Abstract
In coastal environments, eutrophication and ocean acidification both decrease pH, impacting the abiotic conditions experienced by marine life. Infaunal invertebrates are exposed to lower pH conditions than epifauna, as porewater pH is typically lower than the overlying water. We investigated the effects of altering sediment carbonate chemistry, through the addition of transplanted green algae and/or crushed shell hash, on an infaunal community. This factorial field experiment was conducted on an intertidal mudflat in the Bay of Fundy, New Brunswick, from July to September of 2020. After 1 month, sediment pH was increased across all depths (0.09 ± 0.03 pH units, or 0.84–2.5%) by the shell hash, but was not affected by the algae, while the multivariate community composition was impacted by an interaction between algae and experimental block (6.9% of variation) as well as shell hash treatment (2.7% of variation). After month 2, all responses to the treatments disappeared, likely due to tidal currents washing away some of the shell hash and algae, suggesting reapplication of the treatments is needed. Most of the variation in the community composition was explained by spatial variation in the treatment replicates among the treatment blocks (33.5% of variation). Despite the small effects of the experimental treatments on sediment carbonate chemistry, distance-based linear modeling indicated that sediment pH may be an important driver of variation in the infaunal community. Given the complexity of the processes driving sediment chemistry in coastal environments, further experiments exploring changing environmental conditions that drive infaunal marine community structure are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infaunal invertebrates in coastal zones live in a chemically dynamic habitat and are thus exposed to variable pH in both the sediment and water column. Sediment porewater pH in non-disturbed habitats is driven primarily by decomposition of organic matter by microorganisms, leading to more acidic and variable pH than the overlying water column with pH values ranging from 6.5 to 8.2 (Kristensen 2000; Widdicombe et al. 2011). In coastal ecosystems, organic matter enrichment due to terrestrial runoff has the potential to play a significant role in carbonate chemistry cycling due to the role of microbial activity (Widdicombe et al. 2011). Additionally, changes to water column pH, especially water column acidification, will result in changes to the sediment pH. As ocean acidification impacts become more apparent, water column pH is anticipated to decrease by as much as 0.43 pH units in the open ocean by 2100 (Bindoff et al. 2019). This reduction in water column pH is predicted to also result in a decrease in sediment pH because of the pelagic-benthic coupling in carbonate chemistry (Clements and Hunt 2018; Waldbusser and Salisbury 2014). Currently, infaunal invertebrates can grow and thrive within the sediment, despite its lower pH (Widdicombe et al. 2011; Sizmur et al. 2019). It is unknown, however, if the predicted decrease in sediment porewater pH will have significant effects on infaunal invertebrates.
Nutrient enrichment is often considered another problem associated with ocean acidification in coastal ecosystems and often results in increases in abundance of macroalgae and bacteria (Nixon 1995; Wallace et al. 2014; Carstensen and Duarte 2019). As organic matter, such as macroalgae, undergoes degradation, sediment pH decreases due to the release of CO2 from respiration by the microorganisms breaking down the organic matter into nutrients (Freitas et al. 2021). Organic enrichment results in increases in biofilm and benthic algae which in turn results in decreases in oxygen, and therefore pH, as algae populations increase. Dissolved oxygen and pH are highly coupled in coastal ecosystems so as dissolved oxygen levels decrease, pH decreases (Wallace et al. 2014; Carstensen and Duarte 2019). Green algae mats can cause increases in available organic matter and decreases in oxygen exchange between the sediment surface and water column (Rossi 2006). This reduction in oxygen exchange on the sediment surface can then lead to hypoxia and even anoxia in extreme cases, resulting in long-term negative effects for benthic communities (Pearson and Rosenberg 1978; Gobler and Baumann 2016; Gerwing et al. 2016; Drylie et al. 2020; Gerwing et al. 2023a).
A potential remediation technique that has been suggested to counter anticipated decreases in sediment pH due to ocean acidification is the application of crushed shell hash. The addition of crushed shell hash to intertidal mud and sandflats has been shown to increase sediment alkalinity, pH, and aragonite-saturation state within 2–3 weeks of application (Green et al. 2009; Greiner et al. 2018; Curtin et al. 2022; but see Drylie et al. 2019; Doyle and Bendell 2022). However, studies have not addressed the duration of these impacts on sediment carbonate chemistry.
The effects of shell hash addition on organisms within the sediment have varied among studies. Hard clam Mercenaria mercenaria (0.2–0.6 mm) settlement has been shown to increase when crushed shell hash was applied to sediment (Green et al. 2013) but in another study, it had no effect on recruitment of M.mercenaria or soft-shell clam Mya arenaria larger than 1 mm (Beal et al. 2020). Drylie et al. (2019) found that the addition of shell hash had no effect on either sediment pH or biodiversity of infaunal invertebrates. These differing results show that there is a great deal not known about the effects of shell hash on infaunal communities.
The effects of sediment pH on benthic invertebrate communities have not been studied as thoroughly as the effects of water column pH. However, where studied, these communities are consistently negatively impacted by declines in sediment pH (Widdicombe et al. 2009; Drylie et al. 2019; Appolloni et al. 2020). Shallow-water hydrothermal vents, also referred to as CO2 volcanic seeps, are often studied as natural laboratories as the vents exhibit much lower sediment pH due to their constant release of CO2 (Appolloni et al. 2020). At a vent in the Gulf of Naples, Italy, areas close to the vent had the lowest sediment pH (7.56 compared to 8.1 at the control sites), as well as the lowest abundance and diversity of benthic invertebrates but the highest evenness, suggesting a more homogenized infaunal community (Appolloni et al. 2020). The relatively high level of evenness observed in lower sediment pH areas of the vents is due to the benthic communities becoming more homogenized as the environmental conditions become more extreme (Agostini et al. 2018; Brustolin et al. 2019; Appolloni et al. 2020). Negative effects of decreased pH have also been observed in experiments where sediment pH was manipulated either through the addition of CO2 gas in the laboratory (Widdicombe et al. 2009) or through the use of fertilizer, and subsequent increase in organic matter, in the field (Drylie et al. 2019). This shift to more homogenized communities may be a consequence of future ocean acidification, depending on the severity of pH decrease, but more work needs to be done to fully understand how infaunal communities respond to changes in sediment carbonate chemistry.
Macroalgae and other plant matter naturally wash up in the intertidal during high tides, which results in increases in nitrogen and phosphorus levels as the macroalgae decomposes over time (Rossi 2006). As nutrients, in the form of macroalgae (Ulva spp.) and fertilizer, become more readily available, deposit-feeding invertebrates often experience increases in abundance (O’Brien et al. 2010). O’Brien et al. (2010) found that the addition of fertilizers resulted in significant decreases in porewater pH while the addition of macroalgae resulted in increases in Capitella sp. abundance. Polychaetes in the family Capitellidae are a disturbance-selective taxon as their abundances increase in the presence of organic enrichment (Pearson and Rosenberg 1978; Gerwing et al. 2016; Gerwing et al. 2023b). However, too much decomposition due to large amounts of macroalgae results in anoxic conditions occurring that can then lead to decreases in abundance of all non-anoxic tolerant species (O’Brien et al. 2010). In contrast, in other studies the addition of macroalgae did not cause significant changes to the community composition (Rossi 2006), due potentially to a high amount of grazing activity by highly mobile species of snails (e.g., Rossi 2006; O’Brien et al. 2010). The extent to which algae decomposition is a driver in benthic communities’ responses to additional macroalgae presence in the intertidal can vary (Rossi 2006; O’Brien et al. 2010; Drylie et al. 2020).
There are a limited number of studies which experimentally tested the effects of either organic matter, in the form of macroalgae, or the application of crushed shell hash on infaunal benthic species or communities and these studies have found variable effects. There are only two that consider both factors combined. Greiner et al. (2018) looked at how a single species, the clam, Ruditapes philippinarum, responded to the addition of shell hash when there was macroalgae naturally present versus absent on intertidal beaches. Macroalgae presence reduced sediment pH while shell hash presence increased sediment pH (Greiner et al. 2018). R. philippinarum had greater growth in the presence of macroalgae but shell hash had no impact (Greiner et al. 2018). Drylie et al. (2019) examined the effects of addition of fertilizer and shell hash on benthic community structure and ecosystem functioning in an experiment on an intertidal sandflat in New Zealand. Benthic community structure was significantly impacted by fertilizer, which increased the number of species and decreased total abundance, but not by shell hash addition (Drylie et al. 2019). Drylie et al.’s (2019) and Greiner et al.’s (2018) experiments were conducted on sandy beaches and as far as we are aware, there have been no studies that experimentally manipulated shell hash and macroalgae in combination to determine the combined effects of macroalgae and shell hash on both sediment chemistry and infauna.
Factors that influence carbonate chemistry of sediment can impact not only abundance but also ecosystem functioning and biological trait prevalence. Effects on ecosystem functioning have generally not been addressed in previous experiments that added organic matter or shell hash, with the exception of Drylie et al. (2020) who found that functional evenness increased as organic matter levels increased. Biological trait analysis (BTA) uses life history, behavioral, and morphological characteristics of a species assemblage to investigate ecological functioning (Bremner et al. 2006; Violle et al. 2014). BTA can be used to evaluate impacts of disturbances and environmental change (Bremner et al. 2006; Pacheco et al. 2011; Gogina et al. 2014; Teixidó et al. 2018; Villnäs et al. 2018; Beauchard et al. 2021), which can then be incorporated into conservation and management practices (Miatta et al. 2021). Beauchard et al. (2021) found that biological trait combinations can be responsive to trawling disturbances and can be useful for developing vulnerability indicators. It is becoming especially important to consider functional diversity as some ecosystems are experiencing rapid changes in the community composition due to those changes in environmental conditions. Understanding how functional diversity may change in future oceanic conditions is important as changes in ecosystem services can have long-lasting effects (Teixidó et al. 2018). As environmental change is rapidly occurring, examining biological traits in combination with traditional analyses can allow researchers to determine if ecosystem services are impacted by these environmental changes (Tomanova et al. 2008; Rand et al. 2018).
We conducted a field experiment on an intertidal mudflat in the Bay of Fundy, Canada, to investigate how the infaunal invertebrate community and sediment carbonate chemistry responded to the addition of transplanted green algae and/or crushed shell hash. The addition of shell hash was expected to cause an increase in sediment pH while the addition of green algae was predicted to result in a decrease in sediment pH through its decomposition. Our objective was to see how changes in sediment carbonate chemistry would affect infaunal invertebrate communities by using multivariate and univariate analyses to determine how abundance, composition, and biological traits of the infaunal invertebrate community were impacted by the algae and shell hash treatments. We also examined how much multivariate variation in the community was explained by natural variation in sediment characteristics including pH.
Methods
Experimental Setup
A field experiment was conducted on an intertidal mudflat in the Bay of Fundy in Lepreau, NB (45°07′51.8″N, 66°28′27.8″W), from July to September of 2020 (Fig. 1). The tidal range at this site was approximately 6.5 m. On this intertidal mudflat, there are two distinct sides separated by a freshwater inflow. We set up our experiment on the western half of the mudflat (approximately 4500 m2) as the other side of the mudflat was experiencing a macroalgal bloom prior to the start of the experiment. The western half did not show any signs of macroalgal bloom when the experiment began nor by the end of the experiment. Additionally, the western half of the mudflat had a clear neap high tide mark due to the presence of salt marsh grass, which allowed us to identify the mid-tide region of the mudflat more clearly. The mudflat in Lepreau has an average sediment grain size of 73.7 μm with low organic content (2.65%). The infaunal communities at intertidal sites in the Bay of Fundy are commonly comprised of the soft-shell clam Mya arenaria, the amphipod Corophium volutator, Nematoda spp., the oligochaete Clitellio arenarius, and polychaetes Hediste diversicolor and Streblospio benedicti, among many other species (Steele 1983; Gerwing et al. 2015, 2016; McGarrigle and Hunt 2024). Deposit and suspension-feeding infaunal species were the dominant feeding groups that occurred in Bay of Fundy infaunal communities.
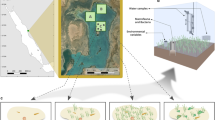
Map of study site and diagram of experimental design for 2-month field experiment where crushed Mya arenaria shell and/or green algae was transplanted to the experimental plots (n = 8 randomized replicate blocks). A Map of Canada showing where the study was completed, B map of region of Bay of Fundy where study area was located, C area where experiment was conducted on the western side of the mudflat, D location of treatment blocks (6 m long × 1.5 m wide; on the mudflat), E placement of the 10 treatment plots (randomly assigned treatments; area = 0.29 m2) within the treatment block design. Note: diagram not to scale
There were two treatment factors in our factorial experiment, shell hash and green algae addition, with three levels per factor (none, low, and high). There was thus a total of nine combinations of the two factors as well as an undisturbed control. The undisturbed control was compared against the procedural control (treatment combination of no shell hash and no algae) in which the sediment was manually disturbed in a similar fashion as the shell hash and algae addition treatments to determine if the disturbance of the sediment resulted in any changes to the sediment carbonate chemistry or infaunal community. There were eight replicates of each treatment combination set up in a randomized block design with one replicate per block (Fig. 1). The blocks were the unit of replication for the statistical analysis.
The addition of shell hash was expected to cause an increase in CaCO3 concentration resulting in the increased availability of CO3−2, which would then cause a feedback loop where HCO3− ion and H+ ions also increased, resulting in an increase in pH and a decrease in the variability of the sediment pH due to the buffering ability of shell hash. For shell hash, we used shell fragments (primarily soft-shell clam, Mya arenaria) remaining from a historical clam processing plant on a different part of the same mudflat, which were collected, crushed (mean size of 1.31 cm2), and then applied to the low and high shell hash treatments. The low treatment had 1.00 kg/m2 of shell hash added to the treatment plots while the high treatment had 2.00 kg/m2 of shell hash added. These amounts were chosen based on Green et al. (2009), the only other study that has looked at amounts of shell hash added to the sediment, in which 1.20 kg/m2 was added in a shell hash addition experiment to cover 45% of their treatment plot surface. We wanted to fully cover the treatment plots in a thin layer of shell hash without interfering in the burrowing behavior of organisms living near the sediment surface; therefore, we increased the shell hash concentration to 2.00 kg/m2 for the high shell hash treatment. The shell hash was sprinkled over the plot and then gently pressed into the surface of the mud.
The second treatment was the addition of tubular/filamentous green algae. The green algae addition was predicted to reduce pH through the increase and subsequent decomposition of organic matter. As with the shell hash, the green algae used in the experiment was collected from the eastern side of the mudflat and then attached to the treatment plots using stainless steel wire pegs. The western side of the mudflat had very little green algae present at the start of the experiment. A mixture of two species of green algae was used: Ulva intestinalis and Chaetomorpha melagonium. The low algae treatment plots had 0.76 kg/m2 of algae added while the high algae treatment plot had 1.52 kg/m2 of algae added. The amount of spatial coverage of the algae was the primary consideration when selecting the chosen concentrations. Plots were never 100% covered, with the average depth of the algae added being less than 1 cm to reduce the potential for anoxia unrelated to decomposition.
Eight blocks were randomly placed in two rows of four blocks on the western side of the mudflat in the mid- to high-intertidal zone (Fig. 1). The locations of the blocks were selected to capture the full range of sediment grain size present on the flat as well as to vary the proximity to the salt marsh at the top of the shore. Blocks were placed approximately 5–10 m apart. Within each block, there was one plot (0.29 m2) randomly assigned to each of the 10 treatment combinations (randomized block design), including one undisturbed control. Within each block, plots were randomly arranged in a column to provide space on each side, to avoid stepping in the treatment plots when sampling. Each plot was 31 cm away from its neighboring plot to allow a buffer between treatment plots in the event of spillover shell hash or algae.
Field Sampling
All treatment plots were sampled twice at low tide, at the end of months 1 (August 4–6, 2020) and 2 (September 5–7, 2020), for the infaunal community and pH profiles. One sediment core (diameter 6.4 cm × 5 cm deep) was collected per treatment plot using a plastic cylinder. Cores were then placed in plastic containers of the same size and transported back to the lab on ice. These cores were used for both pH profiles and infaunal community analysis. At the end of the experiment, an additional sediment core (diameter 6.4 cm × 5 cm deep) was collected from each treatment plot to be used for sediment characteristic analysis to measure mean grain size, sorting, skewness, kurtosis, organic matter content, and carbonate content. These samples were collected using the same plastic cylinder as the biodiversity/pH profile cores and frozen for later analysis.
Due to the length of time required in the lab to complete the pH profiles, not all treatment plots were sampled on the same day. Instead, they were sampled on three consecutive days. All treatment plots within a block were sampled on the same day. The order in which the blocks were sampled was randomly selected for each month to avoid bias. Sampling on the treatment plots was conducted on the one side of the plot in 1 month and then on the other side in the second month. The side that was sampled first was chosen randomly to reduce potential for sampling bias.
Laboratory Processing
pH profiles were measured in each of the sediment cores using a Unisense microelectrode pH-500 probe and Unisense pH/Redox Uniamp meter at 0.5-cm intervals from surface (0 cm) to 3-cm depth within the core. A Velmex Unislide system was used to position the probe at precise depth measurements. Three profiles were measured in each sediment core. The cores were stored on ice in a cooler and then allowed to warm slightly to complete pH profiles at a similar temperature (13–20 °C) as the mudflat. All pH profiles were completed within 6 h of collection. These measurements cannot be made in situ due to the fragility of the pH microelectrode tool used and the necessity of the positioning system to achieve measurements every 0.5 cm. There was the potential that transporting the cores back to the lab impacted the pH of the samples. While the extent to which this delay in measurement changed the pH is unknown, our measurements were made within the timeframe recommended for measurements of water pH (Pimenta and Grear 2018).
After the pH profiles were completed, sediment cores were rinsed over a 500-μm sieve with freshwater to separate invertebrates from most of the sediment. A 500-μm sieve was chosen to focus on smaller macrofauna, given their importance to ecosystem services. The remaining material > 500 μm in size was transferred into a jar and preserved with 95% ethanol. These samples were then examined under a dissecting scope for invertebrates, which were identified to the lowest taxonomic level possible, based on morphological features, and enumerated. The taxonomic level the invertebrates were identified to varied from species level (74%) to phylum level (5%) (Supplementary Information 2 Table S1). Identification of infauna to different taxonomic levels is unlikely to affect the results of ecological analyses of infauna (Gerwing et al. 2020).
The invertebrate species composition and abundance were then used to determine univariate biological metrics and to carry out multivariate analysis of community composition, and functional traits (see “Biological Trait Analysis (BTA)”).
Sediment samples were processed to conduct sediment grain size analysis as well as determine percent organic matter and percent carbonates. Approximately 150–175 g of wet sediment was placed in a drying oven at 70 °C for at least 48 h and then weighed to determine the porosity of each sample based on the change in weight of the sample. The samples were then placed into a sediment shaker for the grain size analysis for 20 min and the sediment in each sieve was then weighed. The sieve sizes used were 4 mm, 2 mm, 1 mm, 710 μm, 500 μm, 250 μm, 125 μm, and 63 μm. Mean (± standard deviation) grain size, skewness, and kurtosis were calculated in R (function “granstat” from “G2Sd” package, Fournier et al. 2014) based on the sediment grain size analysis. The percent organic content and carbonate matter in the sample were estimated using the loss on ignition method described by Heiri et al. (2001). A 2–3-g subsample of each sediment sample was dried for 48 h at 70 °C, weighed, and then placed into a muffle furnace for 4 h at 550 °C. Once the sample cooled, it was reweighed, and the percent loss was used as the estimated percent organic content in the sample. To estimate the percentage of carbonate matter within the sample, the percent loss of weight of the subsample was then determined after being placed into the muffle furnace for an additional 2 h at 950 °C.
Biological Traits
Biological trait analysis can be used to evaluate ecosystem functioning. We were interested in looking at traits related to bioturbation potential (sediment reworking mode and position in relation to the surface of the sediment), resilience stability (longevity, maximum size), feeding mode, dispersal potential (reproductive frequency, larval type, and duration of planktonic larval stage), hypoxia exposure (exposure potential, position in sediment, habitat structure, and motility), hypoxia sensitivity, and AMBI (AZTI’s Marine Biotic Index) score (Borja et al. 2000). The traits used were selected based on work by Pacheco et al. (2011), Gogina et al. (2014), and Villnäs et al. (2018) due to their importance to the environmental stability of the mudflat habitat. Hypoxia exposure and sensitivity as well as the AMBI (Borja et al. 2000) were included to capture the potential impacts of coastal acidification and eutrophication on the species present on the mudflats. The AMBI is used to quantify the response of soft-bottom communities to changes in environmental conditions related to human disturbance (Borja et al. 2000). The AMBI is determined based on the sensitivity of a species to an increasing stress gradient according to the AZTI-AMBI statistical software (Borja et al. 2000). Traits were determined for each species using bibliometric analysis (reviewed in Lam-Gordillo et al. 2020), utilizing previous studies on the species or related species (see Supplementary Materials 2, Table S1 for full list of traits and literature used).
To analyze the biological trait information collected, the traits assigned to the individual species (modalities) had to be converted to numbers. The trait modalities were scored, ranging from 0 to 5, according to their contribution to the overall trait of interest, as based on the framework of Villnäs et al. (2018). Trait modalities were determined using published classification and taxonomic sources of information, depending on the level of information available in the literature. For example, sediment reworking mode was used as a metric for measuring bioturbation potential. If the species did not transport any sediment, then it was assigned “no transport” and scored a 0, while a surficial modulator was scored a 1, a tube-dwelling species was scored a 2, a biodiffuser scored a 3, and a gallery diffuser scored a 5. This was done for every modality for every trait of interest (see Supplementary Information 2, Table S2).
Statistical Analysis
Multivariate Analysis
Primer-e v.7 (PRIMER-e, Quest Research Ltd, New Zealand) was used to complete permutational ANOVAs (PERMANOVAs) to determine significant effects of the shell hash and algae treatments on the infaunal community composition and biological traits (see “Biological Traits”). The data were dispersion-weighted and then square-root transformed before Bray–Curtis similarity matrices were calculated. The community data was dispersion-weighted to reduce the contribution of dominant taxa and those with clumped spatial distributions while considering the variance of individual species. The square-root transformation balanced the need to reduce the contribution of the dominant taxa while also maintaining the importance of the rare species. Block was a random factor in these analyses with shell treatment and algae treatment as fixed factors and 999 permutations were used. The two months were analyzed together as well as separately, so month was a fixed factor in some of the PERMANOVAs. Non-significant interactions (p-value > 0.10) were pooled and interpreted as contributing to the proportion of variation not accounted for by the treatments (pers. comm., Marti Anderson). Any significant interactions (p-value < 0.05) were followed up with pairwise PERMANOVAs. Pairwise comparisons were not adjusted for familywise error rates.
PRIMER-e was also used to complete distance-based linear modeling (DISTLM) to determine the amount of variation observed in the multivariate community composition of the different treatment plots explained by sediment pH and sediment characteristics. DISTLMs were run for month 1 and month 2 separately as well as combined. Models were run for month 1 for both procedural control plots and for all plots to determine any difference in the explanatory power of the abiotic conditions for natural variation versus the experimental conditions. This analysis was not completed for month 2 due to the lack of any effects of the experimental treatments on the infaunal invertebrate community. There were five variables included in the DISTLMs: sediment pH at 3.0 cm, mean grain size, sorting (the spread of grain sizes around the mean), percent organic matter, and percent carbonates. Sediment pH was highly correlated between depths (measured every 0.5 cm from 0–3 cm), so only the depth which explained the most variation (3.0 cm) was included in the models. This was determined by building single variable DISTLM models for each sediment depth and comparing these using AICc scores with a AICc threshold of 2 AIC units (Burnham and Anderson 2004; Posada and Buckley 2004; Richards 2005; Burnham et al. 2011). Then, the model with the best AICc score was chosen (depth 3.0 cm) as it explained the most variation in the infaunal community composition. Marginal tests were used to determine the proportion of variance in invertebrate community composition that was explained by each sediment characteristic. The best model was then determined based on AICc scores.
Biological Trait Analysis
The numerical trait assignments of each species were then used to create an abundance-weighted trait by plot matrix. This was done using matrix multiplication of the species by trait and species by plot abundances matrices. This resulted in a matrix showing the abundance of the traits in each treatment plot. The new trait by plot matrix was then used to analyze the similarities in the trait modality composition between the treatment plots in month 1, month 2, and both months combined. PERMANOVAs were used to determine if the trait compositions were significantly impacted by the algae and shell hash treatments or varied among blocks or months (see “Multivariate Analysis”). Any significant interactions were followed up with pairwise PERMANOVAs. Pairwise comparisons were not adjusted for familywise error rates.
Univariate Analysis
Data were analyzed in R Studio v. 1.3.1093 with a significance level of 0.05 used for all analyses. Generalized linear mixed models (GLMM) were constructed to determine the effects of the shell hash and algae treatments on univariate abiotic and biotic variables. The dependent abiotic variables examined included sediment pH, porosity, mean grain size, skewness, kurtosis, proportion of organic matter, and proportion of shell hash. The dependent variables for the univariate biological metrics were species richness and total abundance. It should be noted that not all organisms were identified to the species level, meaning that the biological metric species richness actually represented taxa richness. We will refer to taxa as species as most of the organisms were identified to the species level. The abundances of the ten most abundant species were analyzed separately to determine the effects of the algae and shell hash treatment on these species. We chose to analyze the top ten species vs. another number based on the presence of these species across the treatment blocks in a shade graph. The remaining species identified within the samples were not present in all treatment blocks and were therefore not analyzed individually. In both the abiotic and biotic univariate statistical models, algae treatment, shell hash treatment, and all possible interactions were fixed effects; depth was an additional fixed factor in the sediment pH models. Months were analyzed separately due to the differences in the abiotic conditions, specifically sediment pH, between the two months. “Block” was a random effect in all the GLMM models as we wanted to account for the spatial variability across the blocks, but we were not explicitly interested in comparing the individual treatment blocks. For sediment pH models, “core” was also a random intercept in the GLMM models due to the multiple pH measurements per core. “Block” was the unit of replication for the statistical analysis, therefore n = 8. The “glmer” function in the “lme4” package (Bates et al. 2015) and “glmmTMB” function in the “glmmTMB” package (Brooks et al. 2017) were used to build the models.
For each dependent variable, a series of models with all fixed and random factors were constructed with the potential distribution families (Gaussian, Poisson, and negative binomial for discrete data; normal, beta, and gamma for continuous data). The models with different distributions were then compared to identify the best fit for each dependent variable based on Akaike information criterion (AICc) scores with an AICc value threshold of 2 AIC units. Once the best full model was identified for each dependent variable, residual plots were checked to confirm homogeneity of variances and normality (where applicable). Then, the p-values of the likelihood ratio tests were determined using the “Anova” function in the “car” package, for the model coefficients were used to identify significant effects of fixed factors. Generalized linear models (GLMs; “glm” function, “stats” package) were also completed to examine the spatial variation in abiotic characteristics; therefore, block was a fixed factor in these analyses to compare the abiotic characteristics between the different blocks.
Undisturbed and Procedural Controls
To identify if there were significant differences in the two control treatments (undisturbed and procedural control), for species richness, total abundance, and abiotic variables (pH profiles and sediment characteristics), linear mixed models (LMM) were built in R using the “lme” function in the “lme4” package, with a random intercept of “block” and “treatment” as a fixed factor. LMM were used rather than GLMM as there were no violations of the assumptions of normality and homogeneity of variances. PERMANOVAs were also used to compare the two control treatments for multivariate analyses, both the biological trait analysis and community composition analysis (see “Multivariate Analysis”). The LMM and PERMANOVAs determined that there were no statistical differences in abiotic characteristics or infaunal community between the undisturbed and procedural controls after months 1 and 2 (p > 0.05). Consequently, only analyses including the procedural control are presented.
Results
Abiotic Conditions
At the start of the experiment, the percent cover of shell hash on the treatment plots was greater in the high shell hash treatment than the low and the no shell hash treatments (Fig. 2). This pattern was clearest in the no algae treatments where shell hash was more visible. There was greater algal coverage in the high and low algae treatments than in the no algae treatment (Fig. 2). By the end of month 1, there was a large reduction of shell hash (Fig. 2), likely due to the smaller pieces of shell hash washing away with the strong tidal currents. By the end of month 2, shell was more visible in the plots due to a reduction in the percent algal coverage, both applied and naturally occurring (Fig. 2), but much of this was larger chunks of naturally present shell, not the experimentally applied crushed shell. The algae percent cover declined over time across all treatments until there was less than 5% algal coverage across all treatments after month 2 (Fig. 2).
There were clear differences in the treatment effects on sediment pH data between months 1 and 2. Sediment pH was successfully manipulated in our treatment plots but only in the first month of the experiment. There was a statistically significant interaction between shell hash and depth on sediment pH after month 1 (DF = 2, Chi-Sq = 6.54, p-value = 0.04), but no significant effect of algae or other interactions (Table 1). As the amount of shell hash increased, the pH increased in the upper 1.0–1.5 cm of each core (Fig. 3). In the bottom 2.0 cm of each core, this trend reversed with the high shell hash treatment having the lowest rather than the highest pH at the bottom of the core, resulting in more similar pH values across depths in the high shell hash treatment than in the other two shell hash treatments (Fig. 3). Tukey’s tests found no significant differences in the sediment pH between the shell hash treatments at any depth, only significant differences in pH between some depths for the 0 and low shell hash treatments, but not in the high shell hash treatment. However, this indicates that the shell hash treatment did affect pH as the differences between depths in pH disappeared at the highest level of shell hash. The treatment effects observed in the first month of the experiment disappeared by the end of the experiment. After month 2, there was only a significant effect of depth on sediment pH (Table 1) with pH increasing slightly with depth (Fig. 3), indicating there were no long-lasting effects of either the shell hash or algae treatments on sediment pH (Fig. 3).
Box plot of sediment pH after A month 1 and B month 2 in the 2-month field experiment from early July to early September 2020 in Lepreau, New Brunswick, where crushed Mya arenaria shell and/or green algae was transplanted to the experimental plots (n = 8 randomized replicate blocks). Shell hash treatments included 0, L (1 kg/m2), and H (2 kg/m2) while algae treatments included 0, L (0.76 kg/m2), and H (1.52 kg/m2)
In the GLMs testing the effects of the treatments on sediment pH (Table 1), the amount of spatial variation explained by block was smaller than that explained by core nested in block. GLMs were completed to specifically address potential spatial variation in the abiotic conditions of the differing blocks, which indicated that there was a highly significant difference in the sediment pH among the blocks after month 1 (DF = 7, Chi-Sq = 109.06, p < 2.2 × 10−16) and month 2 (DF = 7, Chi-Sq = 159.35, p < 2.2 × 10−16).
At the end of the experiment (2 months), there was no significant effect of shell hash, algae, or interaction of the two on any sediment characteristics (Fig. S1, Table S1). Across all treatment plots, the mean grain size averaged 0.60 ± 0.595 mm and the mean organic content was 2.77 ± 0.07%. There was some variability between blocks for some of the sediment characteristics while others were consistent (Fig. S1).
Multivariate Community Composition
The metric MDS plot showed a clear separation in the infaunal communities at month 1 and month 2, indicating temporal variation in the community composition (Fig. S2). As such, the 2 months were analyzed separately as well as together to examine the temporal variation in the communities in the field experiment to consider if there were differences in the effects of the two treatments on the communities between the 2 months. When looking at both months combined, the infaunal community composition was statistically significantly impacted by month (pseudo-F1,7 = 3.942, p-value = 0.018) and the interaction of the shell hash and algae treatments (pseudo-F4,28 = 1.665, p-value = 0.031; Table S2). The infaunal community composition was also significantly impacted by a block and month interaction (pseudo-F7,28 = 3.155, p-value = 0.001; Table S2). The large variation (36.9%) among blocks shows that an experimental design incorporating spatial variation was necessary (Table S2). Additionally, the significant interaction between block and month indicates that the spatial variation in communities among blocks differed in each month. The infaunal community was significantly impacted by a three-way interaction between block, algae, and shell treatments (pseudo-F28,28 = 1.290, p-value = 0.021; Table S2), indicating that there were spatial differences in the way the algae and shell hash treatments influenced the infaunal invertebrate community composition. There were also multiple two-way interactions: algae × shell, block × month, and block × algae (Table S2). There were no significant pairwise comparisons for the shell hash and algae treatment interaction (Table S3).
When month 1 was analyzed separately, there were three significant terms that helped to explain the variation in the multivariate community composition among samples (Table 2). First, there was a significant effect of block (pseudo-F7,28 = 6.670, p-value = 0.0001; Fig. 4A, Table 2). Spatial variance among the blocks explained the largest amount (33.5%) of the community composition variation (Table 2), as observed in the separation between the plots of the different blocks in the MDS plot (Fig. 4A). The block by algae interaction was also significant (pseudo-F14,28 = 1.386, p-value = 0.032) and explained approximately 7% of the variation (Table 2). This was illustrated in the MDS plot, where for some of the blocks, there were groupings of plots that shared the same algae treatment level (Fig. 4A). There was also a small but statistically significant effect of shell hash treatment (pseudo-F2,14 = 2.222, p-value = 0.020) on community composition that explained approximately 2.7% of the variation (Table 2). There was a slight distinction between the shell hash treatments in Fig. 4B, as the high shell hash treatment was slightly separated from the other two levels of the shell hash treatments. This was consistent with the pairwise tests, which indicated that the high shell hash treatment was significantly different from the zero shell hash treatment (p = 0.0275) and marginally non-significantly different from the low shell hash treatment (p = 0.0594). Approximately 53.2% of the variation in community composition was unexplained by the factors considered (Table 2).
Metric MDS plots of infaunal community composition focusing on the effect of A month 1 block by algae interaction (legend colors represent treatment block while shapes represent algae treatment), B month 1 shell hash treatment, and C month 2 block by algae interaction (for comparison to month 1) (legend colors represent treatment blocks while shapes represent algae treatment) (n = 8 experimental blocks) in a 2-month field experiment from early July to early September 2020. Shell hash treatments included 0, L (1 kg/m2), and H (2 kg/m2) while algae treatments included 0, L (0.76 kg/m2), and H (1.52 kg/m2)
When month 2 was analyzed separately, there was a significant block effect (pseudo-F7,28 = 8.915, p-value = 0.0001) explaining 45.6% of the variation but no significant effects of algae or shell hash treatments or their interactions (Table 2, Fig. 4C), matching the lack of any treatment effects observed in the sediment pH at month 2. Approximately 51.8% of the variation in community composition was unexplained by the factors considered (Table 3).
Connection between Abiotic Conditions and Multivariate Community Composition
The best DISTLM models for both procedural control plots and for treatment plots for month 1 included all variables. The best model for month 1 procedural control plots explained a larger amount of the variation in the community (R2 = 0.38) than the best model for all the treatment plots (R2 = 0.18) including procedural controls. The sediment pH at 3.0 cm and mean grain size were significantly related to the community composition observed in the treatment plots but not the control plots (Table 3). Mean grain size and sediment pH explained the greatest proportion of the variance with grain size explaining 7.6% in the controls and 7.0% in the treatment plots while sediment pH explained 6.9% in the controls and 6.4% in the treatment plots (Table 3).
Biological Trait Analysis (BTA)
The biological trait composition for months 1 and 2 displayed some groupings in relation to the algae and shell treatments (Fig. 5, Fig. S3). The PERMANOVA for the combined months found a significant interaction between algae and shell hash (pseudo-F4,92 = 2.419, p-value = 0.045; Table S4; for pairwise tests, see Table S5) and a significant interaction between block and month (pseudo-F7,92 = 6.295, p-value = 0.0001; Table S4). This interaction between block and month indicated variation among the blocks differed between the months and led us to analyze months 1 and 2 separately.
Metric MDS plot looking at biological trait composition in A month 1 and B month 2 in relation to algae (A: 0, L, H) and shell hash (S: 0, L, H) treatments in the 2-month field experiment from early July to early September 2020. Circles are 0 algae, triangles low algae, and squares high algae. Red points are zero shell, blue points low shell, and green points high shell. Shell hash treatments included 0, L (1 kg/m2), and H (2 kg/m2) while algae treatments included 0, L (0.76 kg/m2), and H (1.52 kg/m2)
In month 1, block explained the greatest amount of variation at 58%, with 40% of the variation unexplained (Table 4). The PERMANOVA on the biological trait composition for month 2 found that the only significant effect was a block effect, explaining 29.4% of the variation (Table 4, Fig. 5B). The unexplained variation at month 2 was 38.3%, which was similar to month 1. In month 1, there was a significant interaction between algae and shell hash (pseudo-F4,28 = 2.419, p-value = 0.045); however, it only explained 1.9% of the variation (Table 4, Fig. 5A). A pairwise PERMANOVA indicated that there was a single significant difference between pairs, which occurred in the low algae treatments between the low and high shell hash treatments (Table S6). It should be noted that this single pairwise interaction was one of 12 comparisons, meaning there is a potential for this significant test to be a type I error, and we must interpret this interaction with caution. The variation explained by the interactions of the algae and shell treatments with block increased substantially between months 1 and 2, from 1.9 to 12.4% (Table 4).
Univariate Biological Metrics
Two univariate biological metrics (species richness, total abundance) were calculated to examine how the algae and shell treatments potentially impacted the infaunal community. Mean species richness was 6 ± 2 (x̅ ± SD) and mean abundance was 71 ± 37 infaunal invertebrates per core sample across both months. After months 1 (Table 5, Fig. 6) and 2 (Fig. S3, Table S4), there was some variation in species richness among treatments but no statistically significant differences in species richness among the treatments or interaction between them. Total abundance was impacted by a significant interaction between the treatments after months 1 (DF = 4, Chi-Sq = 111.888, p-value < 2.2 × 10−16; Table 5, Fig. 6) and 2 (DF = 4, Chi-Sq = 42.736, p-value = 1.174 × 10−08; Fig. S4, Table S7). For total abundance, Tukey’s tests indicated that there were significant differences among some treatment combinations after both month 1 (Table 6) and month 2 (Table S8); however, there was no consistent pattern in the differences between the combinations of the different levels of the two treatments.
Box plots of univariate biological metrics (A) species richness and (B) total abundance of invertebrates from month 1 of the 2-month field experiment from early July to early September 2020 where crushed Mya arenaria shell and/or green algae was transplanted to the experimental plots (n = 8 randomized replicate blocks). Shell hash treatments included 0, L (1 kg/m2), and H (2 kg/m2) while algae treatments included 0, L (0.76 kg/m2), and H (1.52 kg/m2)
Abundances of Individual Species
Six of the ten most abundant species collected from the treatment plots belonged to class Clitellata (oligochaetes), class Polychaeta, and phylum Nematoda (Table 7). The remaining four species were two bivalves and two amphipods (Table 7). Among the ten most abundant species, only the oligochaete C. arenarius and the polychaete S. benedicti were significantly impacted by either treatment at the end of month 1 of the experiment (Table 6). After month 1, abundance of C. arenarius had a significant interaction between shell hash and algae treatments (DF = 4, Chi-Sq = 13.036, p-value = 0.0004) and was lowest in the low algae, zero shell treatment and highest in the high algae, zero shell treatment combination (Fig. S5, Table 7). For S. benedicti, there was only a significant effect of algae on abundance (DF = 2, Chi-Sq = 12.276, p-value = 0.002; Table 7). There was a significant increase in the abundance of S. benedicti as the amount of algae added to the plots increased (Fig. S5; Tukey’s test, p < 0.05). After month 2, only S. benedicti and Nematoda spp. had significant differences between the algae treatments (S. benedicti: DF = 2, Chi-Sq = 7.715, p-value = 0.021; Nematoda spp.: DF = 2, Chi-Sq = 10.377, p-value = 0.006; Fig. S6, Table S9). Tukey’s tests (p < 0.05) indicated a small significant difference between only the zero and low algae treatments at month 2; however, there was a lot of variability in abundance among the treatment combinations.
Discussion
In our study, we were able to slightly manipulate sediment pH through the addition of shell hash but not algae, while both treatments affected infaunal communities individually. The manipulations did not have long-lasting effects on pH, however, and since there was not a persistent change in sediment pH, this likely contributed to the small response by the infaunal community. Spatial variation at the 10 s of meter scale, represented by block, explained more infaunal community variation than any other factor, while the shell hash and algae experimental treatments explained a relatively small percentage of the variation (0–3%). Spatial variation among blocks also influenced the response of the infaunal communities to the algae treatments as algae treatments had an impact in some blocks and not in others. Spatial factors commonly explain the majority of infaunal community variation in marine intertidal systems (e.g., Gerwing et al. 2016; Sizmur et al. 2019; Gerwing et al. 2023a, b). The factors that could be driving the spatial variation in the infaunal community in our experiment include natural patchiness, distance from freshwater discharge, and distance from marsh edge. The differing sediment characteristics and sediment pH observed among the blocks explained around 18% of the total variation in infaunal communities, which is consistent with the significant spatial variation in sediment characteristics among the blocks for all but one sediment grain size characteristic. Gerwing et al. (2016) found that infaunal invertebrate communities are highly influenced by temporal and spatial variation, explaining approximately 79% of the variation in community composition. A follow-up study by Gerwing et al. (2023b) also found that spatial variation accounted for a large percentage of variation in community composition but that elemental concentrations accounted for an even larger amount of variation. Spatial variation has been shown to be more significant than temporal variation for some species of infauna, accounting for 50–60% of the total variation in species abundances in estuarine soft-sediment intertidal habitats in the Netherlands (Ysebaert and Herman 2002). These studies are consistent with our results as spatial variability was the greatest explanatory variable. The differences in community composition among blocks are likely due to a combination of sediment characteristics and other variables that were not measured including abiotic, such as other carbonate chemistry variables, and biotic, such as microphytobenthos biomass, variables.
The presence of shell hash had a statistically significant impact on the benthic invertebrate community on the mudflat after one month; however, it explained only 2.74% of the variation in community structure. Previous literature has investigated the potential of shell hash as a remediation technique against predicted coastal acidification and those studies have found mixed results with there being instances of shell hash positively impacting communities (e.g., Wilding and Nickell 2013; Brustolin et al. 2022) and some instances where there was no effect (Drylie et al. 2019; Beal et al. 2020; Doyle and Bendell 2022). Brustolin et al. (2022) suggested that there is site-level variation in the effectiveness of shell hash as a remediation technique, where the addition of shell hash enhanced species richness at some sites but not others. The effectiveness of shell hash addition may be driven by environmental conditions, such as sediment characteristics, or by biotic factors, such as high abundance of bioturbating species, which may have difficulty burrowing. As our study was only completed at one site, we cannot test this potential explanation. In Wilding and Nickell (2013), the presence of shell hash influenced macrobenthic diversity with species richness increasing as the amount of shell hash increased. This suggests that shell hash was beneficial for the benthic species found in their samples. Their observational study had a potential confounding variable, however, as organic matter enrichment also co-varied with distance from mussel beds and shell hash content (Wilding and Nickell 2013). Our experiment did not have an increase in species richness associated with the addition of shell hash. This suggests that perhaps the increase in species richness in Wilding and Nickell (2013) with increasing shell hash may have been linked to the co-varying organic matter. The presence of shell hash has also been documented as promoting sediment heterogeneity in physical properties, especially in muddy habitats, thus promoting more diversity in community composition (Brustolin et al. 2022).
Changes in sediment carbonate chemistry, especially the depth at which these changes occur, may be another cause for the effects observed on community composition in other studies as infaunal species inhabit the sediment at different depths. Therefore, changes to one depth may impact one species while other species will not be affected. In the present study, there was a significant interaction between shell hash addition and sediment depth on sediment pH, indicating that the shell hash treatment altered sediment chemistry by reducing the change in pH with depth. The sediment pH between the surface and 3-cm depth increased noticeably less in the high shell hash treatment (0.7%) than in the low shell hash (1.5%) and the zero shell hash (1.2%) treatments. These observed changes in sediment pH were very small. A study done in salt marshes also found that the addition of crushed shell increased pH and reduced variability in pH (M.P. Kollegger, unpub. data). In contrast, Drylie et al. (2019) and Beal et al. (2020) both found that sediment pH of intertidal mudflats was not influenced by shell hash no matter the concentration of shell hash added. Doyle and Bendell (2022) found that sediment pH increased by 0.1 pH units from the application shell hash at only one site, indicating spatial variation in the effects of shell hash on pH. Changes in sediment pH or the physical properties of the added shell hash may be mechanisms that led to a small change in the benthic community detected in the present study.
Although algae addition did not alter sediment pH, the presence of algae changed the multivariate infaunal community composition including through interactions between algae and other factors. However, the algae treatment did not explain a large amount of the variation compared to the effect of block. The algae may have acted as either a food source or refuge for potential food sources for several infaunal species, such as polychaete and oligochaete worms, which were observed in greater abundance in those treatments. In the present study, the total abundance of infauna in the high algae, zero shell treatment combination was the highest of any of the treatment combinations, another indication that the simple presence of algae altered the community. Algal cover has been observed to explain variation in infaunal invertebrate abundance on intertidal mudflats (e.g., Campbell et al. 2019, 2020). The naturally occurring algal cover in Campbell et al. (2019) and Campbell et al. (2020) explained approximately 11% of the variation in the infaunal invertebrate community and thus appeared to be more influential on the invertebrate community than the algal treatment in the present study. Another study conducted on an intertidal mudflat found that the addition of macroalgae resulted in an increase in the abundance of deposit-feeding infauna (O’Brien et al. 2010). They observed that the increase in abundance was related directly to the increase in the available nutrients (O’Brien et al. 2010). In contrast, Rossi (2006) found that the addition of Ulva spp. algal detritus did not cause major changes to benthic communities, similar to our study as we only found a small effect. Rossi (2006) suggested that the response of a soft-bottom community to algal detritus will be entirely dependent on how every single species is impacted by the direct and indirect effects of the algae. Some species may benefit from the increased availability of food while others are negatively impacted by the potential anoxia due to the algal mats (Rossi 2006). The reduced influence of algae on the infaunal community in our study may be due to the form of green algae (filamentous) chosen to transplant within the mudflat, the low density of macroalgae added to the plots, and the washing away of some algae over time. The algal species used were chosen due to their high abundance in the area.
Contrary to what was expected, the addition of green algae to the treatment plots did not result in a change in the sediment pH in either month of the experiment. It had been expected that the decomposition of green algae would result in a decrease in pH (Rossi 2006). In Drylie et al. (2019), the introduction of organic matter, in the form of fertilizer that induced increased organic matter, caused a decrease in porewater pH from 7.3 to 6.4, after 9 weeks, at the highest concentration treatment. In our study however, there was no effect of algae addition on sediment pH, despite the observation of clear algal decay on algal addition plots. However, anoxia in the sediment was observed, through the appearance of black sediment on the surface of the mudflat, throughout our study site after 2 months, not limited to the experimental plots, and therefore not connected to the experimental algae addition treatment. This type of anoxia event was an unusual event at this site and the cause of the event is unknown. There is potential that the widespread naturally occurring anoxia affected the range of pH overall and may have impacted our ability to detect an effect of algae addition in our experiment. However, other studies have also found no effect of algae addition on sediment pH. A study conducted in a salt marsh in Jamaica Bay, New York, also looked at how the addition of algae would impact sediment pH and similarly found that the addition of algae did not impact sediment pH at either of their sites (B.C.M. Kahn, unpub. data). In the present study, unlike month 1, after month 2, there was no significant multivariate effect of either the algae or shell hash treatments on the infaunal community. There was only a slightly higher amount of green algae remaining on the plots after month 2 for the high algae than the low algae treatment plots. Temporal variation in algal coverage is a natural phenomenon that occurs as a response to availability of nutrients and sunlight (Wallace and Gobler 2021) as well as due to algae washing away from the tidal currents. It was expected that there would be some washing away of the shell hash and algal coverage due to the semi-diurnal tidal cycle with an average tidal height of 6.5 m. Metal pegs were used to retain the algae treatment on the treatment plots. While there was some washing away and consumption of the algae over time, the majority of the algae decomposed over time, which was anticipated. Sediment pH did not decrease as expected as the algae decomposed and it is not clear why this did not occur, perhaps suggesting that a larger amount of algae is needed for sediment pH to decrease, filamentous algae is less likely to cause anoxia and changes in sediment pH, or strong water flushing from tides reduced the effect of the algae.
Few individual species examined were affected by the algae or shell hash treatments. However, the oligochaete C. arenarius and the polychaete S. benedicti were significantly affected by an interaction of algae and shell hash treatments and the algae treatment, respectively. The interaction observed with C. arenarius, where the abundance of C. arenarius increased as the algal concentration increased but only in the zero shell hash treatment, matches the pattern observed with the total abundance, suggesting the significant effect of the treatment interaction on total abundance was partly due to the response of C.arenarius. However, this pattern in abundance of C. arenarius was primarily driven by a single block in the experiment, suggesting a patchy distribution. S. benedicti abundance increased as algal treatment increased as well. The transplanted green algae may have been acting as a refuge for potential food sources for C. arenarius and S. benedicti as both species are deposit feeders (Giere and Pfannkuche 1982; Levin et al. 1987; Llansó 1991; Sebesvari et al. 2006). Four other species of worms and the four other most populous species, belonging to Arthropoda and Bivalvia, were not impacted by either the algae or shell hash treatments. In Rossi (2006), C. volutator decreased in abundance when macroalgae was buried. The burial of algae also resulted in a non-significant decrease in more rare species, such as M.arenaria (Rossi 2006). In our study, we did not observe any difference in the abundance of C. volutator or M. arenaria in the differing algae or shell hash treatments.
Biological traits were not greatly affected by the algae and shell hash treatments. There was a significant interaction between algae and shell hash after month 1. However, the single pair of treatment combinations, out of 18 pairwise tests, displaying a significant interaction was quite possibly either a type 1 error or another example of the spatial variation across the mudflat as this interaction may have been driven by responses of select blocks. The significant interaction between algae and shell hash treatments was only observed after month 1 of the experiment, consistent with the general lack of effects after month 2. Temporal variation in trait prevalence has been observed in previous studies (e.g., in the Baltic Sea, Gogina et al. 2014). As species have different reproductive seasons, their populations will rise and fall as environmental conditions change, thus resulting in changes to trait prevalence.
Due to the small amount of variation in the infaunal community explained by the algae and shell hash experimental treatments, we wanted to look at what environmental conditions may have contributed to the natural spatial variation in community composition observed in the experiment. The amount of variation explained by the sediment characteristics was reduced in the treatment plots (32.5% explained in the control plots vs. 18.3% in all the treatment plots) but the cause of the reduced amount of variation explained in the model for all treatment plots is not clear. The abiotic conditions that had the largest influence on multivariate community composition were mean grain size and sediment pH in both the procedural control treatment plots and all treatment plots (including procedural controls).
The importance of sediment grain size has long been documented in the literature as different species have different preferences (e.g., Snelgrove and Butman 1994; Morse and Hunt 2013; Gerwing et al. 2016; Barbeau et al. 2019). Gerwing et al. (2016) found that in upper Bay of Fundy mudflats, abiotic variables, such as sediment grain size, exposure time, and oxygen availability within the sediment, explained 11% of the variability in the infaunal invertebrate community composition. Besides sediment grain size, other abiotic factors, such as sediment pH, can drive variation in infaunal communities. Shallow-water hydrothermal vents provide a natural example of how increased CO2 impacts sediment pH and community composition, such as the vent in the Bay of Naples studied in Appolloni et al. (2020). They found that closest to the vent, sediment pH was 0.5 pH units lower and community diversity was lowest and that as sediment pH increased the diversity increased (Appolloni et al. 2020). In the present study, the importance of the sediment pH to multivariate community composition is likely not related to the experimental manipulation of shell hash and algae as the experimental effects of the shell hash were only seen at month 1 but the importance of pH is found in models for both months and for the procedural controls. Therefore, despite the small amount of pH manipulation through our treatments, our results indicate that sediment pH is influential on infaunal invertebrate communities. In addition to the abiotic factors driving infaunal communities, there are also biotic factors that influence these communities, such as predation, competition, and availability of food (reviewed in Huettel et al. 2014), which also should be addressed more in future studies.
Our study is the first to manipulate benthic communities on a mudflat by adding shell hash and algae and focus on impacts of these treatments on both sediment pH and infaunal invertebrates. We found that the addition of shell hash had a significant effect on sediment pH, but it was a small and short-term effect. The largest contributor to the variation observed in the community composition was the spatial variation among the blocks. This small-scale spatial variation also led to differences in the responses of the infaunal community to the shell and algae treatments. Algae addition influenced community composition and the abundance of one species, but not sediment pH, suggesting the presence of algae may have directly impacted the infaunal invertebrates present on the plots. There was little evidence that the abundance of biological traits was affected by the experimental treatments. There is potential for crushed shell hash to enhance sediment carbonate chemistry conditions, but it requires repeated application to have long-standing effects. It is necessary for further field experiments to find a longer-term way to manipulate sediment pH to better understand responses to ocean and sediment acidification. This is especially true for the addition of shell hash as a remediation technique for coastal acidification as inducing long-term increases to sediment pH is essential to impacting benthic communities. Additionally, there needs to be further study on what carbonate chemistry parameters drive changes in benthic communities. If the individual carbonate chemistry parameters that influence benthic invertebrate communities are determined, then future studies can investigate how changes to those parameters may be influenced by future oceanic conditions.
Data Availability
The datasets are available from the corresponding author upon request.
References
Agostini, S., B.P. Harvey, S. Wada, K. Kon, M. Milazzo, K. Inaba, and J.M. Hall-Spencer. 2018. Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical−temperate transition zone. Scientific Reports. https://doi.org/10.1038/s41598-018-29251-7.
Appolloni, L., D. Zeppilli, L. Donnarumma, E. Baldrighi, E. Chianese, G.F. Russo, and R. Sandulli. 2020. Seawater acidification affects beta-diversity of benthic communities at a shallow hydrothermal vent in a Mediterranean marine protected area (Underwater Archaeological Park of Baia, Naples, Italy). Diversity 12.
Barbeau, M.A., L.A. Grecian, E.E. Arnold, D.C. Sheahan, D.J. Hamilton, M.A. Barbeau, L.A. Grecian, E.E. Arnold, D.C. Sheahan, and D.J. Hamilton. 2019. Spatial and temporal variation in the population dynamics of the intertidal amphipod Corophium volutator in the upper Bay of Fundy, Canada. Journal of Crustacean Biology 29: 491–506. https://doi.org/10.1651/08-3067.1.
Bates, D., M. Mächler, B.M. Bolker, and S.C. Walker. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. https://doi.org/10.18637/JSS.V067.I01.
Beal, B.F., C.R. Coffin, S.F. Randall, C.A. Goodenow, K.E. Pepperman, and B.W. Ellis. 2020. Interactive effects of shell hash and predator exclusion on 0-year class recruits of two infaunal intertidal bivalve species in Maine, USA. Journal of Experimental Marine Biology and Ecology 530–531. https://doi.org/10.1016/j.jembe.2020.151441.
Beauchard, O., A. Brind’Amour, M. Schratzberger, P. Laffargue, N. Hintzen, P. Somerfield, and G. Piet. 2021. A generic approach to develop a trait-based indicator of trawling-induced disturbance. Marine Ecology Progress Series 675: 35–52. https://doi.org/10.3354/meps13840. Inter-Research Science Center.
Bindoff, N.L., W.W.L. Cheung, J.G. Kairo, J. Arístegui, V.A. Guinder, R. Hallberg, N. Hilmi, et al. 2019. Changing ocean, marine ecosystems, and dependent communities. In In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, ed. H.-O. Pörtner, D.C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, and K. Mintenbeck, 447–587. UK and New York, NY, USA: Cambridge University Press.
Borja, A., J. Franco, and V. Pérez. 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin 40: 1100–1114. https://doi.org/10.1016/S0025-326X(00)00061-8.
Bremner, J., S.I. Rogers, and C.L.J. Frid. 2006. Methods for describing ecological functioning of marine benthic assemblages using biological traits analysis (BTA). Ecological Indicators 6: 609–622. https://doi.org/10.1016/j.ecolind.2005.08.026.
Brooks, M.E., K. Kristensen, K.J. van Benthem, A. Magnusson, C.W. Berg, A. Nielsen, H.J. Skaug, M. Mächler, and B.M. Bolker. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal 9: 378–400. https://doi.org/10.32614/RJ-2017-066. Technische Universitaet Wien.
Brustolin, M.C., I. Nagelkerken, C.M. Ferreira, S.U. Goldenberg, H. Ullah, and G. Fonseca. 2019. Future ocean climate homogenizes communities across habitats through diversity loss and rise of generalist species. Global Change Biology 25: 3539–3548. https://doi.org/10.1111/gcb.14745.
Brustolin, M., R. Gladstone-Gallagher, J. Hewitt, A. Lohrer, and S. Thrush. 2022. The importance of shell debris for within-patch heterogeneity and disturbance-recovery dynamics of intertidal macrofauna. Marine Ecology Progress Series 700: 53–64. https://doi.org/10.3354/meps14186. Inter-Research Science Center.
Burnham, K.P., and D.R. Anderson, eds. 2004. Model selection and multimodel inference. New York, NY: Springer. https://doi.org/10.1007/b97636.
Burnham, K.P., D.R. Anderson, and K.P. Huyvaert. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65: 23–35. https://doi.org/10.1007/s00265-010-1029-6.
Campbell, L., T. Sizmur, F. Juanes, and T.G. Gerwing. 2019. Passive reclamation of soft-sediment ecosystems on the North Coast of British Columbia, Canada. Journal of Sea Research 155: 101796. https://doi.org/10.1016/j.seares.2019.101796. Elsevier.
Campbell, L., S.E. Dudas, F. Juanes, A.M. Allen Gerwing, and T.G. Gerwing. 2020. Invertebrate communities, sediment parameters and food availability of intertidal soft-sediment ecosystems on the north coast of British Columbia, Canada. Journal of Natural History 54: 919–945. https://doi.org/10.1080/00222933.2020.1770886. Taylor & Francis.
Carstensen, J., and C.M. Duarte. 2019. Drivers of pH variability in coastal ecosystems. Review-article. Environmental Science and Technology 53: 4020–4029. https://doi.org/10.1021/acs.est.8b03655. American Chemical Society.
Clements, J.C., and H.L. Hunt. 2018. Testing for sediment acidification effects on within-season variability in juvenile soft-shell clam (Mya arenaria) abundance on the northern shore of the bay of fundy. Estuaries and Coasts 41: 471–483. https://doi.org/10.1007/s12237-017-0270-x.
Curtin, T.P., N. Volkenborn, I.P. Dwyer, R.C. Aller, Q. Zhu, and C.J. Gobler. 2022. Buffering muds with bivalve shell significantly increases the settlement, growth, survival, and burrowing of the early life stages of the Northern quahog, Mercenaria mercenaria, and other calcifying invertebrates. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2021.107686. Elsevier Ltd.
Doyle, B., and L.I. Bendell. 2022. An evaluation of the efficacy of shell hash for the mitigation of intertidal sediment acidification. Ecosphere. https://doi.org/10.1002/ecs2.4003.
Drylie, T.P., H.R. Needham, A.M. Lohrer, A. Hartland, and C.A. Pilditch. 2019. Calcium carbonate alters the functional response of coastal sediments to eutrophication-induced acidification. Scientific Reports. https://doi.org/10.1038/s41598-019-48549-8. Springer US.
Drylie, T.P., A.M. Lohrer, H.R. Needham, and C.A. Pilditch. 2020. Taxonomic and functional response of estuarine benthic communities to experimental organic enrichment: consequences for ecosystem function. Journal of Experimental Marine Biology and Ecology 532: 151455. https://doi.org/10.1016/j.jembe.2020.151455. Elsevier.
Fournier, J., R.K. Gallon, and R. Paris. 2014. G2Sd: a new R package for the statistical analysis of unconsolidated sediments. Geomorphology 20: 73–78. https://doi.org/10.4000/geomorphologie.10513.
Freitas, F.S., S. Arndt, K.R. Hendry, J.C. Faust, and A.C. Tessin. 2021. Benthic organic matter transformation drives pH and carbonate chemistry in Arctic marine sediments. Global Biogeochemical Cycles. https://doi.org/10.1029/2021GB007187.
Gerwing, T.G., D. Drolet, M.A. Barbeau, D.J. Hamilton, and A.M. Allen. 2015. Resilience of an intertidal infaunal community to winter stressors. Journal of Sea Research 97: 40–49. https://doi.org/10.1016/j.seares.2015.01.001. Elsevier B.V.
Gerwing, T.G., D. Drolet, D.J. Hamilton, and M.A. Barbeau. 2016. Relative importance of biotic and abiotic forces on the composition and dynamics of a soft-sediment intertidal community. PLOS ONE 11. Public Library of Science: e0147098. https://doi.org/10.1371/journal.pone.0147098.
Gerwing, T.G., K. Cox, A.M. Allen Gerwing, L. Campbell, T. Macdonald, S.E. Dudas, and F. Juanes. 2020. Varying intertidal invertebrate taxonomic resolution does not influence ecological findings. Estuarine, Coastal and Shelf Science 232: 106516. https://doi.org/10.1016/j.ecss.2019.106516.
Gerwing, T.G., L. Campbell, D.J. Hamilton, M.A. Barbeau, G.S. Norris, S.E. Dudas, and F. Juanes. 2023a. Structuring forces of intertidal infaunal communities on the northern coast of British Columbia, Canada: assessing the relative importance of top-down, bottom-up, middle-out, and abiotic variables. FACETS 8: 1–14. https://doi.org/10.1139/facets-2022-0199. Canadian Science Publishing.
Gerwing, T.G., A.M.A. Gerwing, M.M. Davies, K. Dracott, L. Campbell, F. Juanes, S.E. Dudas, et al. 2023b. Sediment geochemistry influences infaunal invertebrate community composition and population abundances. Marine Biology 170: 1–12. https://doi.org/10.1007/s00227-022-04151-7. Springer Berlin Heidelberg.
Giere, O., and O. Pfannkuche. 1982. Biology and ecology of marine Oligochaeta, a review. Oceanography and Marine Biology: An Annual Review 20: 679–820.
Gobler, C.J., and H. Baumann. 2016. Hypoxia and acidification in ocean ecosystems: Coupled dynamics and effects on marine life. Biol. Lett. 12: 20150976. https://doi.org/10.1098/rsbl.2015.0976.
Gogina, M., A. Darr, and M.L. Zettler. 2014. Approach to assess consequences of hypoxia disturbance events for benthic ecosystem functioning. Journal of Marine Systems 129: 203–213. https://doi.org/10.1016/j.jmarsys.2013.06.001.
Green, M.A., G.G. Waldbusser, S.L. Reilly, K. Emerson, and S. O’Donnell. 2009. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnology and Oceanography 54: 1037–1047. https://doi.org/10.4319/lo.2009.54.4.1037.
Green, M.A., G.G. Waldbusser, L. Hubazc, E. Cathcart, and J. Hall. 2013. Carbonate mineral saturation state as the recruitment cue for settling bivalves in marine muds. Estuaries and Coasts 36: 18–27. https://doi.org/10.1007/s12237-012-9549-0.
Greiner, C.M., T. Klinger, J.L. Ruesink, J.S. Barber, and M. Horwith. 2018. Habitat effects of macrophytes and shell on carbonate chemistry and juvenile clam recruitment, survival, and growth. Journal of Experimental Marine Biology and Ecology 509: 8–15. https://doi.org/10.1016/j.jembe.2018.08.006. Elsevier.
Heiri, O., A.F. Lotter, and G. Lemcke. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110.
Huettel, M., P. Berg, and J.E. Kostka. 2014. Benthic exchange and biogeochemical cycling in permeable sediments. Annual Review of Marine Science 6: 23–51. https://doi.org/10.1146/annurev-marine-051413-012706.
Kristensen, E. 2000. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 426: 1–24. https://doi.org/10.1023/A:1003980226194.
Lam-Gordillo, O., R. Baring, and S. Dittmann. 2020. Ecosystem functioning and functional approaches on marine macrobenthic fauna: A research synthesis towards a global consensus. Ecological Indicators 115: 106379. https://doi.org/10.1016/j.ecolind.2020.106379.
Levin, L.A., H. Caswell, K.D. DePatra, and E.L. Creed. 1987. Demographic consequences of larval development mode: planktotrophy vs. lecithotrophy in Streblospio benedicti. Ecology 68: 1877–1886.
Llansó, R.J. 1991. Tolerance of low dissolved oxygen and hydrogen sulfide by the polychaete Streblospio benedicti (Webster). Journal of Experimental Marine Biology and Ecology 153: 165–178. https://doi.org/10.1016/0022-0981(91)90223-J.
McGarrigle, S.A., and H.L. Hunt. 2024. Infaunal invertebrate community relationships to water column and sediment abiotic conditions. Marine Biology 171: 3. https://doi.org/10.1007/s00227-023-04318-w.
Miatta, M., A.E. Bates, and P.V.R. Snelgrove. 2021. Incorporating biological traits into conservation strategies. Annual Review of Marine Science 13: 421–443. https://doi.org/10.1146/annurev-marine-032320-094121.
Morse, B.L., and H.L. Hunt. 2013. Impact of settlement and early post-settlement events on the spatial distribution of juvenile Mya arenaria on an intertidal shore. Journal of Experimental Marine Biology and Ecology 448: 57–65. https://doi.org/10.1016/j.jembe.2013.06.016. Elsevier B.V.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219. https://doi.org/10.1080/00785236.1995.10422044. Taylor & Francis.
O’Brien, A.L., L. Morris, and M.J. Keough. 2010. Multiple sources of nutrients add to the complexities of predicting marine benthic community responses to enrichment. Marine and Freshwater Research 61: 1388–1398.
Pacheco, A.S., M.T. González, J. Bremner, M. Oliva, O. Heilmayer, J. Laudien, and J.M. Riascos. 2011. Functional diversity of marine macrobenthic communities from sublittoral soft-sediment habitats off northern Chile. Helgoland Marine Research 65: 413–424. https://doi.org/10.1007/s10152-010-0238-8.
Pearson, T.H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: An Annual Review 16: 229–331.
Pimenta, A.R., and J.S. Grear. 2018. Guidelines for measuring changes in seawater pH and associated carbonate chemistry in coastal environments of the Eastern United States. Narragansett, RI & Washington, DC.
Posada, D., and T.R. Buckley. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. https://doi.org/10.1080/10635150490522304.
Rand, K., E. Logerwell, B. Bluhm, H. Chenelot, S. Danielson, K. Iken, and L. Sousa. 2018. Using biological traits and environmental variables to characterize two Arctic epibenthic invertebrate communities in and adjacent to Barrow Canyon. Deep Sea Research Part II: Topical Studies in Oceanography. https://doi.org/10.1016/j.dsr2.2017.07.015. Synthesis of Arctic Research SOAR Phase II.
Richards, S.A. 2005. Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86: 2805–2814. https://doi.org/10.1890/05-0074.
Rossi, F. 2006. Small-scale burial of macroalgal detritus in marine sediments: effects of Ulva spp. on the spatial distribution of macrofauna assemblages. Journal of Experimental Marine Biology and Ecology 332: 84–95. https://doi.org/10.1016/j.jembe.2005.11.003.
Sebesvari, Z., F. Esser, and T. Harder. 2006. Sediment-associated cues for larval settlement of the infaunal spionid polychaetes Polydora cornuta and Streblospio benedicti. Journal of Experimental Marine Biology and Ecology 337: 109–120. https://doi.org/10.1016/j.jembe.2006.06.007.
Sizmur, T., L. Campbell, K. Dracott, M. Jones, N.J. O’Driscoll, and T. Gerwing. 2019. Relationships between potentially toxic elements in intertidal sediments and their bioaccumulation by benthic invertebrates. PLoS ONE 14: e0216767. https://doi.org/10.1371/journal.pone.02167.
Snelgrove, P.V.R., and C.A. Butman. 1994. Animal-sediment relationships revisited: cause versus effect. Oceanography and Marine Biology: An Annual Review 32: 111–177.
Steele, D. 1983. Coarse sedimentary shores. In Marine and coastal systems of the Quoddy region, New Brunswick, ed. M.L.H. Thomas, 74–94. Ottawa, Ontario: Government of Canada Fisheries and Oceans.
Teixidó, N., M.C. Gambi, V. Parravacini, K. Kroeker, F. Micheli, S. Villéger, and E. Ballesteros. 2018. Functional biodiversity loss along natural CO2 gradients. Nature Communications 9: 5149. https://doi.org/10.1038/s41467-018-07592-1. Springer US.
Tomanova, S., N. Moya, and T. Oberdorff. 2008. Using macroinvertebrate biological traits for assessing biotic integrity of neotropical streams. River Research and Applications 24: 1230–1239. https://doi.org/10.1002/rra.1148.
Villnäs, A., J. Hewitt, M. Snickars, M. Westerbom, and A. Norkko. 2018. Template for using biological trait groupings when exploring large-scale variation in seafloor multifunctionality. Ecological Applications 28: 78–94. https://doi.org/10.1002/eap.1630.
Violle, C., P.B. Reich, S.W. Pacala, B.J. Enquist, and J. Kattge. 2014. The emergence and promise of functional biogeography. Proceedings of the National Academy of Sciences 111: 13690–13696. https://doi.org/10.1073/pnas.1415442111.
Waldbusser, G.G., and J.E. Salisbury. 2014. Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Annual Review of Marine Science 6: 221–247. https://doi.org/10.1146/annurev-marine-121211-172238.
Wallace, R.B., and C.J. Gobler. 2021. The role of algal blooms and community respiration in controlling the temporal and spatial dynamics of hypoxia and acidification in eutrophic estuaries. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2021.112908. Elsevier Ltd.
Wallace, R.B., H. Baumann, J.S. Grear, R.C. Aller, and C.J. Gobler. 2014. Coastal ocean acidification: the other eutrophication problem. Estuarine, Coastal and Shelf Science 148: 1–13. https://doi.org/10.1016/j.ecss.2014.05.027. Elsevier Ltd.
Widdicombe, S., S.L. Dashfield, C.L. McNeill, H.R. Needham, A. Beesley, A. McEvoy, S. Øxnevad, K.R. Clarke, and J.A. Berge. 2009. Effects of CO2 induced seawater acidification on infaunal diversity and sediment nutrient fluxes. Marine Ecology Progress Series 379: 59–75. https://doi.org/10.3354/meps07894.
Widdicombe, S., J.I. Spicer, and V. Kitidis. 2011. Effects of ocean acidification on sediment fauna. In Ocean Acidification, ed. J.-P. Gattuso and L. Hansson, 176–191. Oxford University Press.
Wilding, T.A., and T.D. Nickell. 2013. Changes in benthos associated with mussel (Mytilus edulis L.) farms on the west-coast of Scotland. PLoS ONE. https://doi.org/10.1371/journal.pone.0068313.
Ysebaert, T., and P.M.J. Herman. 2002. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Marine Ecology Progress Series 244: 105–124. https://doi.org/10.3354/meps244105.
Acknowledgements
We would like to thank Marie-Josée Maltais, Crystal Radtke, Joshua de Jong, Samantha Gaitens, Maia Kendel, and Allison Thomas for their assistance with laboratory and field work. Additionally, we would like to thank Marti Anderson for her guidance with the multivariate statistical analysis components of this research.
Funding
This study was funded by an NSERC Discovery Grant to HLH and NBIF and UNB graduate scholarships to SAM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patricia A. Ramey-Balci
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGarrigle, S.A., Francis, M.C. & Hunt, H.L. Effects of Experimental Addition of Algae and Shell Hash on an Infaunal Mudflat Community. Estuaries and Coasts 47, 1617–1636 (2024). https://doi.org/10.1007/s12237-024-01378-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01378-z