Abstract
Introduction
Implant is a promising delivery system for chronically used drugs. Excipients play an important role in physicochemical properties and more importantly drug release profile from the implant system; therefore, selecting appropriate materials in matrix formulation is an important issue.
Objective
The main purpose of the present study is to explore the role of various excipients on the physicochemical characteristic of risperidone (Ris) implants. In this study, various Ris implant formulations with polyethylene glycol (PEG) as hydrophilic and cholesterol (Chol) as hydrophobic excipients were fabricated and evaluated.
Methods
Ris implants were fabricated by casting method. Mathematical modeling was employed to explore the release mechanism of various formulations. In order to analyze the mechanical strength of implants, texture analysis was performed. The physical state of Ris in implants matrix was analyzed by differential scanning calorimetry (DSC) and X-ray diffraction (XRD) analysis. Scanning electron microscopy (SEM) was used for the morphology investigation of implants. Fourier transform infrared spectroscopy (FTIR) was used to explore any changes in the chemical structure of the drug in formulation.
Results
Implant formulations with Chol showed sustained release of Ris as long as 59 days relative to 32 days with PEG. Mathematical evaluation of Ris release showed an erosion-based mechanism for implant formulations with Chol, whereas implants with PEG followed a diffusion release mechanism. Texture analysis of implants showed higher mechanical strength for the formulation with Chol. Both DSC and XRD studies confirmed partial conversion of crystalline Ris to amorphous form in formulations with Chol. The water uptake and matrix bulk erosion of implants showed lower erosion, and the water uptake for formulations with Chol in comparison to formulations with PEG. FTIR analysis showed no changes in the chemical structure of Ris in all formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic drug therapy is one of the most challenging issues in most diseases like diabetes and psychiatric disorders. Frequent administration of drug and the chronic nature of these kinds of diseases make patients less comfortable; hence, their compliance during pharmacotherapy will decrease as well [1]. By developing biodegradable polymers in the past two decades, several drug delivery systems were also developed for long-term delivery of different drugs. Among the various drug delivery systems, advanced controlled release implants are attractive systems which have been used both for hydrophobic and hydrophilic drugs with acceptable clinical outcomes [2, 3]. Basically, implants are composed of polymer(s) and drug(s) with or without excipients. The implant drug delivery system has several advantages including, decreasing the administration frequency by incorporating the total amount of the needed drug in just one dose, excluding the missed dose especially in geriatrics, bypassing the first pass metabolism, and also reducing hospitalization and further medical care [4]. Although implants have some disadvantages like painful injection and also rigidity in dose management in some cases, these limitations do not reduce their acceptability and research in this field of drug delivery is still in progress.
During the implant formulation, several materials like poly L-lactide-co- glycolic acid (PLGA) [5], polycaprolactone (PCL) [6], and lipids [7] have been used as matrix former. Lipids are widely used material in different drug delivery systems like liposomes [8], solid lipid nanoparticles [9], and also implants [10]. Many researchers reported favorable characteristic for lipid implants. For example, Kreye et al. reported desired release profile of propranolol and theophylline from triglyceride-based implant [11]. Sax and co-workers reported that the triglyceride implants showed promising erosion behavior and also good in vivo degradability [12]. Siepmann et al. reported the release mechanism of interferon from lipid implants [13]. They showed that drug diffusion occurs through water-filled pores and crystalline lipid matrix is impermeable to water. Therefore, the water uptake of this matrix is limited, which leads to minimum matrix erosion and results in prolonged release of drug. Despite the satisfactory physicochemical characteristics of triglyceride-based implants, lipids like triglycerides and their derivatives are susceptible to auto-oxidation and produce hazardous materials like aldehydes and malondialdehyde [14]. In vivo investigations have shown that exposure to this kind of chemical causes DNA alteration [15]. These toxic effects caused the Medical Device Agency of the UK (now called Regulating Medicines and Medical Devices, MHRA) to release a hazardous notice (AN 1999(01)) for Trilucent® breast implant which was composed of soy bean oil at in May 2000 due to genotoxic lipid peroxidation. Hence, the product was withdrawn from the market [16].
Poly L-lactide-co-glycolic acid (PLGA) is a polyester polymer which is approved by the FDA [17] and widely used in different drug delivery formulations due to its biocompatibility. Moreover, different implant formulations were developed and launched into market like Norplant® and Jadelle® which were approved by FDA for contraception in 1990 and 1996, respectively, by means of PLGA [18]. PLGA degrades to water and CO2 after contact with an aqueous media and the resultant products decrease the micro environment pH which is harmful for some drugs and especially peptides [19]. Apart from this issue which is mostly important for peptides and proteins, PLGA is the best candidate as a matrix former in the formulation of biodegradable implants.
Extensive research has been performed on implant formulations but there are less focus on the application of excipients and the exploration of their roles in physicochemical properties and release behavior. Excipients could act as release modifiers and also enhance the mechanical properties or may affect the matrix erosion of implants [20]. Some materials like polysaccharides including cyclodextrin [21], polyols like polyethylene glycol (PEG) [22], and fatty acids like palmitic acid [23] were studied as excipients in implant formulation. Wang et al. reported significant improvement in the release kinetic of β-lapachone from PLGA implants with different grades of cyclodextrins as an excipient [24]. Among different compounds, Chol has impressive characteristics which may alter the physicochemical properties of implants, when applied as an excipient in matrix-forming materials. Chol is a naturally occurring lipid in the body and has several important roles in cell functions [25]. More importantly, unlike unsaturated fatty acids, there are no aldehydes and malondialdehyde residues in the decomposed products of Chol that may cause DNA alteration [26]. In one study reported by Khan et al., it was demonstrated that Chol has no toxic effect when used as an excipient in matrix formulation in mice model [27]. Moreover, in this study, Chol showed minimum matrix erosion in comparison with other lipids. Regarding these properties, Chol can be considered as a suitable excipient in implant formulation along with PLGA as a matrix polymer. Although lots of studies demonstrated the release profile of drugs from implant with different matrices, there are few reports focused on the effect of excipients on the physicochemical properties and more importantly the mathematical exploration of drug release kinetics.
Ris, a dopamine antagonist, is the most prescribed drug in schizophrenia treatment guideline [28]. Long-term administration of this drug during therapy and also the chronic nature of schizophrenia make it an attractive candidate for implant drug delivery systems. In a previous study, the Ris implant formulation was studied but the role of different excipients on physicochemical properties and also on in vitro release were not explored [29]. The main concept of the present study was to explore the role of excipients on the physicochemical properties of implant formulations and also mechanistically evaluate model drug (Ris) release profile from different formulations. In this research, different formulations of Ris implant with PLGA as a matrix polymer and Chol or PEG as hydrophobic and hydrophilic excipients, respectively, were fabricated and the physicochemical characterization and in vitro release kinetic were studied. Moreover, the release mechanism of each series of formulations was investigated mathematically.
Materials and Methods
Materials
Risperidone was a kind gift from Dr. Abidi pharmaceutical company (Iran). PLGA (50:50) was purchased from Purac (the Netherland). Polyethylene glycol (PEG) 6000 and cholesterol were purchased from Sigma-Aldrich (USA). All other reagents and solvents were of analysis grade and used as received.
Methods
Fabrication of RIS Implants
Ris implants were fabricated via the injection molding method with cylindrical-shaped stainless steel (AISI 316L) molding device. Different amounts of PLGA and 60 mg of Ris (20 mg for each implant) were dissolved in 10 ml of dichloromethane with either Chol or PEG. The resultant solution was heated on mild heating to evaporate dichloromethane, and viscous paste was formed. The paste was injected into the die of the molding device via 1 ml polypropylene syringe followed by immediate solvent removal. After 30 min, the cylindrical implant with the length of 30 mm and 2 mm diameter was casted and cut into three equal rods of 10 mm. Each implant was wrapped up in aluminum pack and kept at room temperature for further analysis.
Drug Loading Efficiency and Content Uniformity Evaluation
In order to investigate the drug loading in each implant and also content uniformity analysis, each implant was dissolved in 25 mL of dichloromethane and filtered through 0.22-μm filter. Fifty microliters of sample was taken for analysis via RP-HPLC (Agilent Technologies®). The mobile phase was methanol with a flow rate of 1 ml/min through C18 column (150 × 4.6 mm, ODS 35 μm) at 25 °C and peak detection wave length of 275 nm. Drug loading was calculated by the following equation:
In vitro Release Studies
To evaluate the release profile of Ris, different formulations were placed on a shaker bath (100 rpm) in 500 mL PBS (pH = 7.4, T = 37 °C). At predetermined time intervals, 1.0 ml aliquot of each sample was withdrawn for analysis by RP-HPLC as mentioned above. After each sample removal, 1.0 ml of fresh buffer was added to maintain sink condition.
Mathematical Study of Release Kinetic
In order to explore the release kinetic mechanism of different implant formulations, various release models were studied. Equation (1) reveals the zero-order kinetic release in which the amount of the released drug is independent of drug concentration in the formulation. In this equation,
M t /M ∞ is considered to be the fraction of the released drug in time t, K 0 represents the zero-order kinetic constant, and t is related to time [30]. The first-order kinetic release posits that the amount of released drug is dependent on the loaded drug in the formulation [31] and is expressed as,
where K 1 is related to the first-order kinetic release. Equation (3) represents the Higuchi model in which the amount of released drug is proportional to the square root of time. This kinetic model describes diffusion release mechanism on the basis of Fick’s first law from different matrices [32]. In this equation,
K H represented the Higuchi constant release. The last release model is based on Korsmeyer-Peppas which explains complex kinetics mechanisms. Equation (4) describes the Korsmeyer-Peppas model,
in which K p and n represent the release and power law constant, respectively. Korsmeyer-Peppas or power law equation represents non-Fickian release in which the mechanism of release depends on the power constant, where, if n = 0.45, the release mechanism is Fickian; if n is greater than 0.89, the release mechanism follows case 2 or super case 2 which explains the polymer erosion; and if 0.45 ˂ n ˂ 0.89, the release is anomalous or non-Fickian [33].
In order to mathematically analyze the best fitted model errors in each series of formulations, sum squares of error (SSE), sum squares of regression (SSR), and also sum squares of total variation (SST) were calculated by Eqs. (5), (6), and (7). Correlation coefficient values for each mathematical model were calculated by Eq. (8). In each mathematical model, the level of significance was 0.05 (p < 0.05);
where \( \widehat{Y_i} \) is the vector of predicted value of Y based on the given X i , Y i is the vector of actual value, \( \overline{Y} \) is corresponded to the average of the original Y values, and e represented the error vector. The relative amounts of sums of squares presented the regression quality in terms of fitting the calibration data. If the regression is perfect, then SSE becomes zero and R 2 will be 1. All practical release data were fitted into the above formula using SigmaPlot® 12.0 software.
Water Uptake and Implant Matrix Erosion Study
To investigate water uptake, different formulations of Ris implants were placed in 500 ml of PBS (pH = 7.4) and after 30 days, each sample was withdrawn and weighted [wet mass (t)] and dried in an oven at 37 °C [dry mass (t)]. The water content (%) (t) was calculated as follows:
To determine the implant matrix erosion, the following formula was used in which [dry mass (0)] is related to the weight of each implant just after fabrication and [drug released (t)] is related to the cumulative amount of drug, released at time t.
Physical Characterization of the Implants System
Mechanical Properties of Implants
The mechanical properties of implant formulations were evaluated using a texture analyzer device. Implant formulations (F2 and F4) were placed on the metal plate of the device in an upright position. A cylindrical flat probe was fixed on load cell (50 kg) and driven downwards at the speed of 0.1 mm/s. The analysis was continued until implant rupture. The force versus displacement was calculated by the software of the device, and the mechanical strength curve was plotted.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) study was performed for PLGA, Ris, Chol, and ground implants matrices using Metler-toledo (Greifensee; Switzerland) differential scanning calorimeter. The analysis was performed via aluminum hermetic pan in which 15 mg of each sample was placed and covered with lid, purging nitrogen gas to prevent any oxidation reaction. Samples were heated from 20 to 300 °C with heating rate of 10 °C min-1.
X-ray Diffraction Analysis
X-ray diffraction (XRD) analysis was performed in order to investigate the physical state of Ris in different implant matrices. XRD pattern of implant formulations (F2 and F4) were analyzed by X'Pert Pro MPD® (The Netherlands) using Cu Kα radiation (40 kV, 30 mA), and the scanning speed was 2 deg min1. Data were analyzed by means of X'Pert High Score Plus® software.
Fourier Transform Infrared Spectroscopy
In order to explore any destructive reactions during implant fabrication, Fourier transform infrared spectroscopy (FTIR) spectra of PLGA, Chol, Ris, and implants were analyzed using Magna-IR550 Nicolet FTIR spectrophotometer. Approximately 10 mg of each sample and 50 mg of KBr were ground via mortar and pestle and a thin tablet was made for analysis. The spectra were scanned at room temperature in the 500 to 4000 cm-1 wavelength range with a resolution of 4 cm-1.
Implant Morphology Studies
In order to investigate the matrix changes during release study, scanning electron microscopy (SEM) images were obtained from cross section of each implant. Three millimeters of each implant was cut by scalpel and dried in oven (40 °C) followed by coating with thin layer of gold by means of sputter coater (SCD 005, Bal–Ted, Switzerland) for 60 s before imaging. Images were obtained at 5 kV voltages and 20 mA current using SEM XL 30, Philips (The Netherlands).
Results and Discussion
Implant Formulations
Four kinds of cylindrical implants were fabricated with different formulations but the same dimension and drug loading. In order to evaluate the exact role of excipients, the amount of drug and also implant dimension were both kept constant in all formulations. The role of lipophilic and hydrophilic excipients on the physicochemical properties and also release mechanism of Ris were investigated mathematically. The differences between formulations were in matrix excipient and also the amount of PLGA as a matrix polymer. Two series of formulations were composed of same excipient but different amounts of PLGA, and the other two formulations were composed of PEG and Chol as a hydrophilic and hydrophobic excipient, respectively (Table 1). The drug loading of each series of implants is almost 100 % and also each rod shape implant was composed of the same amount of Ris (20 ± 1.2 mg) which showed acceptable content uniformity of each implant unit.
In vitro Release Study and Release Mechanism
The cumulative release profile of Ris in all series of implant formulations was less than 20 % in the initial days of release study which showed no significant burst release. This finding shows that almost all fed drug was entrapped in the polymeric matrix, and there was no significant drug enrichment on the implant surface. The release profile of Ris was different in each series of implants with different matrix compositions and PLGA amounts. In order to better understand the underlying release mechanism in each series of implant formulations, the release data of all formulations were fitted by the zero-order, first-order, Korsmeyer-Peppas, and Higuchi equations. As illustrated in Table 2, the best fitted model for formulations F1 and F2 were consistent with the Higuchi model (p < 0.001) while the release pattern of formulation F3 and F4 followed the Korsmeyer-Peppas and zero-order kinetic, respectively (p < 0.001). From the best fitted model in different implant formulations, it can be seen that the main release mechanism of formulations F1 and F2 was based on diffusion but for F3 and F4 formulations, matrix erosion was the most probable involved mechanism. Dissolving and leaching out of PEG in formulation F1 and F2 led to more water diffusion into the body of the implant which led to Ris release. This mechanism is confirmed by the Higuchi model which is based on diffusion. Less diffused water through implant formulations F3 and F4 showed the important role of Chol in changing Ris release mechanism from diffusion to erosion model as described by Korsmeyer-Peppas and zero-order models. Table 3 shows the mathematical analysis of the best fitted model errors along with the correlation coefficient of each implant formulation (p < 0.001). As seen in this table due to the less difference in the R 2 value of Korsmeyer-Peppas and zero-order kinetics, both model can be considered for release mechanism of formulation F4 but due to the minimum amount of SSE for zero-order kinetic, it may demonstrate the underlying mechanism better in terms of data fitting.
It is important to explain that this erosion mechanism for formulation F3 and F4 is based on “surface erosion.” As mentioned above, the matrix erosion for formulation F3 and F4 was at the minimum level during 30 days of the experiment (Table 4). This matrix erosion is mainly “bulk erosion” which happened following leaching and dissolving of PEG and penetrating water molecules into the implant body of formulation F1 and F2. After water penetration, PLGA polymer hydrolysis to lactic acid and glycolic acid and the body of the implant degraded accordingly. In surface erosion mechanism, implants (F3 and F4) were degraded from the upper layers and minimum degradation in the body of implants occurred.
Effect of Matrix Composition
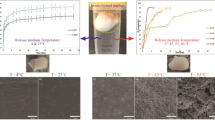
The cumulative release of Ris showed significant differences between implants with the same amount of PLGA but different kinds of excipients. The cumulative release of Ris at the first time points and also other time points was decreased by adding Chol as an excipient in the formulation of implant matrix. However, by adding PEG instead of Chol, cumulative release of Ris was increased impressively at the same time points. Figures 1 and 2 show the cumulative release profile of Ris from different implant formulations with different kinds of excipients. Due to the high solubility of PEG in aqueous media, it leaches out of the implant formulation and channels formed accordingly which are suitable for diffusion of water to the implant matrix and so the dissolution of Ris. Moreover, PEG acts as a co-solvent in the release medium and increases the solubility of Ris during the release experiment [22]. These characteristics of PEG lead to higher Ris release in formulations which are fabricated with this kind of excipient. Figure 3a, b show the cross-sectional SEM image of formulation F1 and F2, respectively, after 20 days of release study. It can be found that by dissolving PEG, a complex network of interconnected channels was formed which enhanced the water diffusion into the body of the implant matrix and increased the release rate of Ris. Siepmann et al reported a mathematical modeling for the release of PEG from implants with different amounts of PEG in matrix former formulation [13]. They showed that by increasing the amount of PEG from 5 to 20 %, the cumulative release of this excipient increased as well. By PEG leaching out of the implant formulation, the number and volume of channels are increased and so water penetrates to the implant body, which leads to the enhancement of the drug release. In another experiment which was done by Cheng et al., it was reported that almost more than 50 % of incorporated PEG in implant formulation was dissolved and led to an increase in the cumulative release of praziquantel [34]. So, PEG is considered as “pore former” in implant formulation.
The cumulative release of Ris from implants fabricated with Chol was significantly less than implants formulated with PEG. Figure 2 shows the cumulative release of Ris from implants with Chol. Comparing of cumulative release of implants with PEG and Chol (Figs. 1 and 2, respectively), it is demonstrated that the release profile of Ris from implant with Chol was as long as 59 days while the same amount of Ris was released from PEG implant just in 32 days. Due to highly crystalline and hydrophobic characteristics of Chol, the diffusion of water into the implant matrix would be negligible in comparison with PEG. So, less water penetration into the matrix of this formulation lead to the formation of fewer pores and channels, and as a result, lower amounts of Ris are released accordingly. Figure 3c, d illustrates the cross-sectional SEM images of formulations F3 and F4 after 20 days of release experiment, respectively. Unlike the SEM image of formulations F1 and F2, there is no evidence of pores and interconnected channels in the matrix of formulations F3 and F4 which indicates the role of Chol in preventing water diffusion into the body of the implant. Moreover, in these images, the crystalline structure of Chol is seen. Taking the water uptake of PEG and Chol implants into account (“Water Uptake and Matrix Bulk Erosion Studies” section), it can be assumed that the water content of PEG implant is so much higher than that of Chol implants in the same time points (Table 4). In the previous report by Kreye et al., it was shown that using lipid matrix formers sustained the release of both hydrophilic and hydrophobic drugs [11]. Moreover, they showed that the release of model drugs had a minimum burst effect. In another study performed by Opdebeeck and Tucker, they succeeded in getting the sustained release profile of bovine serum albumin from Chol implants during 42 days of their experiment [35]. Unlike PEG, which acts as a pore former in matrix formulation, Chol does not have the same function as PEG. Moreover, the structure of matrix remains more intact in comparison with PEG by prevention of water diffusion into the body of the implant.
Effect of PLGA Amount
Cumulative release of Ris from the implants with the same excipient but different amount of PLGA was studied. As shown in Figs. 1 and 2, the cumulative release of Ris was dramatically decreased when the amount of PLGA was increased from 100 to 200 mg. By increasing the PLGA amounts, the drug release was reduced, which showed the important role of the main polymer in the matrix formulation. Comparing the cumulative release of formulations F3 and F4, it is demonstrated that with the same amount of Chol, the extended release of Ris was achieved by increasing the amount of polymer (PLGA) to 200 mg. This comparison shows the same trend for formulations F1 and F2; however, due to the aforementioned role of PEG in matrix formulation, the extended release of Ris, more than 32 days, was not achieved even with 200 mg of PLGA. These results indicate the important role of Chol in the formulation of these kinds of implants.
Water Uptake and Matrix Bulk Erosion Studies
Table 4 shows the water uptake and matrix bulk erosion of different formulations of Ris implants. The water uptake and matrix bulk erosion of implants with higher PLGA amounts (F2 and F4) were less than that of formulation with lower amounts of matrix former polymer (F1 and F3) which indicates the role of hydrophobic PLGA in water diffusion into the implant matrix. The water uptake and also matrix bulk erosion of implants with PEG (F1 and F2) were dramatically higher than that of implants with Chol. This phenomenon implies the solubility of PEG in water and its release from the implant matrix into the aqueous media. The erosion of implant formulations with PEG was also higher due to more diffusion of water into the implant matrix and more cleavages of PLGA to lactic and glycolic acid.
Physical Characterization of Implants
Mechanical Properties
Figure 4 shows the texture analysis results of formulation F2 and F4. As it is seen in this graph, the amount of force which was needed to displace the implant formulation with Chol is significantly higher than that of the implant with PEG. This indicates the greater energy needed for breakage of Chol implant relative to PEG one. Chol, due to its highly crystalline nature, makes the implant stiffer and so more energy is needed for its breakage. Most probably, PEG acts as a lubricant between PLGA chains and causes displacement of implants using lower energy. It has also been reported that implants with Precirol ATO 5 (glyceryl palmitostearate) as a matrix former need more energy for breakage [19]. As a result, lipids and lipid derivatives enhanced the mechanical property of implants due to their crystalline structure.
Differential Scanning Calorimetry
Differential scanning calorimetry has been used to investigate the physical state of molecules in delivery systems. Differential scanning calorimetry (DSC) thermograms of Ris, Chol, PLGA, and implant formulations (F2 and F4) are shown in Fig. 5. Ris (Fig. 5c) is a highly crystalline compound with degradation temperature of 170 °C. Chol (Fig. 5e) also showed a crystalline structure with melting point of 147 °C whereas PLGA (Fig. 5a) has an amorphous structure. DSC analysis of formulation F2 (Fig. 5d) showed that the crystalline state of Ris was preserved in the implant matrix. However, the sharp endothermic Ris peak disappeared in formulation F4 (Fig. 5b). It can be concluded that Ris dissolved in Chol molecules and converted to its amorphous state, but maintained its crystalline nature in PLGA matrix (formulation F2). One previous study reported that Ris crystals were converted to amorphous form in lipid matrix, which indicates the dissolution of Ris in melted matrix [36]. Moreover Rahman et al. reported that the crystalline state of Ris changed due to solubility in glyceryloleate matrix [37]. In the thermogram of formulation F4, one endothermic peak is seen around 125 °C which is most probably related to the Chol. Most probably, the melting point of Chol was decreased upon dissolving of risperidone molecules. Silva and co-workers reported depilation in enthalpy and also melting point of lipidic molecules (888 ATO) after dissolving risperidone molecules in solid lipid nanoparticles. They also monitored these changes by different amounts of loaded risperidone and found out that the magnitude of this depletion in melting points of lipid was correlated to the amount of loaded risperidone. In another hand, by increasing the amount of risperidone, more depilation in the melting point of lipid was seen [38].
X-ray Powder Diffraction Study
In order to confirm the physical state of Ris in implant matrix, X-ray powder crystallography study was also performed. Ris showed several peaks at 2θ value of 19.7°, 20.1°, 22.54°, 24.59°, 27.33°, and around 32° [39]. As seen in Fig. 6a, Chol has crystalline peak at 2θ value of 2.67°, 5.28°, 15.1°, and 23.5°. Ris peaks describe the crystalline state of this drug in implant formulation F2 (Fig. 6b). Figure 6c showed the x-ray powder diffraction (XRD) pattern of formulation F4 in which Ris crystalline peaks at 27.33° and 32.9° disappeared, which reveals some changes in the crystalline nature of Ris molecules. These changes in XRD pattern along with DSC results suggested partial conversion of Ris molecules from a crystalline state to an amorphous one which was previously reported [40]. Moreover, apart from the crystalline conversion of Ris molecules, dispersion and dissolving of drug molecules in Chol would be another reason for changes in the XRD pattern of implant formulation F4.
Fourier Transform Infrared Spectroscopy Analysis
In order to evaluate Ris stability during formulation and fabrication, Fourier transform infrared spectroscopy (FTIR) analysis was performed. Ris showed absorbance peak related to C-N stretching at 1000–1200 cm-1 and also stretching peaks between 2759 and 3000 cm-1 corresponded to C-H bonds at CH2 groups. Ris also represented a sharp absorbance peak at 1651 cm-1 which is related to C = O stretching of aromatic ketone [41]. Comparing the Ris FTIR spectra within different implant formulations (Fig. 7), it is observed that there were no major changes in the chemical structure of Ris during the formulation process and most probably no destructive chemical reactions occurred between formulation components and drug.
Conclusion
A series of risperidone implant formulations were fabricated and evaluated for physicochemical properties. In this study, two different types of excipients were used in the fabrication of Ris implants. The findings of the present study suggested that Chol as a hydrophobic excipient sustained the release of the drug up to 59 days while PEG delayed the release of Ris maximum up to 32 days with the same amount of Chol . Mathematical investigations of release profile from different formulations represented that Ris implants with Chol follow the zero-order release model while implants with PEG showed diffusion-based release which is in agreement with the Higuchi release kinetic. Moreover, implants with Chol showed higher mechanical strength relative to implants with PEG. Although the crystalline state of Ris changed due to solubilization in Chol molecules, its chemical structure remained intact; this revealed the compatibility of formulation components and the fabrication method. On the basis of the present study, it can be concluded that apart from polymer mass in implant formulation, excipients play a major role in the release profile of the drug and also affect other implant properties like water uptake and mechanical strength. Moreover, by selection of proper excipient(s) in implant formulation, the appropriate release mechanism and desired release profile would be achievable.
References
Agarwal P, Rupenthal ID. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today. 2013;18(7):337–49.
Bourges J, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58(11):1182–202.
Halliday AJ, Moulton SE, Wallace GG, Cook MJ. Novel methods of antiepileptic drug delivery—polymer-based implants. Adv Drug Deliv Rev. 2012;64(10):953–64.
Dash A, Cudworth G. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol. 1998;40(1):1–12.
Kempe S, Metz H, Pereira PG, Mäder K. Non-invasive in vivo evaluation of in situ forming PLGA implants by benchtop magnetic resonance imaging (BT-MRI) and EPR spectroscopy. Eur J Pharm Biopharm. 2010;74(1):102–8.
Lan S-F, Kehinde T, Zhang X, Khajotia S, Schmidtke DW, Starly B. Controlled release of metronidazole from composite poly-ε-caprolactone/alginate (PCL/alginate) rings for dental implants. Dent Mater. 2013;29(6):656–65.
Ho EA, Vassileva V, Allen C, Piquette-Miller M. In vitro and in vivo characterization of a novel biocompatible polymer–lipid implant system for the sustained delivery of paclitaxel. J Control Release. 2005;104(1):181–91.
Ogawa Y, Kodaka M, Okuno H. Trigger lipids inducing pH-dependent liposome fusion. Chem Phys Lipids. 2002;119(1):51–68.
Wong HL, Rauth AM, Bendayan R, Wu XY. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur J Pharm Biopharm. 2007;65(3):300–8.
Sax G, Winter G. Mechanistic studies on the release of lysozyme from twin-screw extruded lipid implants. J Control Release. 2012;163(2):187–94.
Kreye F, Siepmann F, Willart J, Descamps M, Siepmann J. Drug release mechanisms of cast lipid implants. Eur J Pharm Biopharm. 2011;78(3):394–400.
Sax G, Kessler B, Wolf E, Winter G. In-vivo biodegradation of extruded lipid implants in rabbits. J Control Release. 2012;163(2):195–202.
Siepmann J, Siepmann F. Mathematical modeling of drug release from lipid dosage forms. Int J Pharm. 2011;418(1):42–53.
Raghuveer K, Hammond E. The influence of glyceride structure on the rate of autoxidation. J Am Oil Chem Soc. 1967;44(4):239–43.
Fang J-L, Vaca CE, Valsta LM, Multanen M. Determination of DNA adducts of malonaldehyde in humans: effects of dietary fatty acid composition. Carcinogenesis. 1996;17(5):1035–40.
Williams G, Caldwell J, Armstrong D, Bartsch H, Bevan R, Browne R, et al. Multicenter study to assess potential hazards from exposure to lipid peroxidation products in soya bean oil from Trilucent™ breast implants. Regul Toxicol Pharmacol. 2009;53(2):107–20.
Parent M, Nouvel C, Koerber M, Sapin A, Maincent P, Boudier A. Plga in situ implants formed by phase inversion: critical physicochemical parameters to modulate drug release. J Control Release. 2013;172(1):292–304.
Peterson HB, Curtis KM. Long-acting methods of contraception. N Engl J Med. 2005;353(20):2169–75.
Kreye F, Siepmann F, Siepmann J. Drug release mechanisms of compressed lipid implants. Int J Pharm. 2011;404(1):27–35.
Desai KGH, Mallery SR, Schwendeman SP. Effect of formulation parameters on 2-methoxyestradiol release from injectable cylindrical poly (DL-lactide-co-glycolide) implants. Eur J Pharm Biopharm. 2008;70(1):187–98.
Wang F, Saidel GM, Gao J. A mechanistic model of controlled drug release from polymer millirods: effects of excipients and complex binding. J Control Release. 2007;119(1):111–20.
Herrmann S, Winter G, Mohl S, Siepmann F, Siepmann J. Mechanisms controlling protein release from lipidic implants: effects of PEG addition. J Control Release. 2007;118(2):161–8.
Wang PY. Palmitic acid as an excipient in implants for sustained release of insulin. Biomaterials. 1991;12(1):57–62.
Wang F, Blanco E, Ai H, Boothman DA, Gao J. Modulating β‐lapachone release from polymer millirods through cyclodextrin complexation. J Pharm Sci. 2006;95(10):2309–19.
Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610(2):271–80.
Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225(1):73–80.
Khan MZI, Tucker IG, Opdebeeck JP. Cholesterol and lecithin implants for sustained release of antigen: release and erosion in vitro, and antibody response in mice. Int J Pharm. 1991;76(1):161–70.
Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psych. 1994.
Rabin C, Liang Y, Ehrlichman R, Budhian A, Metzger K, Majewski-Tiedeken C, et al. In vitro and in vivo demonstration of risperidone implants in mice. Schizophr Res. 2008;98(1):66–78.
Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5(1):23–36.
Huang J, Wigent RJ, Bentzley CM, Schwartz JB. Nifedipine solid dispersion in microparticles of ammonio methacrylate copolymer and ethylcellulose binary blend for controlled drug delivery: effect of drug loading on release kinetics. Int J Pharm. 2006;319(1):44–54.
Higuchi T. Mechanism of sustained‐action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(12):1145–9.
Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364(2):328–43.
Cheng L, Guo S, Wu W. Characterization and in vitro release of praziquantel from poly (ɛ-caprolactone) implants. Int J Pharm. 2009;377(1):112–9.
Opdebeeck J, Tucker I. A cholesterol implant used as a delivery system to immunize mice with bovine serum albumin. J Control Release. 1993;23(3):271–9.
Rahman Z, Zidan AS, Khan MA. Risperidone solid dispersion for orally disintegrating tablet: its formulation design and non-destructive methods of evaluation. Int J Pharm. 2010;400(1):49–58.
Rahman Z, Zidan AS, Khan MA. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm. 2010;76(1):127–37.
Silva AC, Kumar A, Wild W, Ferreira D, Santos D, Forbes B. Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Inter J Pharm. 2012;436(1):798–805.
Sattanathan P, Babu JM, Vyas K, Reddy R, Rajan S, Sudhakar P. Structural studies of impurities of risperidone by hyphenated techniques. J Pharm Biopharm Anal. 2006;40(3):598–604.
Hu Z, Liu Y, Yuan W, Wu F, Su J, Jin T. Effect of bases with different solubility on the release behavior of risperidone loaded PLGA microspheres. Colloids Surf B:Biointerfaces. 2011;86(1):206–11.
Daniel JSP, Veronez IP, Rodrigues LL, Trevisan MG, Garcia JS. Risperidone–solid-state characterization and pharmaceutical compatibility using thermal and non-thermal techniques. Thermochim Acta. 2013.
Acknowledgments
The authors would like to acknowledge the Iranian National Science Foundation (INSF) for the grant No 89004212.
Conflict of Interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saadat, E., Abdollahi, A. & Dorkoosh, F.A. Fabrication and Characterization of Risperidone Implants as an Extended Antipsychotic Delivery System, Exploring the Role of Excipients. J Pharm Innov 10, 118–129 (2015). https://doi.org/10.1007/s12247-015-9212-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-015-9212-y