and BI) [37] indicating a linear increase in PLDN solubility as a function of β-CD concentration suggesting the formation of water soluble complexes. A synergistic effect on PLDN solubility was observed due to addition of KDN. The apparent stability constant (KC) and CE were calculated. The Kc values for binary and ternary systems were found to be 906.58 and 1106.87 M−1, respectively. The significantly higher Kc value for ternary system can be attributed to the presence of KDN suggesting formation of more stable complex. The CE values for binary and ternary systems were found to be 0.071 and 0.088, respectively. The higher CE value for ternary system suggests the improved complexation efficiency of β-CD due to addition of KDN. The slope values of binary and ternary systems were less than unity indicating formation of 1:1 stoichiometry complexes [38].
Saturation Solubility of PLDN, PMs, and ICs in Water
The results of the saturation solubility of PLDN, physical mixtures, and inclusion complexes in water are depicted in Fig. 4. The formation of binary and ternary inclusion complexes of PLDN with β-CD resulted in improved water solubility of PLDN. The extent of solubility of PLDN in ternary complexes was more than that in binary complexes and their corresponding physical mixtures. The solubility enhancement of PLDN in binary and ternary complexes was found to be ̴ 17- and ̴ 30-folds, respectively, with respect to saturation solubility of pure PLDN in water (i.e., 0.0326 mg/mL). The higher solubility of PLDN in ternary system compared to binary system can be attributed to enhanced complexation efficiency of β-CD due to addition of PLDN.
X-ray Powder Diffraction
X-ray powder diffraction (XRPD) study was carried out to confirm formation of new solid system. The diffraction pattern of PLDN showed several sharp high intensity peaks at diffraction angles (2θ) of 10.24, 10.78, 14.54, 14.94, 18.64, 19.16, 21.98, 24.62, 25.0, 28.54, and 31.16 °C indicating its crystalline nature. The x-ray diffractograms of β-CD also showed several intense peaks due to its crystalline nature, however, KDN has not shown any prominent peaks suggesting its amorphous character. The diffractograms of PLDN, β-CD, KDN, PMs, and ICs are shown in Fig. 5. The crystallinity of PLDN in PMs and ICs was determined by comparing the intensity of peaks, presence/absence of some representative peaks in the diffractograms of PMs and ICs with those of a PLDN.
The intensity of some characteristic diffraction peaks of PLDN was significantly reduced in binary and ternary complex compared to their physical mixtures. Moreover, the XRD graph of ternary complex showed disappearance of many characteristic peaks of PLDN which were observed in XRD pattern of binary complex and PMs. Thus, it is indicative that the ternary complexation has resulted in the formation of more amorphous solid complex through increasing the complexation ability of β-CD.
Scanning Electron Microscopy
SEM photomicrographs of PLDN, β-CD, KDN, PMs, and ICs are shown in Fig. 6. The SEM images indicated PLDN as irregular particles and β-CD as crystalline molecule. The products of binary and ternary complexes appeared as amorphous aggregates suggesting a considerable change in the original morphology of the PLDN, β-CD, and KDN. This change in the morphology of PLDN in binary and ternary complexes was indicative of the formation of new solid complexes.
Fourier-Transform Infrared Spectroscopy
FT-IR is a useful analytical tool to predict a possible interaction of guest (drug) with host (cyclodextrin) molecules in solid state. The characteristic peaks due to various functional groups in guest molecule are affected due to an interaction between guest and host molecule [39]. The FTIR spectrum of PLDN shows the presence of characteristic bands at 3296.48 (−OH stretch), 2786.01 (=C–H stretch), 1628.02 (C = C stretch), 1536 (C = N stretch), 1270 (C–H bending), and 1131.80 cm−1 (C–F stretch). The FT-IR spectrum of β-CD indicates bands at 3300–3500 cm−1 due to O–H stretching vibrations and the bands due to -CH and CH2 groups appears in 2800–3000 cm−1 region. The IR spectrum obtained for binary and ternary IC showed shift in IR band, reduction in intensity, and/or disappearance of some characteristic bands of PLDN. The significant shift in IR bands of PLDN at 3296.48, 2935.78, 1628.02, 1536.65, 1270, and 1131.8 cm−1 suggests the interaction of PLDN with β-CD and KDN. Further, the IR band shift was more distinct in ternary IC compared to binary IC and physical mixtures indicating enhanced complexation of PLDN with β-CD due to addition of KDN. The shifts in the IR bands of PLDN and β-CD in the PLDN-β-CD IC and PLDN-β-CD-KDN systems are shown in Table 1, and the FT-IR spectras of are illustrated in Fig. 7.
Differential Scanning Calorimetry
The DSC thermal analytical technique can be used to study an interaction between drug (guest) and host molecule. The DSC thermograms of samples are shown in Fig. 8. PLDN showed a characteristic endothermic peak corresponding to its melting point at 181.46 °C, whereas the DSC graph of β-CD showed an endotherm at 122.94 °C. DSC graphs of binary and ternary ICs investigated that the intensity of endothermic peak of PLDN at its melting point is considerably reduced compared to their PMs. However, the thermal peak of PLDN in ternary IC was reduced to more extent compared to binary IC indicating strong interaction of PLDN with β-CD and KDN. This could be attributed to increased complexation efficiency of β-CD due to addition of KDN.
In Vitro Dissolution Study
The in vitro dissolution test was carried out to study the effect of improved solubility of PLDN on dissolution rate of solid inclusion complexes. The results of drug release study are illustrated in Fig. 9. An increase in dissolution rate was achieved with the binary and ternary inclusion complexes. As illustrated in figure, about 82.0 and 99.0 % of PLDN was released in 20 min from the binary and ternary complex, respectively. However, <25 % drug release was obtained at the same time interval from pure PLDN. The % dissolution values after 30 min depicted in Table 2 suggests a satisfactory drug release [40] from binary and ternary ICs compared to pure PLDN and physical mixtures of binary and ternary system. The increased dissolution rate of PLDN in complexes may be due to increased interaction of drug with the dissolution medium. This can further be attributed to the ability of cyclodextrin and hydrophilic polymer to improve drug wettability and water solubility. The significantly improved dissolution rate of PLDN in ternary complex compared to binary complex can be attributed to the presence of water soluble polymer in ternary system resulting in enhanced complexation of PLDN with β-CD.
Conclusions
The characterization studies of binary and ternary complexes such as SEM, DSC, XRPD, and FTIR revealed that noticeable changes were observed in all characterization methods, indicating the formation of new solid inclusion complexes. The ternary complex has resulted remarkable improvement in solubility and dissolution compared to binary complex. The apparent stability constant (Kc) and complexation efficiency (CE) of β-CD were significantly improved due to addition of KDN to binary system of drug and β-CD suggesting a more stable complex formation with increased complexation efficiency of β-CD. Thus, from the experimental observations, it can be concluded that the presence of KDN may lead to appreciable improvement in the solubilizing and complexing ability of β-CD through the formation of ternary inclusion complex with PLDN. Hence, ternary complexation of PLDN with β-CD and KDN can be used a novel approach to improve its solubility and dissolution performance.
References
Gribbon P, Sewing A. High-throughput drug discovery: what can we expect from HTS? Drug Discov Today. 2005;10:17–22.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Ghulam M. Solubility enhancement of simvastatin: a review. Acta Pol Pharm. 2012;69:581–90.
Jug M, Kos I. The pH-dependent complexation between risperidone and hydroxypropyl-b-cyclodextrin. J Incl Phenom Macrocycl Chem. 2009;64:163–71.
Cecile D, Nathalie A, Cedric C, Pascal O, Bernard M, Claude V. Comparative study of the complex forming ability and enantioselectivity of cyclodextrin polymers by CE and 1H NMR. Carbohydr Polym. 2013;92:2282–92.
Cecile D, Nathalie A, Albane B, et al. Study of the complexation of risperidone and 9-hydroxyrisperidone with cyclodextrin hosts using affinity capillary electrophoresis and 1H NMR spectroscopy. J Chromatogr A. 2008;1215:185–93.
Kim C, Park J. Solubility enhancers for oral drug delivery. Am J Drug Deliv. 2004;2:113–30.
Brewster ME, Loftsson T. Cyclodextrin as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
Magnusdottir A, Masson M, Loftsson T. Cyclodextrins. J Incl Phenom Macrocycl Chem. 2002;44:213–8.
Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Loftsson T. Pharmaceutical applications of cyclodextrins. J Pharm Sci. 1996;85:1017–25.
Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;6:30–42.
Martin EM, Valle D. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46.
Sami F, Philip B, Pathak K. Effect of auxiliary substances on complexation efficiency and intrinsic dissolution rate of gemfibrozil-beta-CD complexes. AAPS PharmSciTech. 2010;11:27–35.
Ribeiro L, Carvalho RA, Ferreira DC, Veiga FJB. Multicomponent complex formation between vinpocetine, cyclodextrins, tartaric acid and water-soluble polymers monitored by NMR and solubility studies. Eur J Pharm Sci. 2005;24:1–13.
Taupitz T, Dressman JB, Buchanan CM, Klein S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behavior of poorly soluble drugs. Case example: Itraconazole. Eur J Pharm Biopharm. 2013;83:378–87.
Loftsson T, Masson MJ. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J Drug Delivery Sci Technol. 2004;14:35–43.
Valero M, Carrillo C, Rodriguez LJ. Ternary naproxen: beta-cyclodextrin: polyethylene glycol complex formation. Int J Pharm. 2003;265:141–9.
Loftsson T, Fridriksdottir H, Sigurdardottir AM, Ueda H. The effect of water-soluble polymers on drug-cyclodextrin complexation. Int J Pharm. 1994;110:169–77.
Ribeiro L, Loftsson T, Ferreira D, Veiga F. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes. Chem Pharm Bull. 2003;51:914–22.
Mura P, Faucci MT, Bettinetti GP. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-beta-cyclodextrin. Eur J Pharm Sci. 2001;13:187–94.
Loftsson T, Frikdriksdottir H. The effect of water-soluble polymers on the aqueous solubility and complexing abilities of β-cyclodextrin. Int J Pharm. 1998;163:115–21.
Fauci MT, Melani F, Mura P. 1H-NMR and molecular modelling techniques for the investigation of the inclusion complex of econazole with alpha-cyclodextrin in the presence of malic acid. J Pharm Biomed Anal. 2000;23:25–31.
Granero G, Garnero C, Longhi M. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2003;20:285–93.
Garnero C, Longhi M. Study of ascorbic acid interaction with hydroxypropyl-β-cyclodextrin and triethanolamine, separately and in combination. J Pharm Biomed Anal. 2007;45:536–45.
Mura P, Maestrelli F, Cirri M. Ternary systems of naproxen with hydroxypropyl-beta-cyclodextrin and aminoacids. Int J Pharm. 2003;260:293–302.
Bramhane DM, Saindane NS, Vavia PR. Inclusion complexation of weakly acidic NSAID with β-cyclodextrin: selection of arginine, an amino acid, as a novel ternary component. J Incl Phenom Macrocycl Chem. 2011;69:453–60.
Thi T. Comparison between 2-hydroxypropyl-b-cyclodextrin and 2-hydroxylpropyl-γ-cyclodextrin for inclusion complex formation with danazol. J Incl Phenom Macrocycl Chem. 2011;71:137–47.
Janicak PG, Winans EA. Paliperidone ER—a review of the clinical trial data. Neuropsychiatr Dis Treat. 2007;3:869–97.
Pierre C, James C. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383–97.
Kozielska M. Pharmacokinetic- pharmacodynamic modeling of the D2 and 5-HT2A receptor occupancy of risperidone and paliperidone in rats. Pharm Res. 2012;29:1932–48.
Cada D. Paliperidone. Hosp Pharm. 2007;42:637–47.
Danela C, Azaroualb N, Brunela A, et al. Study of the complexation of risperidone and 9-hydroxyrisperidone with cyclodextrin hosts using affinity capillary electrophoresis and 1H NMR spectroscopy. J Chromatogr A. 2008;1215:185–93.
Pandey A, Rath B, Dwivedi A. Dissolution rate and bioavailability enhancement of co-ground mixtures of paliperidone, with different hydrophilic carriers. Inter Curr Pharm J. 2013;3:70–7.
Pandey A, Rath B, Dwivedi AK. Enhancement of dissolution rate and bioavailability of Paliperidone by hot melt extrusion technique. J Sci Ind Res. 2014;73:680–5.
Sherje A, Londhe V. Inclusion complexes of hydroxypropyl-β-cyclodextrin and paliperidone: preparation and characterization. Curr Drug Discov Technol. 2015;11:271–8.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–22.
Jullian C, Morales-Montecinos J, Zapata TG, Aguilera B, Rodriguez J, Aran V. Characterization, phase-solubility, and molecular modeling of inclusion complex of 5-nitroindazole derivative with cyclodextrins. Bioorg Med Chem. 2008;16:5078–84.
Amareshwar K, Rai DK. Spectroscopic studies of some antidiabetic drugs. Spectrochim Acta A. 2003;59:1673–80.
Dash AK. Solid dosage forms. In: Dash A, Singh S, Tolman J. Pharmaceutics- basic principles and application to pharmacy practice. Elsevier Inc.: Academic Press; 2014. pp. 177.
Acknowledgments
The authors are thankful to Inventia Healthcare Pvt. Ltd. Thane (West), India and Gangwal Chemicals Pvt. Ltd., Mumbai, India for providing the gift samples of paliperidone (PLDN) and β-CD, respectively. We are also thankful to Tata Institute of Fundamental Research, Mumbai, India for facilitating SEM analysis of the samples.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Sherje, A.P., Londhe, V. Ternary Inclusion Complex of Paliperidone with β-Cyclodextrin and Hydrophilic Polymer for Solubility and Dissolution Enhancement. J Pharm Innov 10, 324–334 (2015). https://doi.org/10.1007/s12247-015-9229-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-015-9229-2