Abstract
Tidal marsh plant communities in the Pacific Northwest are characterized by tall, perennial graminoids (TPGs), which provide forage for herbivores such as Canada geese. Excessive grazing by Canada geese leads to loss of marsh habitat, and removal of grazing pressure is required for the vegetation to recover. Grazing exclosures (fences) are used to allow time and space for vegetation to recover following intensive herbivory; however, their effects on native plant community recovery has not been tested. Generalized linear models were used to compare TPG abundance in aboveground vegetation and surface seed banks in 1-year-old and 10-year-old exclosures at Nanaimo River Estuary (NRE) and Little Qualicum River Estuary (LQRE), respectively, to areas of the marshes that had no known history of grazing (undisturbed) and areas still actively grazed (grubbed). Compared to undisturbed sites, grubbed sites had 187.3% less mean TPG vegetation cover and 190.7% lower proportion of TPG seeds. The 1-year-old exclosures at NRE had 105.0% less mean TPG vegetation cover and 193.2% lower proportion of TPG seeds. The 10-year-old exclosures at LQRE had 7.0% greater mean TPG cover and 55.7% greater proportion of TPG seed than all undisturbed sites; however, these exclosures had 110.0% greater mean relative abundance of non-native TPGs than undisturbed sites. These results indicate vegetation may not recover towards comparable historic conditions through grazing exclusion alone, and that active restoration methods may be required following intensive grazing, especially in estuaries where the vegetation community and surface seed bank has a high abundance of non-native, invasive species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries around the world are subject to disturbance and cumulative effects of stressors from a variety of natural and anthropogenic disturbances (Lotze 2010; Zedler 2017). Sources of disturbance or stressors may occur on a variety of spatial and temporal scales, from extensive conversion of estuarine floodplain for municipal and industrial use (Finn et al. 2021) to local disturbances such as intensive storms or tidal surges (Pasternack 2009). These cumulative impacts can have negative consequences on plant communities comprising estuarine habitat, such as native species biodiversity loss or species homogenization, leading to altered community structure and functional processes (Price et al. 2020; Simberloff et al. 2013). Not only does this directly impact the biodiversity and function of an estuary, but it can also erode resistance to subsequent disturbance as historically abundant species become locally extirpated (Diefenderfer et al. 2021; Johnstone et al. 2016; Schaefer 2011).
Following disturbance, life history strategies of colonizing species may drive alternative successional trajectories, leading to alternative species composition and abundance in the recovering habitat (Connell & Slatyer 1977; Ricklefs 2008). If life history traits are sufficiently competitive, early colonizers may inhibit recruitment of species diversity present in the pre-disturbance community, thus shifting it to an alternative stable state (Connell & Slatyer 1977; Scheffer & Carpenter 2003). How successional trajectories proceed in estuarine plant communities depends on abiotic conditions (e.g., elevation, salinity), as well as availability of viable plant propagules. Propagules may be present in the form of seeds deposited into the sediment surface (hereafter, surface seed bank) or vegetative clonal growth at the site. Local propagule inputs may be derived from seed rain from parent plants or extension of clonal stems within the site and dependent on species-specific growth strategies such as seed limitation in favor of clonal growth (Morzaria-Luna & Zedler 2007). However, dispersal of propagules from distant sites is possible through water, with seed or clonal fragments of some species able to float on tidal currents for weeks to months to reach new colonization sites (Koutstaal et al. 1987). Novel species (e.g., non-native or invasive species) locally encroaching from terrestrial habitat or dispersed through water into an estuary may shift propagule loads and thus shift potential recovery trajectories of a site via priority effects (Connell & Slatyer 1977) before a disturbance event occurs. Following disturbance, propagule pressure may overshadow recruitment advantages of historically dominant species (Lavorel & Lebreton 1992).
Post-disturbance propagule recruitment and establishment, especially in tidal marshes, depend on abiotic conditions including disturbance-free temporal niches, termed windows of opportunity (Balke et al. 2014), where germinating seeds must anchor and elongate their roots before they are subject to dislodging during subsequent tidal inundation. Because seedlings are susceptible to dislodging or drowning during tidal inundation, successful establishment favors clonal fragments over seed-based establishment at tidal elevations with greater erosion and inundation stress (Silinski et al. 2016). However, increases in the frequency or duration of local disturbances can impact the recruitment window of opportunity for historically successful propagules (Hu et al. 2015). For example, stronger storms or tidal currents due to changing climate, or increased human disturbances such as boat waves, can become a compounding feedback loop between altered propagule availability and altered windows of opportunity for successful species to establish. This feedback loop can serve as an environmental filter influencing which species may successfully establish within the new windows of opportunity. Some natural disturbance events, like grazing by ungulates or waterfowl, may occur as either discrete, short-term events, or as long-term disturbance agents in an ecosystem. Historically, grazing in estuaries would have occurred as species like waterfowl moved through on migration routes, and forage preference would be related to seasonal plant phenology (Buchsbaum & Valiela 1987). Under these grazing regimes, plant communities were able to passively recover through natural recruitment and succession (Meli et al. 2017). However, intensive or persistent grazing in conservation areas such as estuaries is becoming more common with the introduction of novel grazers within a region, compounded by the loss of conservation habitat due to anthropogenic landscape changes (Clausen & Percival 1998; Prowse et al. 2019). These new grazing pressures can effectively reset successional processes through total removal of mature vegetation by opening space with reduced or absent competition and thus potentially set the plant community on a recovery trajectory towards an alternative stable state via seed bank recruitment (Abernethy & Willby 1999; Srivastava & Jefferies 1996). Through alternative competitive strategies, these novel species can shift recovery trajectories to a new compositional palette.
Estuaries around the Salish Sea in the Pacific Northwest of North America are dominated by swards of graminoids (sedges, rushes, grasses) whose competitive strategies include clonal vegetative reproduction and tall (> 1 m) canopy cover, interspersed with a diversity of broadleaf, flowering species (“forbs”) (Borde et al. 2020). Many estuaries in this region are overgrazed by non-native, hyperabundant Canada geese (Branta canadensis ssp. Fulva) (Dawe et al. 2011; Dawe & Stewart 2010). In addition to removing leafy aboveground vegetation, Canada geese will rip out or “grub” starchy rhizomes capable of clonal reproduction, which in turn increases erosion of marsh sediments and their seed banks. Estuary plant communities impacted by intensive goose herbivory and associated sediment disturbances can recover either by clonal expansion from adjacent remnant patches or through seed recruitment from seed dispersed and retained on the eroded site. For graminoids that are seed-limited, such as Carex sp. (Kettenring & Galatowitsch 2011; Schütz 2000), recovery of these species to pre-disturbance abundance may be more dependent on the presence of intact, clonally dominated communities adjacent to the grazed areas.
Our main objective in this study was to understand propagule composition and availability within surface seed banks and aboveground vegetation composition changes at discrete stages since exclusion of grazing by Canada geese in two Salish Sea estuaries. Traditional succession models predict the most competitive species will increasingly dominate the plant community as time since disturbance increases (Tilman 1990). This would particularly be the case in a community dominated by clonal species, where recovery is driven by species spreading clonally from adjacent undisturbed patches, in addition to potential recruitment from the seed bank. If succession is happening the way inhibition models explain, then:
-
1.
Composition and abundance of dominant tall, perennial graminoids in aboveground vegetation at older disturbance sites will have greater similarity to undisturbed (reference) vegetation than recently disturbed sites. Alternatively, the grazing disturbance to remove vegetation and altered propagule availability can lead to alternative succession pathways, where new species can achieve competitive dominance through seed or clonal recruitment, derailing the slow clonal encroachment of historically dominant species from neighboring sites. If this is the case, we expect compositional abundance of competitively dominant species in the older disturbance sites will be significantly different from that of undisturbed sites.
-
2.
Because a longer post-grazing recovery period should allow for recruitment of more species diversity into the aboveground vegetation and surface seed bank, we expect composition and abundance of surface seed banks should closely resemble that of aboveground vegetation in recently disturbed sites (e.g., via direct seed rain) and become more species-rich and dissimilar from aboveground vegetation (i.e., via dispersal) with time since disturbance.
Methods
Study Area and Site History
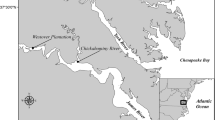
The Little Qualicum River Estuary (LQRE) and Nanaimo River Estuary (NRE) are situated on the east coast of Vancouver Island along the Strait of Georgia, British Columbia, Canada (Fig. 1), and are on the ancestral, unceded territories of Coast Salish Peoples, including the K’ómoks, Snuneymuxw, Qualicum Band, and Nanoose Nations. Prior to European colonial settlement, traditional practices by Indigenous Peoples around the Salish Sea would have included managing estuaries as root gardens to promote the abundance of broadleaf flowering species with starchy roots, rather than the perennial graminoids that competitively dominate the estuaries today (Turner et al. 2013). Colonial settlement of the LQRE began in 1887, with cattle grazing, log booming, and sawmill operations being the chief uses of the estuary until 1974 when 29 ha of the estuary were donated to the Canadian Wildlife Service for conservation purposes, and subsequently designated as a provincially protected National Wildlife Area in 1977 (Clermont 2010). The NRE was similarly settled through the late nineteenth and early twentieth centuries and heavily augmented through construction of a ferry terminal, logging operations, and coal mining. While the NRE is part of the UNESCO-designated Mount Arrowsmith Biosphere Region and portions fall under conservation management, it is not provincially protected through designation as a WMA.
The extent of watershed catchments flowing into the Salish Sea (A, outlined in green) spans the US-Canadian border on the Pacific Coast of North America (A, inset). Sampling took place within the polygons outlined in gold dash at the Little Qualicum River Estuary (B) and Nanaimo River Estuary (C) along the southeast coast of Vancouver Island, near the towns of Qualicum Beach and Nanaimo, respectively
Goose Introduction and Grazing Exclusion
Canada geese (Branta canadensis spp. fulva) were historically a migratory species to Vancouver Island, although its likely there were small resident breeding populations on northern Vancouver Island; however, in the late twentieth century, populations from eastern North America were introduced to the island to promote hunting (Dawe & Stewart 2010). These populations have since established resident populations, with regional seasonal migration patterns along the eastern coast of Vancouver Island and around the Salish Sea (Pearce & Demers 2019). In the absence of sufficient hunting pressure or predation, these resident populations have grown hyperabundant (Dawe & Stewart 2010) and exert year-round pressure on estuaries through herbivory and grubbing (the digging and uprooting of plant roots and rhizomes; Fig. 2C). This sustained pressure leads to complete loss of above- and belowground plant structures, denuded marsh platforms, and subsequent sediment and seed bank loss through erosion.
Reference vegetation for this study was defined as areas with no known grazing disturbance and dominated by native species, including Carex lyngbyei in the Little Qualicum River Estuary (A) and Juncus balticus or Deschampsia caespitosa in the Nanaimo River Estuary (B). Intensive grubbing (C) by Canada geese (Branta canadensis) removes rhizomes and contributes to sediment loss, resulting in large areas of bare or “denuded” mudflat (D). Exclosures were constructed around the edges of these grubbed/grazed areas to promote vegetation recovery in 2010 in the Little Qualicum River Estuary (E) and Nanaimo River Estuary in 2020 (F). Photo credits: A D. Clermont, 2021; B S. Lane, 2021; C G. Fairbrother, date unknown; D G. Ashley, 2021; E N. Dawe, 2011; F T. Clermont, 2020
Fences or “exclosures” are commonly used to exclude herbivores, including Canada geese, from continued grazing. A history of exclosure construction since 2010 in the LQRE (Clermont 2015) and recent history of exclosure construction in NRE in 2020 (Tim Clermont, personal comm.) afford the opportunity to develop a coarse chronosequence of recovery at discrete time periods since grazing disturbance, including grubbed (denuded mudflat), 1-year-old exclosures, 10-year-old exclosures, and areas that have no known history of grazing by Canada geese (undisturbed or “reference”) (Table 1). Exclosures are constructed along the edges of vegetation impacted by grazing activity (Fig. 2D, E, F), with the rationale that remnant vegetation should recover towards a desired undisturbed or “reference” condition (Fig. 2A, B) once grazing pressure is relieved (Guardians of Mid-Island Estuaries Society 2021).
Site Ecological Assessment
Salinity was measured (FieldScout Direct Soil EC Meter, 2265FSTP) at depths of 10–15 cm as part of initial site evaluation. It was difficult to obtain measurements in grubbed sites and some 1-year-old exclosures, as sediment loss exposed underlying gravel/cobble layers, resulting in several of these sites only sampled in the top 1–3 cm. Despite these challenges, all salinities recorded fell within the range of mesohaline marsh types in which the same plant communities may be expected (Odum 1988; Fig. S1).
We estimated elevation differences of approx. 30 cm (estimated range 20–75 cm) between the highest elevation (undisturbed) and lowest elevation (grubbed) sites, and all sites were at or below elevations inundated at mean higher high water (MHHW), as estimated in the field during tidal maxima during site selection June–July 2021. All species recorded in this study are typically found between mudflat and MHHW elevations (Janousek et al. 2019).
Vegetation Sampling
Aboveground vegetation was surveyed across all vegetation plots (Table 1) once in mid-July 2021. Two 1-m2 vegetation plots were placed within the exclosures (“sites,” n = 4 per disturbance condition in each estuary; Table 1), at least 1 m from the bank edge and any exclosure boundary, and at least 3 m apart within the exclosure. Quadrats were placed so that one side of the plot was parallel to the bank.
All vascular species were identified according to Hitchcock and Cronquist (2018). Species were considered in the plot if at least half of their basal stem(s) were inside the quadrat boundary; overhanging vegetation originating from basal stems outside the plot was not considered. Aerial vegetated cover was estimated to the nearest3% (1/32 m2). For any species present with less than 3% cover, species were assigned 2% cover if > 20 individuals were present, 1% cover if 2–20 individuals were present, and 0.1% cover for single individuals. Bare ground was estimated as the remainder of the plot area not covered by aboveground vegetation. Any plots with > 100% cover were standardized relative to 100% to allow for comparison across plots and to constrain values to fit statistical distributions. To characterize plant structure, all species were assigned to a height category tall (> 1 m), medium (50–100 cm), or short (< 50 cm) based on their maximum reported height in the Illustrated Flora of British Columbia (Douglas et al. 1998). Our key species of interest, tall perennial graminoids (TPGs), were defined as any grass (Poaceae), sedge (Cyperaceae), or rush (Juncaceae) with a biennial or perennial life history and mature height of at least 1 m.
Surface Seed Bank Sampling and Germination
Two surface seed bank samples were taken from each plot (n = 16 per disturbance condition in each estuary; Table 1) in summer (July 2020), fall (October 2020), and spring (March 2021). A 10-cm-diameter handheld bulb planter (e.g., Husky 9 in. stainless Steel Bulb Planter, Home Depot, USA) was used to excise sediment 1 cm deep to capture the most recent seeds deposited into the marsh sediment, which we call the “surface seed bank.” Vegetative roots, rhizomes, or other viable rooted material were removed before placing the sample in a plastic zipper bag. All surface seed bank samples from the same estuary and disturbance condition were then homogenized in a clean bucket with 100 mL dechlorinated water. Samples were hand-sifted for any remaining root, rhizome, or vegetative material; then, the homogenized sample was transferred to a clean plastic zipper bag. Summer and fall 2020 samples were stored at 4 °C for approx. 12 weeks to simulate overwinter cold stratification to release seed dormancy (Rosbakh et al. 2019); samples collected in the spring of 2021 underwent natural winter conditions within the estuaries and were not subjected to cold stratification.
Germination trials were conducted under greenhouse conditions with 15 h daylength at ~ 20 °C. Seedling pots (9 cm × 13 cm × 5.7 cm (depth)) were filled with moist, sterile potting media (Sunshine Mix No. 4, Sun Gro Horticulture, Agawam, MA, USA). Pots were placed in solid cache trays and constantly bottom-watered with municipal tap water. Seeds were sown by adding 75 mL of sampled sediments to the top of each seedling pot (n = 8 per estuary and disturbance condition) while constantly agitating the homogenized seed bank sample to prevent seeds from settling to the bottom of the sediment mixture. Seeds were allowed to germinate for 5 weeks, at which time all individuals were identified, counted, and removed. The seedling trays were observed for any further germination for another 7–10 days, at which time the samples were discarded. Any species that could not be identified to species at germination were labelled and transplanted into 38 P plug trays with the same growing media and growing conditions until a positive identification could be made.
Statistical Analyses
Because tall, perennial graminoids (TPGs) are the dominant species group in intact tidal marsh plant communities, we focused most of our analyses on this taxonomic group. Mean species richness was calculated for all species in the aboveground vegetation and surface seed banks for each disturbance category. For all species, we defined dominance within the aboveground vegetation plots or surface seed bank samples as species having ≥ 25% mean relative abundance. Although a species may be dominant within the vegetation at a site, dominance may not indicate specificity or fidelity to a specific disturbance category. To understand which species could be used to significantly characterize each grazing disturbance condition in the aboveground vegetation and surface seed, we used indicator species analysis (Indicspecies R package, De Caceres & Jansen, 2016). Species significantly driving compositional abundance in each disturbance category were defined by a biserial correlation coefficient (multipatt func = “r.g.”) and permutational analysis, with “strong” indicators defined as indicator values (IndVal) ≥ 0.5 (Dufrêne & Legendre 1997).
Relative abundance in the aboveground vegetation was calculated as the proportion of one species relative to the total plot cover, defined as the sum of all vegetation cover. Relative abundance in the seed bank sample was calculated as the proportion of one species out of the total number of germinated seedlings. To test whether species relative abundance differed among disturbance recovery categories, we used separate generalized linear models for the aboveground vegetation and surface seed bank, each with a binomial distribution and logit link function from the R stats package (R Core Team, 2022). Our response variable, the proportion of graminoid abundance in each sample of aboveground vegetation or the surface seed banks, was fit against each disturbance category as the primary predictor, with estuary location as a fixed effect to test for geographic differences. Model residuals were visually inspected for normality. For each model, we used the predict() function with type = “response” (stats, R Core Team 2022) to calculate the probability of finding TPG relative abundance proportional to undisturbed aboveground vegetation or surface seed banks. All analyses were performed in R Studio (v. 4.2.2).
Results
Aboveground Vegetation
We found the greatest relative abundance of tall, perennial graminoids (TPGs) in the aboveground vegetation in undisturbed sites at both estuaries (n = 16 plots, 8 plots per estuary), and in 10-year-old exclosures at Little Qualicum River Estuary (LQRE, n = 8 plots); however, 10-year-old exclosures were dominated by non-native, invasive species (Agrostis stolonifera) (Figs. 3 and 4). Mean relative abundance of native TPGs was 6.0% greater in undisturbed sites at LQRE than undisturbed sites at Nanaimo River Estuary (NRE). Meanwhile, mean relative abundance of native or non-native TPGs in grubbed sites at both estuaries (n = 16 plots, 8 plots per estuary) was over 100% lower than any exclosures or undisturbed sites, as these sites contained an average of < 3.5% TPG cover. In LQRE, mean relative abundance of native TPGs in undisturbed sites was 92.0% greater than non-native TPG cover. However, in 10-year-old exclosures at LQRE, relative abundance of non-native TPGs was 83.8% greater than native TPGs. At NRE, mean relative abundance of native TPGs was 88.1% greater in undisturbed sites compared to 1-year-old exclosures (n = 8 plots) and 194.9% greater than grubbed sites (Fig. 3).
Relative abundance of tall perennial graminoids (TPGs) in the aboveground vegetation and surface seed banks at Nanaimo and Little Qualicum River Estuaries. Colored points are individual plots (n = 8 per disturbance condition in each estuary) or seed bank samples (n = 8 per disturbance condition in each estuary), with box and whisker plots indicating interquartile ranges. Notably, there is a nearly equal abundance of non-native and native TPG seed in 10-year-old exclosures and greater relative abundance of non-native than native TPG seed in undisturbed sites in Little Qualicum Estuary
Relative abundance of species identified in indicator species analysis at each estuary sampled in aboveground vegetation (n = 8 plots per disturbance category in each estuary) and surface seed bank (n = 8 samples per disturbance category in each estuary). Colored columns represent means; error bars represent standard error. Species listed alphabetically along x-axis by major clade, with graminoids appearing in black box, and forbs unboxed; non-native species indicated with asterisk. Notably, abundance of key native tall perennial graminoids such as Carex lyngbyei are absent from the seed bank, while others such as Juncus balticus are dominant (> 25% mean relative abundance) in the seed bank but not dominant in aboveground vegetation, such as observed in 10-year-old exclosures at Little Qualicum Estuary
Native species richness in the aboveground vegetation was always greater than non-native species richness, regardless of disturbance category. At NRE, all sites in all disturbance categories had an average of four to five native species, and one to two non-native species. In LQRE, grubbed sites had an average of five native species, undisturbed sites had an average of three native species, and all sites had one to two non-native species (Fig. 5). We considered a species to be dominant within the plot if it was present with ≥ 25% mean relative abundance, although indicator species were not always dominant (Table 2; Fig. 4). Across both estuaries, only a few species were dominant in the aboveground vegetation: native TPG species Carex lyngbyei was dominant in undisturbed sites at both LQRE (51.0 ± 15.5%) and NRE (36.0 ± 12.6%), while non-native TPG Agrostis stolonifera (59.1 ± 6.1%) was the only TPG dominant in the 10-year-old exclosures at LQRE. Native Eleocharis parvula was the only dominant species in grubbed sites at LQRE (43.5 ± 6.5%) and had 22.7 ± 5.8% mean relative abundance in grubbed sites at NRE (Fig. 4).
Species richness of native vs. non-native plants among disturbance categories in both Nanaimo and Little Qualicum River Estuaries for both aboveground vegetation (n = 8 plots per disturbance condition in each estuary, top panel) and surface seed banks (n = 8 samples per disturbance condition in each estuary, bottom panel)
Indicator species indices are the product of a species’ specificity (relative abundance) and fidelity (relative frequency) to a site and may be considered a “strong” indicator if the index (IndVal) calculated by indicspecies::multipatt() is ≥ 0.5 (Dufrêne & Legendre (1997)). Undisturbed sites at both estuaries (n = 8 plots per estuary) were characterized by three indicator species: native TPG Juncus balticus (IndVal = 0.2, p = 0.020), native TPG Carex lyngbyei (IndVal = 0.2, p = 0.026), and native forb Triglochin maritima (IndVal = 0.2, p = 0.042); however, none of these indices was considered strong indicators according to Dufrêne & Legendre (1997). In 10-year-old exclosures at LQRE (n = 8 plots), indicator species analysis identified non-native TPG A. stolonifera as the strongest community indicator (IndVal = 0.4, p = 0.001), which was approximately twice as strong an indicator as native forb Potentilla anserina (IndVal = 0.2, p = 0.036). The indicator species of 1-year-old exclosures at NRE (n = 8 plots) included two native forbs: Spergularia canadensis (IndVal = 0.2, p = 0.022) and Lysimachia maritima (IndVal = 0.2, p = 0.043), while grubbed sites in both estuaries (n = 8 plots per estuary) were indicated by short native graminoid Eleocharis parvula (IndVal = 0.3, p = 0.007) and non-native forb Cotula coronopifolia (IndVal = 0.2, p = 0.047) (Table 2).
We employed a generalized linear model to test the relationship between proportion of TPGs in the aboveground vegetation, using undisturbed sites as the model intercept (estimate = 1.0, p = 0.206, n = 16) and estuary as a fixed effect (Table 3). Compared to undisturbed sites, grubbed sites in both estuaries had 193.0% less mean TPG cover (estimate = − 5.2, p = 0.024, n = 16), while 1-year-old exclosures at NRE had 97.4% less mean TPG cover (estimate = − 1.9, p = 0.091, n = 8) (Table 3; Fig. 6). The 10-year-old exclosures at LQRE had 17.0% greater mean TPG cover than all undisturbed sites (estimate = 0.6, p = 0.611, n = 8) (Table 3; Fig. 6); however, these exclosures had 92.6% greater mean relative abundance of non-native TPGs than in undisturbed sites at LQRE (Fig. 3). Overall, proportion of TPGs in undisturbed sites was 7.4% lower at NRE than at LQRE, but estuary location did not have a strong effect on model outcomes (estimate = − 0.3, p = 0.815).
Actual vs. predicted values for proportion of tall, perennial graminoids in each disturbance category in aboveground vegetation cover (n = 8 plots per disturbance category in each estuary, top panel) and surface seed bank samples (n = 8 samples per disturbance category in each estuary, bottom panel). Actual values plotted as colored points for Little Qualicum River Estuary and Nanaimo River Estuary; estimated mean values shown in black as means (points) with standard error
Surface Seed Banks
In surface seed banks, mean relative abundance of non-native TPG seeds in undisturbed sites at LQRE was 81.2% higher than native TPG seeds (n = 8 plots), while 10-year-old exclosures at LQRE had only 4.4% greater mean relative abundance of native TPGs than non-native TPGs (n = 8 plots) (Fig. 3). Mean relative abundance of native (8.8%) and non-native TPG (6.2%) species were nearly even in undisturbed sites at NRE (n = 8 plots); however, their combined mean abundance was 135.3% lower than mean TPGs in undisturbed sites at LQRE. Seed of TPGs was nearly absent in grubbed sites at both estuaries (n = 16, 8 samples per estuary), and in 1-year-old exclosures at NRE (n = 8 samples per estuary), with < 1.5% relative abundance of native or non-native TPG species in these disturbance categories.
Across all disturbance conditions, mean native species richness in the surface seed bank ranged from four to five species at NRE and three to five species at LQRE. Mean richness of non-native species was lower at both NRE and LQRE, with one to two species found across all disturbance conditions. (Fig. 5) Few species were dominant (> 25% mean relative abundance) in the surface seed bank (Fig. 4): at NRE, only Eleocharis parvula was dominant in the grubbed (91.7 ± 0.7%), 1-year-old exclosures (82.0 ± 0.9%), and undisturbed sites (80.0 ± 3.1%). At LQRE four species dominated across the different disturbance conditions: mean relative abundance of non-native TPG Agrostis stolonifera heavily dominated undisturbed sites (55.7% ± 1.7%) and 10-year-old exclosures (40.2 ± 5.5%), while native TPG Juncus balticus dominated 10-year-old exclosures (41.4 ± 2.8%), but not the undisturbed sites (17.4 ± 2.5%). Grubbed sites at LQRE were dominated by E. parvula (66.9 ± 1.4%) and Spergularia canadensis (30.5 ± 1.2%) (Fig. 4).
Although indicator species analysis of the surface seed bank did not identify species with “strong” fidelity and specificity (IndVal ≥ 0.5 (Dufrêne & Legendre (1997)), several species found in the seed bank may be indicative of each disturbance conditions (Table 2). Across both estuaries, grubbed sites (n = 8 samples per estuary) were best characterized by native forb Salicornia depressa (IndVal = 0.2, p = 0.006). Interestingly, no indicator species were identified for 1-year-old exclosures at NRE. In 10-year-old exclosures (n = 8 samples) at LQRE, two TPGs and one forb were identified: J. balticus was the strongest indicator species (IndVal = 0.4, p = 0.001), while indices for A. stolonifera (IndVal = 0.2, p = 0.006) and Triglochin maritima (IndVal = 0.2, p = 0.043) were approximately similar. Indicator species for undisturbed sites (n = 8 samples) at both estuaries identified native TPGs Carex lyngbyei (IndVal = 0.2, p = 0.022) and J. articulatus (IndVal = 0.2, p = 0.034) and one non-native forb Cotula coronopifolia (IndVal = 0.2, p = 0.023).
We tested the relationship between disturbance condition and proportion of TPGs in the surface seed bank, using undisturbed sites as the model intercept (estimate = 1.2, p = 0.151, n = 16) and estuary as a fixed effect (Table 3). Compared to both undisturbed sites, grubbed sites had 192.1% lower proportion of TPG seeds (estimate = − 5.1, p = 0.055, n = 16), and 1-year-old exclosures had 194.7% lower proportion of TPG seeds (estimate = − 3.4, p = 0.458, n = 8) (Table 3; Fig. 6). Compared to all undisturbed sites, 10-year-old exclosures at LQRE had 55.6% greater proportion of TPG seed (estimate = 0.3, p = 0.794, n = 8). The proportion of TPG seed in undisturbed sites at LQRE was 131.5% greater than in undisturbed sites at NRE, resulting in estuary location having a strong effect on model outcomes (estimate = − 2.9, p = 0.024, n = 24 samples from NRE).
Discussion
We wanted to understand whether tall, perennial graminoids (TPGs) recovered following grazing disturbance by resident Canada geese and whether recovery of surface seed banks resembled aboveground vegetation composition. We found that proportion of TPGs in 10-year-old exclosures was similar to undisturbed sites (Fig. 6); however, non-native species Agrostis stolonifera dominated these exclosures with mean relative abundance of 59.1 ± 6.1% in the aboveground vegetation and 40.2 ± 5.5% in the surface seed banks (Figs. 3 and 5). This was in sharp contrast to the dominance of native TPG Carex lyngbyei in aboveground vegetation (but not surface seed banks) in undisturbed sites at Little Qualicum River Estuary (LQRE, 51.0 ± 15.5%) and at Nanaimo River Estuary (NRE, 36.0 ± 12.6%). We found that TPGs were not dominant in the aboveground vegetation or surface seed banks in grubbed sites at either estuary or 1-year-old exclosures at NRE. In surface seed banks, TPGs were only dominant at LQRE in the 10-year-old exclosures and undisturbed sites; seeds of TPGs were notably absent at NRE (Fig. 6). Besides its dominance in the surface seed banks of 10-year-old exclosures, A. stolonifera comprised 54.7 ± 1.7% of mean relative abundance of seeds in undisturbed sites at LQRE. At LQRE, surface seed banks in the 10-year-old exclosures also had a dominance of native TPG Juncus balticus (41.4 ± 2.8%); however, seeds of this species were dominant in the undisturbed sites (Fig. 4). Given that non-native species A. stolonifera dominate the recovered vegetation and surface seed banks after 10 years of grazing exclusion at Little Qualicum River Estuary (LQRE), we suggest that these disturbed habitats are recovering towards an alternative composition and abundance, reflecting the prevalence of non-native grass A. stolonifera throughout the region.
Non-native TPGs may have a greater competitive recruitment advantage, contributing to this alternative recovery trajectory following disturbance by grubbing. This may be driven at least in part by A. stolonifera’s high seed production, resulting in a “weighted lottery” (Lavorel & Lebreton 1992) of seed propagules from which the plant community can recover. Additionally, clonal reproduction through spreading rhizomes or stolons offers an additional mechanism by which this non-native species may overtake a habitat following disturbance. For example, we found that both A. stolonifera and J. balticus were dominant in surface seed banks in undisturbed sites and 10-year-old exclosures at LQRE (Fig. 4). Despite these two species having similar mean relative abundance of seeds in the 10-year-old exclosures, native J. balticus is not dominant in the aboveground vegetation in these exclosures, suggesting that its seed and/or clonal recruitment strategies were not sufficient to out-compete those of A. stolonifera. If these two species had comparable competitive recruitment traits, we might expect a similar proportion of cover abundance in the above ground vegetation in 10-year-old exclosures (Aicher et al. 2011). At LQRE, we noted that J. balticus had 81.6% greater mean relative abundance of seeds in 10-year-old exclosures than in undisturbed sites, but 43.6% less mean relative abundance in the aboveground vegetation in 10-year-old exclosures. This suggests that seed production or retention in the seed bank may be higher in 10-year-old exclosures than in undisturbed sites at LQRE. We also noted that J. balticus was 86.3% more abundant in the aboveground vegetation in undisturbed sites at NRE than LQRE, although it did not have a dominant presence in the seed bank at NRE. This could indicate site-specific growth strategies to favor clonal reproduction rather than seed reproduction, which may impact recovery trajectories following grazing disturbance. Additionally, seed limitation of some species like Carex lyngbyei result in reliance on recovery from clonal reproduction which may be insufficient to out-compete non-native, invasive species like A. stolonifera (Kettenring & Galatowitsch 2011).
These results illustrate how even when clonal or seed propagules of historically dominant native species remain following disturbance, propagule pressure of highly competitive non-native species can drive alternative successional trajectories. Post-disturbance recovery dominated by novel species may also exacerbate disturbance-driven losses of propagules, and hinder efforts to passively restore native composition (Johnstone et al. 2016). However, such intensely altered propagule loads were site-specific. We noted that the sites sampled at LQRE appear to have a greater threat of non-native invasive species encroachment than sites sampled at NRE (Fig. 4), despite its status as a protected Wildlife Management Area since 1993. This may be due to legacy cattle grazing impacts, such as introduction of the species for grazing forage. Non-native species A. stolonifera was not dominant in the surface seed banks in undisturbed sites in NRE, nor did it appear in the surface seed banks of 1-year-old exclosures in this estuary. While seed dispersal has been demonstrated in the feces and on the feet of migratory birds (Vivian-Smith & Stiles 1994), this may not offer sufficient explanation for the very high abundance observed in the LQRE (but not NRE; Fig. 4) and other estuaries in the region impacted by grazing and agriculture (Lane et al. 2024).
It is notable that indicator species analysis did not identify any species as significantly characterizing 1-year-old exclosures at NRE (Table 3). This suggests that overgrazing and grubbing not only removes vegetative growth but also contributes to the loss of propagules in the surface seed bank via erosion when vegetation is stripped away. This may indicate that there are critical windows of opportunity in which native species propagule loads must recover (Fivash et al. 2021). If they are unlikely to disperse from the surrounding landscape, land managers may need to actively intervene through seed and plant addition to preserve their presence in the landscape. As with many restoration projects in degraded lands, this will likely need to be coupled with suppression of competitive non-native species invasion to offset the disproportionate representation of those species in the arriving propagule pool.
Successful passive restoration methods are dependent on the extent and duration of the disturbance (Meli et al. 2017) and may be inappropriate in ecosystems with a history of invasive species (Shackelford et al. 2019). If propagules of non-native, invasive species are competitively successful within the available windows of opportunity to the point of excluding native species richness and abundance, then passive restoration methods such as grazing exclosures are insufficient to restore habitat conditions comparable to the pre-disturbance state. We suggest that in these estuaries, passive recovery creates a temporal window of opportunity in which non-native invasive species like A. stolonifera can gain dominance, and therefore, active restoration is required for native communities to recover. Because the cumulative impacts of overgrazing and high propagule loads of non-native species may reset the recovery trajectory with unknown consequences for ecosystem function (Mack et al. 2000), we recommend actively restoring a diversity of native species as soon as possible following removal of grazing disturbance to expedite habitat recovery, coupled with grazing exclosures to prevent further herbivory. Sites with a known abundance of non-native, invasive species may benefit from transplanting rooted plants (rather than seed), especially in areas of low to moderate salinity to increase recovery through clonal vegetative expansion (Crain et al. 2008). Seed additions may offer a cost-effective way to increase species diversity recruited to the restoration site; however, reliance on seed alone to restore a disturbed area may not be successful due to uncertain germination and seedling survival rates. While a species may grow robustly across a given salinity and/or elevation gradient, the species’ germination and establishment success is highly restricted by microsite variation in salinity and elevation (Janousek & Folger 2013; Lane 2022).
Our findings suggest there is a window of opportunity to influence the recovery pathway of estuaries following geese removal. The “blank slate” produced by intensive geese grubbing and grazing creates a “weed-shaped hole” (Buckley et al. 2007) unless management intervenes. High levels of non-native species invasion have been found in other protected Pacific Northwest estuaries, and our findings reiterate the need for regular monitoring and active management of estuarine systems (see also Stewart et al. 2023; Lane et al. 2024). Moreover, estuaries throughout the Pacific Northwest comprise complex Indigenous food systems supporting many culturally important plant species often referred to as root gardens (Deur et al. 2013; Turner et al. 2013). Restoration following Canada geese removal creates an opportunity for the re-instatement of Indigenous stewardship practices to revive these ancient food systems and broader land management practices. This will entail partnerships and support of local Indigenous communities as they work to reinstate their cultural practices and achieve food sovereignty.
Data Availability
Data and code for all years of observation are available on GitHub (https://github.com/stefanielane/HabitatRecovery).
References
Abernethy, V. J., and N. J. Willby. 1999. Changes along a disturbance gradient in the density and composition of propagule banks in floodplain aquatic habitats. Plant Ecology 140(2): 177–190. https://doi.org/10.1023/A:1009779411686.
Aicher, R. J., L. Larios, and K. N. Suding. 2011. Seed supply, recruitment, and assembly: Quantifying relative seed and establishment limitation in a plant community context. The American Naturalist 178(4): 464–477. https://doi.org/10.1086/661900.
Balke, T., P. M. J. Herman, and T. J. Bouma. 2014. Critical transitions in disturbance-driven ecosystems: Identifying windows of opportunity for recovery. Journal of Ecology 102(3): 700–708. https://doi.org/10.1111/1365-2745.12241.
Borde, A. B., H. L. Diefenderfer, V. I. Cullinan, S. A. Zimmerman, and R. M. Thom. 2020. Ecohydrology of wetland plant communities along an estuarine to tidal river gradient. Ecosphere 11(9): e03185. https://doi.org/10.1002/ecs2.3185.
Buchsbaum, R., and I. Valiela. 1987. Variability in the chemistry of estuarine plants and its effect on feeding by Canada geese. Oecologia 73(1): 146–153. https://doi.org/10.1007/BF00376991.
Buckley, Y. M., B. M. Bolker, and M. Rees. 2007. Disturbance, invasion and re-invasion: Managing the weed-shaped hole in disturbed ecosystems. Ecology Letters 10(9): 809–817. https://doi.org/10.1111/j.1461-0248.2007.01067.x.
Clausen, P., and S. M. Percival. 1998. Changes in distribution and habitat use of Svalbard light-bellied brent geese Branta bernicla hrota, 1980–1995: Driven by Zostera availability. Skrifter-Norsk Polarinstitutt 200:245–268.
Clermont, H. 2010. Little qualicum river estuary regional conservation area guardian of the estuary 2010–2019 management plan. Regional District of Nanaimo. https://www.rdn.bc.ca/cms/wpattachments/wpID2040atID3337.pdf.
Clermont, H. 2015. Canada Goose (Branta canadensis) management strategy for Mount Arrowsmith bio‐sphere region: Towards the restoration of goose damaged estuaries. Guardians of Mid-Island Estuaries Society. https://www.estuaryguardians.org/canada-goose-management.
Connell, J. H., and R. O. Slatyer. 1977. Mechanisms of succession in natural communities and their role in community stability and organization. The American Naturalist 111(982): 1119–1144.
Crain, C. M., L. K. Albertson, and M. D. Bertness. 2008. Secondary succession dynamics in estuarine marshes across landscape-scale salinity gradients. Ecology 89(10): 2889–2899. https://doi.org/10.1890/07-1527.1.
Dawe, N. K., and A. C. Stewart. 2010. The Canada Goose (Branta canadensis) on Vancouver Island. British Columbia. British Columbia Birds 20:18.
Dawe, N., S. Boyd, R. Buechert, and A. Stewart. 2011. Recent, significant changes to the native marsh vegetation of the Little Qualicum River estuary, British Columbia; a case of too many Canada Geese (Branta canadensis)? Journal of the British Columbia Field Ornithologists 21:11–31.
De Cáceres, M., and F. Jansen. 2016. Indicspecies. http://r.meteo.uni.wroc.pl/web/packages/indicspecies/indicspecies.pdf.
Deur, D., N. Turner, A. Dick, D. Sewid-Smith, and K. Recalma-Clutesi. 2013. Subsistence and resistance on the British Columbia Coast: Kingcome Village’s estuarine gardens as contested space. BC Studies (179): 13–37. https://pdxscholar.library.pdx.edu/anth_fac/77.
Diefenderfer, H. L., G. D. Steyer, M. C. Harwell, A. J. LoSchiavo, H. A. Neckles, D. M. Burdick, G. E. Johnson, K. E. Buenau, E. Trujillo, J. C. Callaway, R. M. Thom, N. K. Ganju, and R. R. Twilley. 2021. Applying cumulative effects to strategically advance large-scale ecosystem restoration. Frontiers in Ecology and the Environment 19(2): 108–117. https://doi.org/10.1002/fee.2274.
Douglas, G. W., D. Meidinger, and J. Pojar (Eds.). 1998. Illustrated flora of British Columbia. Vols. 1–8. B.C. Min. Environ., Lands and Parks, and B.C. Min. For. https://www.cabdirect.org/cabdirect/abstract/20013088729.
Dufrêne, M., and P. Legendre. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67:345–366.
Finn, R. J. R., L. Chalifour, S. E. Gergel, S. G. Hinch, D. C. Scott, and T. G. Martin. 2021. Quantifying lost and inaccessible habitat for Pacific salmon in Canada’s Lower Fraser River. Ecosphere 12(7): e03646. https://doi.org/10.1002/ecs2.3646.
Fivash, G. S., R. J. M. Temmink, M. D’Angelo, J. van Dalen, W. Lengkeek, K. Didderen, F. Ballio, T. van der Heide, and T. J. Bouma. 2021. Restoration of biogeomorphic systems by creating windows of opportunity to support natural establishment processes. Ecological Applications 31(5): e02333. https://doi.org/10.1002/eap.2333.
Guardians of Mid-Island Estuaries Society. 2021. Eco-cultural restoration of the K’ómoks Estuary (COA-F21-F-3333). Fish & Wildlife Compensation Program.https://a100.gov.bc.ca/pub/acat/documents/r59352/COA_F21_F_3333_1638897963422_7E19AD9D7A.pdf. Accessed 17 Apr, 2024.
Hitchcock, C. L., amd A. Cronquist. 2018. Flora of the Pacific Northwest, an illustrated manual (D. E. Giblin, B. S. Legler, P. F. Zika, & R. G. Olmstead, Eds.; 2nd ed.). University of Washington Press.
Hu, Z., J. van Belzen, D. van der Wal, T. Balke, Z. B. Wang, M. Stive, and T. J. Bouma. 2015. Windows of opportunity for salt marsh vegetation establishment on bare tidal flats: The importance of temporal and spatial variability in hydrodynamic forcing. Journal of Geophysical Research: Biogeosciences 120(7): 1450–1469. https://doi.org/10.1002/2014JG002870.
Janousek, C. N., and C. L. Folger. 2013. Inter-specific variation in salinity effects on germination in Pacific Northwest tidal wetland plants. Aquatic Botany 111:104–111. https://doi.org/10.1016/j.aquabot.2013.06.009.
Janousek, C. N., K. M. Thorne, and J. Y. Takekawa. 2019. Vertical zonation and niche breadth of tidal marsh plants along the Northwest Pacific Coast. Estuaries & Coasts. 42(1): 85–98. https://doi.org/10.1007/s12237-018-0420-9.
Johnstone, J.nF., C. D. Allen, J. F. Franklin, L. E. Frelich, B. J. Harvey, P. E. Higuera, M. C. Mack, R. K. Meentemeyer, M. R. Metz, G. L. Perry, T. Schoennagel, and M. G. Turner. 2016. Changing disturbance regimes, ecological memory, and forest resilience. Frontiers in Ecology and the Environment 14(7): 369–378. https://doi.org/10.1002/fee.1311.
Kettenring, K. M., and S. M. Galatowitsch. 2011. Seed rain of restored and natural prairie wetlands. Wetlands 31(2): 283–294. https://doi.org/10.1007/s13157-011-0159-6.
Koutstaal, B. P., M. M. Markusse, and W. de Munck. 1987. Aspects of seed dispersal by tidal movements. In A. H. L. Huiskes, C. W. P. M. Blom, & J. Rozema (Eds.), Vegetation between land and sea: Structure and processes (pp. 226–235). Springer Netherlands. https://doi.org/10.1007/978-94-009-4065-9_18.
Lane, S. L. 2022. Using marsh organs to test seed recruitment in tidal freshwater marshes. Applications in Plant Sciences 10(4): e11474. https://doi.org/10.1002/aps3.11474.
Lane, S. L., N. Shackelford, G. E. Bradfield, M. Denoth, and T. G. Martin. 2024. Plant community stability over 40 years in a Fraser River estuary tidal freshwater marsh. Wetlands 44(3): 1–13. https://doi.org/10.1007/s13157-024-01776-w.
Lavorel, S., and J. D. Lebreton. 1992. Evidence for lottery recruitment in Mediterranean old fields. Journal of Vegetation Science 3(1): 91–100. https://doi.org/10.2307/3236002.
Lotze, H. 2010. Historical reconstruction of human-induced changes in U.S. estuaries. In R. Gibson, R. Atkinson, & J. Gordon (Eds.), Oceanography and marine biology (Vol. 20103650, pp. 267–338). CRC Press. https://doi.org/10.1201/EBK1439821169-c5.
Mack, R. N., D. Simberloff, W. Mark Lonsdale, H. Evans, M. Clout, and F. A. Bazzaz. 2000. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications 10(3): 689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2.
Meli, P., K. D. Holl, J. M. R. Benayas, H. P. Jones, P. C. Jones, D. Montoya, and D. M. Mateos. 2017. A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLOS ONE 12(2): e0171368. https://doi.org/10.1371/journal.pone.0171368.
Morzaria-Luna, H. N., and J. B. Zedler. 2007. Does seed availability limit plant establishment during salt marsh restoration? Estuaries and Coasts 30(1): 12–25. https://doi.org/10.1007/BF02782963.
Odum, W. E. 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics. 19(1): 147–176. https://doi.org/10.1146/annurev.es.19.110188.001051.
Pasternack, G. B. 2009. Chapter 3.Hydrogeomorphology and sedimentation in tidal freshwater wetlands. In A. Barendregt, D. F. Whigham, & A. H. Baldwin (Eds.), Tidal freshwater wetlands (pp. 31–40). Backhuys Publishers.
Pearce, S., and E. Demers. 2019. Abundance, distribution, movement and mortality of Canada Geese (Branta canadensis) in Nanaimo, British Columbia. BC Birds 29:36–43.
Price, E. P. F., G. Spyreas, and J. W. Matthews. 2020. Biotic homogenization of wetland vegetation in the conterminous United States driven by Phalaris arundinacea and anthropogenic disturbance. Landscape Ecology 35(3): 779–792. https://doi.org/10.1007/s10980-020-00978-x.
Prowse, T. A. A., P.J. O’Connor, S. J. Collard, and D. J. Rogers. 2019. Eating away at protected areas: Total grazing pressure is undermining public land conservation. Global Ecology and Conservation 20:e00754. https://doi.org/10.1016/j.gecco.2019.e00754.
R Core Team. 2022. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/.
Ricklefs, R. E. 2008. Disintegration of the ecological community. The American Naturalist 172(6): 741–750. https://doi.org/10.1086/593002.
Rosbakh, S., L. Hülsmann, I. Weinberger, M. Bleicher, and P. Poschlod. 2019. Bleaching and cold stratification can break dormancy and improve seed germination in Cyperaceae. Aquatic Botany 158:103128. https://doi.org/10.1016/j.aquabot.2019.103128.
Schaefer, V. H. 2011. Remembering our roots: A possible connection between loss of ecological memory, alien invasions and ecological restoration. Urban Ecosystems 14(1): 35–44. https://doi.org/10.1007/s11252-010-0138-3.
Scheffer, M., and S. R. Carpenter. 2003. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends in Ecology & Evolution 18(12): 648–656. https://doi.org/10.1016/j.tree.2003.09.002.
Shackelford, N., S. M. Murray, J. R. Bennett, P. L. Lilley, B. M. Starzomski, and R. J. Standish. 2019. Ten years of pulling: Ecosystem recovery after long-term weed management in Garry oak savanna. Conservation Science and Practice 1(10): e92. https://doi.org/10.1111/csp2.92.
Schütz, W. 2000. Ecology of seed dormancy and germination in sedges (Carex). Perspectives in Plant Ecology, Evolution and Systematics 3(1): 67–89. https://doi.org/10.1078/1433-8319-00005.
Silinski, A., J. van Belzen, E. Fransen, T. J. Bouma, P. Troch, P. Meire, and S. Temmerman. 2016. Quantifying critical conditions for seaward expansion of tidal marshes: A transplantation experiment. Estuarine, Coastal and Shelf Science 169:227–237. https://doi.org/10.1016/j.ecss.2015.12.012.
Simberloff, D., J. L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi, and M. Vilà. 2013. Impacts of biological invasions: What’s what and the way forward. Trends in Ecology & Evolution 28(1): 58–66. https://doi.org/10.1016/j.tree.2012.07.013.
Srivastava, D. S., and R. L. Jefferies. 1996. A positive feedback: Herbivory, plant growth, salinity, and the desertification of an Arctic salt-marsh. Journal of Ecology 84(1): 31–42. https://doi.org/10.2307/2261697.
Stewart, D., W. G. Hood, and T. G. Martin. 2023. Undetected but widespread: The cryptic invasion of non-native cattail (Typha) in a Pacific northwest estuary. Estuaries and Coasts. https://doi.org/10.1007/s12237-023-01171-4.
Tilman, D. 1990. Constraints and tradeoffs: Toward a predictive theory of competition and succession. Oikos 58(1): 3–15. https://doi.org/10.2307/3565355.
Turner, N. J., D. Lepofsky, and D. Deur. 2013. Plant management systems of British Columbia’s first peoples. BC Studies: The British Columbian Quarterly 179:179. https://doi.org/10.14288/bcs.v0i179.184112.
Vivian-Smith, G., and E. W. Stiles. 1994. Dispersal of salt marsh seeds on the feet and feathers of waterfowl. Wetlands 14(4): 316–319. https://doi.org/10.1007/BF03160638.
Zedler, J. B. 2017. What’s new in adaptive management and restoration of coasts and estuaries? Estuaries and Coasts 40(1): 1–21. https://doi.org/10.1007/s12237-016-0162-5.
Acknowledgements
We thank P. Chalmers and G. Melchers for their field assistance and D. Bertuol-Garcia for statistical advice. We are grateful to B. Binges, the University of Victoria Glover Greenhouse, and the University of Victoria Centre for Forest Biology for facilitating greenhouse space to conduct seed bank germination. Research access in Little Qualicum River Estuary was supported by a research permit from the Canadian Wildlife Service, and in Nanaimo River Estuary by a Wildlife Permit, both issued to Guardians of Mid-Island Estuaries Society.
Funding
Financial support for 2021 field surveys was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2018–03838 to J. S. Richardson (University of British Columbia). Analysis and manuscript writing were supported by the Liber Ero Chair in Conservation and NSERC RGPIN-2019–04535 awarded to T. G. Martin, as well as by a Mitacs Accelerate Fellowship supported by the Guardians of Mid-Island Estuaries Society to S. L. Lane.
Author information
Authors and Affiliations
Contributions
Study conception, 2021 data collection, analysis, and interpretation were undertaken by Stefanie L. Lane. Nancy Shackelford assisted with ecological theoretical framework; Tara G. Martin assisted with cultural framework. Manuscript was drafted by Stefanie L. Lane; Nancy Shackelford and Tara G. Martin edited draft versions of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Communicated by James Lovvorn
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lane, S.L., Shackelford, N. & Martin, T.G. Passive Recovery Risks Non-native Vegetation Invasion Following Intensive Herbivory by Canada Geese in Two Salish Sea Estuaries. Estuaries and Coasts (2024). https://doi.org/10.1007/s12237-024-01419-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-024-01419-7