Abstract
Marshes play a key role in global nitrogen cycling at the land–water margin. Invasive species are generally considered detrimental as they alter ecosystems they invade, but recent studies have shown some established invasive species can enhance certain ecosystem functions. The European haplotype of Phragmites australis is an aggressive and widespread invasive plant species in North America. We hypothesized that P. australis may play an important role in marsh nitrogen cycling by promoting higher rates of sediment denitrification compared with native marsh species. Seasonal measurements of sediment dissolved gas (N2 and O2) fluxes at three sites within the Albemarle-Pamlico Region of North Carolina compared sediments from invasive P. australis, native Spartina alterniflora, and/or Juncus roemarianus, and unvegetated sediments. In a marine tidal site, annual net denitrification in sediments associated with upland P. australis was highest compared to lower elevation marsh species or unvegetated sediments under ambient (139 μmol N2-N m−2 h−1) and nitrate enriched (219 μmol N2-N m−2 h−1) conditions. N2 fluxes were lower in sediments from two brackish marshes and did not differ between associated species, unvegetated sediments, or between high or low organic matter sites. Treatments with elevated nitrate showed enhanced net denitrification in most sediments at the marine site, suggesting the capacity to remove additional nitrate delivered episodically. Additionally, N2 fluxes measured before and after Hurricane Florence showed an increase in denitrification in P. australis sediments after the hurricane. Ecosystem value for this nitrogen removal service in the marine tidal site was estimated at US$ 266–426 *ha−1*yr−1. These results demonstrate an important role for invasive P. australis in coastal nitrogen cycling in marine environments and provide landscape context for potential biogeochemical impacts of this invasion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities over the past century have resulted in increases in nutrients, such as nitrogen and phosphorus, delivered to coastal ecosystems (Nixon 1995; McClelland and Valiela 1998; Boesch 2002). Although phosphorus and nitrogen limitation can co-occur, nitrogen is often the dominant limiting nutrient in marine systems and is required to support primary production at the base of marine food webs (Sundareshwar et al. 2003; Howarth and Marino 2006; Elser et al. 2007; Conley et al. 2009). However, excess nitrogen loads degrade water quality in estuarine systems through eutrophication (World Resources Institute 2008; Seitzinger et al. 2006; Paerl and Otten 2013), anoxia (Diaz and Rosenberg 2008), and harmful algal blooms (Anderson et al. 2002; Anderson 2009). This issue is heightened during storm events, when large pulses of bioavailable nitrogen are delivered to coastal waters (Paerl et al. 2018).
Marshes are valuable habitats that can function as buffer zones at the land–sea interface (Deegan et al. 2012) and play a key role in global biogeochemical cycles of carbon (Mcleod et al. 2011) and nitrogen (Galloway et al. 2003). Marshes have the capacity to remove and retain excess nutrients (Valiela and Cole 2002; Merrill and Cornwell 2002) including as much as 20–50% of externally sourced nitrogen (Galloway et al. 2004; Seitzinger et al. 2006; Deegan et al. 2012). Nitrogen removal occurs through biological processes, such as denitrification, during which microbial communities in the sediment transform dissolved inorganic nitrate (NO3−) to biologically unreactive dinitrogen gas (N2) (Seitzinger et al. 2006) or physical processes such as the burial of nitrogen in the sediments (Merrill and Cornwell 2002). Seasonally, nitrogen is assimilated by plants and incorporated into biomass. This uptake is largely released when plant biomass decays following the growing season, but some nitrogen is translocated to rhizomes and/or stored in plant materials (Kühl et al. 1997). If excess nitrogen is not intercepted by marshes, it is delivered to adjacent nutrient-sensitive estuaries. Understanding potential threats to marsh function is crucial to maintain and protect environmental and economic values of marsh habitat (Millennium Ecosystem Assessment (Program) 2005).
Hurricanes are known to impact coastal systems in numerous ways, including erosion, habitat removal, and delivery of large loads of nutrients and bacteria (Mallin and Corbett 2006; Miller et al. 2006; Paerl et al. 2020). These storm pulses of nutrients and bacteria from inland systems to coastal waters can negatively impact water quality and persist for weeks or even months following a major storm event (Mallin et al. 1999). In eastern North Carolina, the presence of agriculture and livestock negatively impacts water quality via agricultural runoff (e.g., waste and fertilizers) washed directly into coastal waters (Mallin 2000). Extreme weather events are expected to continue to increase under climate change scenarios (Sainsbury et al. 2018), providing large pulses of agricultural and other non-point source pollution into coastal systems (Zhang et al. 2009). Understanding how coastal systems, and marshes in particular, respond to increased nutrient loads is critical to assessing long-term ecosystem function.
Phragmites australis is native to North America, but an aggressive European haplotype has spread throughout the region over the past several centuries (Saltonstall 2002) and all but completely supplanted its native counterpart, particularly in high-nutrient and disturbed conditions (Minchinton and Bertness 2003). Unlike the native P. australis subspecies, the salt tolerance of invasive P. australis allows it to invade lower areas of the marsh that are typically dominated by native species such as Spartina alterniflora or Juncus roemarianus (Vasquez et al. 2005). Invasive P. australis can be associated with a decrease in the biodiversity of both flora and fauna in the marsh (Vitousek et al. 1996; Sala et al. 2000; Silliman and Bertness 2004) and an overall decline in habitat quality due to its monoculture growth structure (Minchinton and Bertness 2003). Other negative impacts include increased shading of sediments and associated reduction in benthic microalgal biomass (Currin et al. 2003) and reduced soil moisture (Windham and Lathrop 1999). Throughout its range in North America, invasive P. australis is considered an undesirable species and has been subjected to eradication attempts that have demonstrated limited success in sustained removal (Hazelton et al. 2014). This is particularly true along the east and gulf coasts of North America and throughout the Great Lakes Region (Chambers et al. 1999).
Understanding the contributions of invasive P. australis to ecosystem function is critical. Nitrogen regulation by inter- and subtidal habitats is a crucial component of ecosystem function (Piehler and Smyth 2011), and invasive marsh grasses could affect this valuable ecosystem service if associated nitrogen removal capacity varies from that of native grasses. Previous studies have found the removal of P. australis in wetlands reduced denitrification potential (Alldred et al. 2016; Findlay et al. 2003) and nitrogen uptake (Judd and Francoeur 2019). In constructed wetlands where native P. australis was predominant, associated sediments were found to effectively remove bioavailable nitrogen under ambient and nitrate-enriched conditions (Soana et al. 2020). Though some prior work has measured denitrification potential of invasive P. australis in North American salt marshes (Granville et al. 2021; Li et al. 2021; Windham and Meyerson 2003), direct denitrification rates in these systems are poorly constrained.
The objective of this study was to determine the impact of invasive P. australis on the biogeochemical cycling of nitrogen in marshes. Specifically, we sought to quantify the flux of dinitrogen gas (N2) in sediments associated with P. australis in comparison to native marsh plants and unvegetated sediments under ambient nutrient regimes, in response to simulated storm additions of nitrate, and following a hurricane. We hypothesized that P. australis promotes sediment denitrification at higher rates than that associated with native plant species.
Methods
Site Description
The Albemarle-Pamlico System is a large, temperate estuary that extends from southeastern Virginia and continues south along the North Carolina (NC) coast. It has a total surface area of over 8,000 km2, and an average depth of 4.9 m, making it the second largest estuary in the USA after the Chesapeake Bay. Water quality in the Albemarle-Pamlico system is predominantly affected by nutrient loads from agricultural runoff and animal waste discharge, with 32% (~1.9 million hectares) of the total management area’s land use comprised of farmland and livestock operations (USDA 2012).
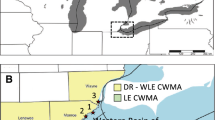
Research was focused primarily on the Rachel Carson National Estuarine Research Reserve in Beaufort, NC, a marine system that experiences a semi-diurnal tidal regime (site name: MAR). Supplemental sites included Currituck Banks National Estuarine Research Reserve and Kitty Hawk Woods Coastal Reserve to encompass a range of geographic areas and tidal regimes (Fig. 1). Both northern sites are brackish and primarily influenced by wind driven tides but differed in sediment organic matter (SOM), which was high in Currituck Banks (site name: BR-H) and low in Kitty Hawk Woods (site name: BR-L). These three reserves span the northern (BR-H), middle (BR-L), and southern (MAR) portions of the system and are respectively located in the Currituck Sound, Albemarle Sound, and Back Sound. Although not formally documented, colonization of P. australis in MAR is estimated at a minimum of 15 years previous to the study (personal communications, North Carolina Coastal Reserve (NCCR)). P. australis presence in BR-H is documented in historical aerial imagery as early as 1980 (North Carolina Coastal Reserve and Fear 2009). No pre-existing data nor historical imagery was available to age P. australis in BR-L; however, based on personal observation it has been well established at this site for a minimum of five years.
Flux incubations were conducted on sediment cores collected from MAR during late spring (11/06/18; T = 25 °C), summer (04/09/18; T = 29 °C), and fall (04/12/18; T = 9 °C) to capture variability through the growing season. In addition, BR-H and BR-L were sampled during late spring (18/06/18; T = 25 °C) and fall (27/11/18; T = 15 °C). Following Hurricane Florence, which impacted the area between September 12 through 15 of 2018, an additional flux measurement was conducted at MAR on September 25, 2018 (T = 22 °C), roughly two weeks after the pre-storm summer flux experiment conducted on September 4.
Sample Collection
Sediment cores were collected in triplicate at each site in clear polyvinyl chloride (PVC) tubes measuring 6.4 cm in diameter and 31 cm in height. Sediment height in each core was approximately 17 cm. Cores were collected within patches of non-native P. australis, native Juncus roemarianus, and/or Spartina alterniflora, and from unvegetated sediments (n = 3 for each). Sediments associated with each treatment will hereafter be referred to by species name, unvegetated sediments, or collectively as “sediment treatments”. Effort was made to exclude live plant material and living organisms (e.g., snails, crabs, etc.) from the cores. Sixty liters of water were collected adjacent to the marsh habitat. Sediment cores were transported to the UNC Institute of Marine Sciences in Morehead City, NC, and placed in tanks of site water maintained at in situ water temperature within an environmental chamber (Bally Refrigerated Boxes Inc., Morehead City, NC). The submerged cores and water were left overnight to ensure temperature equilibration.
Continuous flow-through sediment flux experiments were performed according to the methods detailed in Piehler and Smyth (2011). Gas-tight caps with inflow and outflow ports were placed on each submerged core excluding air bubbles and subsequently connected to the flow through system (Supplemental Fig. 1). This system used a multichannel peristaltic pump to pull unfiltered, aerated site water into the surface water of each core while drawing water just above the sediment surface of each core a rate of 1 mL*min−1 for an approximate turnover time of five hours (i.e., replacement time of water within sediment core tube). Following capping, cores remained in the dark environmental chamber for a minimum of three complete turnovers (~ 15 h) of water within the cores to allow for equilibration. Water samples flowing from the cores and from bypass lines (considered “inflow waters”) were sampled periodically over the next two days, with a minimum of one turnover time between each collection. Though this method does not replicate tidal cycles or extended exposed periods, it is the best method for direct N2 flux measurements (Groffman et al. 2006). Additional water samples were collected, filtered, and frozen for later nutrient analysis of NOx− and NH4+ with a Lachat Quick-chem 8000 (Lachat Instruments, Milwaukee, WI, USA). NOx− represents the sum of nitrate (NO3−) and nitrite (NO2−), although the latter was assumed to be negligible.
Sediment samples (1 cc) were collected directly adjacent to cores at 1 cm depth for determination of benthic chlorophyll-a (chl-a) concentration and percent carbon (C) and nitrogen (N) content. Sediment chl-a was extracted in a solvent mix (45:45:10 ratio of methanol/acetone/deionized water), sonicated, and analyzed with a UV mini 1240 spectrophotometer (Shimadzu Instruments, Columbia MD) using an acidification method (Lorenzen 1967). The molar concentrations of C and N in dried, ground, and hydrochloric acid fumed sediment samples were measured with a Model 2400 Series II CHN analyzer (Perkin Elmer, Waltham, MA; Dalsgaard et al. 2000). Upon completion of core incubations, a 3-cm deep subsample from each core was collected for analysis of sediment organic matter (SOM) via loss on ignition. Efforts were made to minimize the number of roots and rhizomes present in the subsamples used for these analyses.
Nitrogen Enrichment
Ambient concentrations of NOx were close to minimum detection limits and never exceeded 1 μM on any seasonal sample date in any location. Following collection of three sample timepoints on day one of the flux experiment, NaNO3 was added to the inflow waters, raising the initial nitrate concentration by 20 μM NO3− to simulate a storm pulse of nitrogen based on NO3− concentrations in runoff from stormwater outfalls draining into Taylor’s Creek across from the Rachel Carson Reserve (MAR). Following nitrate enrichment, cores were allowed to equilibrate for a minimum of 15 h, approximately three turnover times. Sampling after this period included three collections of water for dissolved gas analysis spaced at five hours and one collection of water for dissolved nutrient analyses as described above.
Hurricane Florence
Coastal North Carolina was impacted by Hurricane Florence during September 2018. Approximately 64 cm of precipitation fell over four days (Sept. 12–15) and caused over two weeks of widespread inland and coastal flooding. Measurements completed one week before the storm represent “summer” in our seasonal sampling scheme, while measurements one-and-a-half weeks after the storm are compared to pre-storm rates but are not included in seasonal analyses. Ambient nitrate concentration in waters collected during the post hurricane experiment was 2.5 μM.
Flux Calculations
Dissolved gas samples were analyzed using a Balzers Prisma QME 200 quadrupole membrane inlet mass spectrometer (MIMS; Bay Instruments, Easton, MD; Kana et al. 1994) for N2/Ar and O2/Ar. N2 and O2 fluxes from the sediments were calculated with the following equation (Kana et al. 1998; Fulweiler and Nixon 2009; Piehler and Smyth 2011):
Using equation 1, a positive number represents net denitrification, and a negative number represents net nitrogen fixation. To convert the O2 flux to sediment oxygen demand (SOD), results were multiplied by –1.
Denitrification efficiency (DNE) was calculated as the percentage of dissolved inorganic nitrogen released from the sediments as N2 gas during the flux experiment. This measure of the portion of net denitrification to the overall nitrogen flux was calculated using the following equation (Eyre and Ferguson 2002):
Statistical Analyses
All data from this study were analyzed using JMP Pro 16.0 and R software packages. Data were assessed for normality using the Shapiro–Wilk test. It was determined that these results were not normally distributed (Shapiro–Wilk p ≤ 0.001). The assumption of normality and homogeneity of variance was also violated with Log and Box-Cox transformations (Shapiro–Wilk p ≤ 0.001; Levene p ≤ 0.001). We ran both nonparametric (Kruskal–Wallis) and parametric analyses (one-way and two-way ANOVAs) and found results did not differ qualitatively, and thus we present only analyses using parametric tests. Tukey HSD and Newman–Keuls tests were used for post-hoc analysis. Correlation analysis was performed between N2 flux and other relevant variables, and only significant results are presented. A p-value less than 0.05 was used to determine statistical significance.
Economic Evaluation of Nitrogen Removal Service Associated with P. australis
The value of nitrogen removal via denitrification was estimated using the rates from the North Carolina Nutrient Offset Credit Program, a regionally derived number developed from significant stakeholder input in its determination. The current trading price of the North Carolina Nutrient Offset Credit Program for the Tar-Pamlico region is $21.67 per kilogram of nitrogen removed (15A North Carolina Administrative Code 2B.0703). To best estimate the annual value of habitat specific nitrogen removal, the range of seasonal rates of net denitrification measured in sediments associated with P. australis at MAR were multiplied by $21.67 and constrained to relate to in situ conditions (3 hr dark/inundation daily during 9 months per year).
Results
MAR (Rachel Carson Reserve)
N2 Flux in P. australis and Native Grasses
Over the course of the year, denitrification rates measured in sediments associated with three species of marsh grass and unvegetated sediments ranged from 32 to 139 μmol N2-N m−2 h−1 under ambient conditions and 72 to 219 μmol N2-N m−2 h−1 under nitrate-enriched conditions (Fig. 2a). P. australis sediments exhibited a significantly higher annual mean rate of denitrification than sediments associated with native species or unvegetated sediments under both ambient and nitrate-enriched conditions (Fig. 2a; Table 1). No significant differences in annual denitrification rates were observed between any of the other three sediment treatments sampled under either ambient or nitrate-enriched conditions (Fig. 2a). Denitrification rates increased following nitrate enrichment in all sediment treatments, though this increase was significant only in unvegetated and J. roemarianus sediments.
Annual mean and standard error for N2 flux grouped by vegetation under ambient and nitrate-enriched conditions at a) MAR b) BR-H and c) BR-L. Positive values represent net denitrification, and negative values indicate net nitrogen fixation. Significance for all comparisons was set at p < 0.05. Asterisks (*) indicate significant differences between ambient and nitrate enriched conditions for each species or unvegetated sediments. Lower case letters indicate significant differences between sediment treatments under ambient conditions and Upper-case letters indicate significant differences between treatments under nitrate enriched conditions
P. australis sediments exhibited higher SOM and lower benthic chl-a relative to native species and unvegetated sediments (Table 1). A weak but significant positive relationship was observed between SOM and N2 flux for P. australis sediments (r2 = 0.29), and no significant relationships between SOM and N2 flux were observed for other species (Fig. 3). Benthic chl-a concentrations decreased significantly with increasing elevation of marsh vegetation, and P. australis sediments had a significantly higher % N than unvegetated or native species (Table 1).
Seasonal Patterns in N2 Flux
Comparing seasonal N2 fluxes provided context for variations in microbial processing of nitrogen throughout the growing season among marsh species. For each season, denitrification rates in P. australis sediments surpassed rates measured in native species and unvegetated sediments under both ambient and nitrate enriched conditions. However, in contrast to annual means, these differences were only significant between P. australis and S. alterniflora during the spring enriched conditions and between P. australis and all other treatments during summer for both ambient and enriched conditions (Fig. 4). No significant differences were observed between treatments during the fall (Fig. 4). In the spring and summer, native species and unvegetated sediments showed an increase in denitrification with nitrate enrichment over respective ambient rates, which was significant for unvegetated sediments in the spring and for all native and unvegetated sediments in the summer (Fig. 4). Denitrification in P. australis sediments showed a significant increase with nitrate addition during the spring only (Fig. 4). Seasonal variation in N2 flux was only significant in S. alterniflora sediments between spring and fall (ANOVA, p < 0.001).
a) Seasonal mean and standard error of N2 flux at MAR grouped by sediment type under ambient and nitrate-enriched conditions. N2 flux for summer preceded Hurricane Florence and post hurricane rates measured under ambient nitrate conditions are outlined in red with * denoting significant (p < 0.05) differences between pre- and post-hurricane for respective treatment sediments. Significant differences (p < 0.05) between ambient and nitrate-enriched rates associated with each species are denoted by *. Lower-case letters indicate significant differences between species during each season under ambient conditions. Upper-case letters denote significant differences between N2 flux within each season under nitrate-enriched conditions. N.D. indicates no data
Hurricane Florence
Both before and after Hurricane Florence, all sediment treatments exhibited net denitrification, and significantly higher rates of ambient denitrification were measured in P. australis sediments compared with native species and unvegetated sediments (Fig. 4). Denitrification rates after Hurricane Florence were significantly higher than pre-storm rates for P. australis sediments only (Fig. 4). Following Hurricane Florence, the average denitrification rate in P. australis sediments was 322 µmol N2-N m−2 h−1 under ambient conditions (Fig. 4) which surpassed the mean annual rates observed under ambient (139 µmol N2-N m−2 h−1) or enriched (219 µmol N2-N m−2 h−1) conditions in these sediments.
Regional Comparison
BR-H (Currituck Banks) and BR-L (Kitty Hawk Wood) Reserves
Ambient denitrification rates in P. australis sediments were lower at BR-H and BR-L (the brackish-high and -low sediment organic matter sites) compared with MAR (ANOVA; p ≤ 0.001), and there were no differences in N2 flux between sediment treatments within either brackish site on an annual scale (Fig. 2b, c). At BR-H, unvegetated sediments and J. roemarianus sediments exhibited net nitrogen fixation under ambient conditions, and N2 flux significantly increased in these sediments following nitrate enrichment (Fig. 2b). P. australis was the only sediment treatment in BR-H to exhibit annual net denitrification but had no significant response to nitrate enrichment (Fig. 2b). In BR-L, the low organic site, no differences in mean annual N2 fluxes were observed between sediment treatments under ambient or nitrate enriched conditions (Fig. 2c).
Sediment attributes and fluxes for P. australis sediments compared between three sites showed that P. australis at MAR was distinct from the two brackish sites in terms of higher SOD and N2 flux but benthic chl-a did not differ for this species among sites (Tables 1 and 1s). Elevated NH4 flux in P. australis sediments at MAR in the spring and summer relative to native and unvegetated sediments resulted in lower DNE for P. australis sediments (61%) compared with native and unvegetated sediments (69–84%; Table 1). In the brackish sites, NH4 fluxes associated with P. australis were not distinct from native or unvegetated sediments (Table 1s). The range in DNE for all sediment treatments were high in BR-L (90- 99%) and lower in BR-H (31–64%) (Table 1s).
P. australis Nitrogen Removal as an Ecosystem Service
Value of nitrogen removal service provided by P. australis in MAR was estimated as a range of US$0.32 to $0.52 *ha−1*hr−1 based on the annual range of denitrification rates measured under ambient nitrate conditions. Neither inundation times nor habitat area for P. australis was available for this site. Scaling with assumptions to approximate in situ conditions (3 hr of dark/inundation period daily for 9 months per year) resulted in annual cost for replacing this service of US$266-$426 *ha−1*yr−1 although inundation of P. australis sediments at MAR may be restricted to extreme high tides and flooding events. The valuation assessment would be improved by measurement of additional site-specific characteristics as well as quantification of denitrification rates for sediments exposed to air and light that were not possible with the methodology employed here.
Discussion
This study found that sediments associated with invasive P. australis had higher mean annual rates of denitrification than those of native marsh species or unvegetated sediments in a tidal marine setting (MAR) in ambient conditions. Rates of denitrification in P. australis sediments exceeded those of native and unvegetated sediments at MAR under nitrate-enriched conditions as well. The difference in denitrification rates between invasive and native marsh sediments at MAR was particularly distinct following Hurricane Florence, when N2 flux in P. australis sediments surpassed all other measured rates.
Differences Between P. australis and Native Grasses
At MAR, P. australis sediments appear to play a key role in nitrogen removal. The marsh species within MAR are clearly defined by tidal zonation, and the higher denitrification rates observed may be driven by bacterial communities associated with P. australis benefitting from characteristics of upland vegetation zones that include higher carbon availability (Cao et al. 2008), less competition with benthic microalgae (Sullivan and Currin 2002), and potentially higher rates of remineralization and nitrification at this higher elevation (Table 1; Garner and Allen 2013; Marton et al. 2015).
P. australis is known to increase sediment accretion rates in tidal marshes, more effectively trapping litter and particulate matter than adjacent native vegetation due to dense stands formed by the plant (Rooth et al. 2003). P. australis grows at the highest elevation in MAR and accretion in this upland section of the marsh is likely affected by retention of litter following seasonal die off as well as sediment trapping of runoff during storm events and flooding during spring tides (Asaeda et al. 2002; Bedford 2005). As readily decomposable organic matter drives denitrification by providing the electron donor for nitrate reduction (Burford and Bremner 1975), the organic matter trapped by P. australis and made available to the underlying sediments is likely a key factor in the consistently elevated rates associated with this species in MAR and is supported by the positive relationship observed between net N2 flux and sediment organic matter for P. australis sediments (Fig. 3).
Despite higher rates of denitrification and % N in P. australis sediments, ammonium flux from P. australis sediments was variable, which resulted in the lowest denitrification efficiency of the sediments studied, though efficiencies across all sediment treatments were extremely high. The driver of this variability is unclear but suggests that P. australis sediments can both act as a pathway for nitrogen release as ammonium, indicated by the higher ammonium flux in the spring and summer, and as a sink for nitrogen, such as in the fall when the ammonium flux is low.
Seasonal Influence on Denitrification Rates in MAR
The lower rates of denitrification observed in the spring could reflect competition for nitrogen between the marsh plants and microbial communities (Burdick and Konisky 2003). Though minimal above-ground plant material was present in the sampled cores, we speculate that the high growth rates of all marsh species in the spring may lower nitrogen availability in the sediments and thus limit denitrification (Kuzyakov and Xu 2013). Increases in denitrification following nitrate enrichment for most treatments in the spring support this interpretation. The absence of differences in denitrification following nitrate enrichment in P. australis sediments during the summer suggests alternate limitation of denitrification associated with this species during the middle of the growing season (Fig. 4) or alternate, unmeasured fates of nitrate, such as dissimilatory nitrate reduction to ammonium (DNRA) or release of N2O. The decrease in sediment organic matter in P. australis sediments between the spring and summer and low sediment organic matter in the fall may indicate seasonal carbon limitation for denitrification (Table 1; Fig. 4; Van de Broek et al. 2016).
These data suggest that marine sediments associated with P. australis have the capacity to remove greater loads of bioavailable nitrogen as it enters the system in pulses (i.e., associated with larger storm events) than native counterparts, particularly during the early growing season when the microbial communities are likely otherwise nitrogen limited (Fig. 4). Net removal may be offset by higher ammonium fluxes compared to sediments in S. alterniflora, J. roemarianus, or unvegetated sediments in the early part of the growing season (Table 1).
Mitigation of Storm Nitrogen Inputs to Estuarine Systems
Though coastal marshes cannot entirely negate the impacts of major storm events, the ecosystem services offered by this habitat can mitigate these impacts (Arkema et al. 2013; Smith et al. 2018; Adame et al. 2019). This is directly observed in our data, with significantly higher rates of denitrification in P. australis’ sediments at MAR following sustained flooded conditions from Hurricane Florence than rates measured under ambient or nitrate-enriched conditions in any season. The response of P. australis sediments to Hurricane Florence is likely driven by an advantageous land position in a tidal marsh with established marsh species zonation. Specifically, the combination of P. australis’ high density and habitat position promote increased trapping of sediment and wrack which may be reflected in a (non-significant) increase in sediment organic matter following the storm compared with sediment samples collected two weeks prior. As storm surge receded, the position of P. australis at the wrack line of freshly deposited organic matter likely fueled the high denitrification rates, as aboveground litter has been shown to drive denitrification in sediments underlying P. australis (Li et al. 2021). This suggests P. australis sediments perform a key ecosystem service to mitigate storm impacts during a vulnerable period.
Denitrification Variability by Site
The higher rates of denitrification associated with P. australis relative to native species observed in the marine system (MAR) were not evident at the brackish sites (BR-H and BR-L), where irregular, wind-driven tides and smaller range in elevation did not result in clear zonation of marsh species. BR-H is characteristic of marshes fringing the northern sounds with regard to organic matter while BR-L presented the opportunity to examine denitrification associated with P. australis in a disturbed context, where it is known to colonize quickly and extensively (Bart et al. 2006). Additionally, BR-L is subject to high wave energy due to open water exposure and proximity to residential canals where boat wakes are frequent.
In the high sediment organic matter site (BR-H), ambient N2 flux in unvegetated sediments and native J. roemarianus were negative, indicative of nitrogen fixation, but switched to net denitrification following nitrate enrichment. This suggests nitrate limitation, possibly resulting from competition with macrophytes in this highly productive habitat (Kuzyakov and Xu 2013). We speculate that diminished nitrification during extended periods of anoxia caused by sustained inundation at this site may also be a factor (Bollmann and Conrad 1998). The low organic matter site (BR-L) showed no variation in denitrification rates between species nor with nitrate enrichment for each species. Although age and origin of this marsh are unknown, the site appeared to be a recent colonization of a sandy over-wash or dredge spoil. It is likely that carbon availability is limiting denitrification in sediments for all marsh species at this site (Smith and Duff 1988). If P. australis density and extent increase, sediment organic matter may eventually accumulate at this site (de Groot et al. 2011). If so, denitrification is predicted to increase and ameliorate storm loaded nitrogen, but the evidence from BR-H does not support a distinct effect of P. australis on this process.
The observed regional differences in denitrification rates associated with P. australis sediments are likely driven by landscape position of P. australis in these marshes. Though P. australis can survive and grow in salinities up to and exceeding 32 ppt (Achenbach et al. 2013), it shows a decline in growth rate and germination in salinities higher than 10 ppt (Mauchamp and Mesleard 2001) and thus is typically found at higher elevations in marshes which experience full salinity (Lissner and Schierup 1997). By contrast, the interspersion of marsh species over a smaller elevation range in the brackish marshes likely results in less distinct differences in microbial communities associated with the sediments of particular marsh species (Barry et al. 2021).
An additional consideration of the landscape position of P. australis in the marine setting is inundation frequency and duration. Inundation patterns that varied among sites have implications for denitrification rates that were measured only under simulated flooded conditions in this study. For instance, wind-driven tides, characteristic of the brackish sites, result in longer periods of both exposure and inundation. Extended exposure would favor aerobic processes including mineralization and nitrification, while inundation could enhance denitrification of available nitrate, but limit coupled nitrification–denitrification. In contrast, semi-diurnal tides foster a regular balance of these processes that shift along the elevation gradient. It is likely that experimental inundation of upland P. australis at MAR resulted in favorable anoxic conditions for denitrification that are less frequent at this higher elevation but relevant to extreme tide and storm events. The differences in denitrification associated with P. australis at both BR-H and BR-L relative to MAR appear to be driven most directly by tidal regime (Knights et al. 2017) that affects landscape position (Kaplan et al. 1979) and distribution of sediment organic matter (Eyre et al. 2013). However, denitrification associated with the presence of P. australis in all sites showed this ecosystem service to be at least on par with that associated with native species.
Management Implications
Traditional ecosystem management has focused on attempts to control or eradicate P. australis from marsh habitats. These eradication attempts are often costly, harmful to adjacent native communities, and have varying degrees of success (Hazelton et al. 2014; Quirion et al. 2018; Lombard et al. 2012; Back et al. 2012). In the rare cases of successful eradication, there is limited literature assessing longer-term success of restoration efforts (Hazelton et al. 2014).
Between 2005 and 2009, an estimated US$ 4.6 million was spent annually managing ~80,000 hectares of invasive P. australis, equating to a cost of approximately US$ 58 *ha−1*yr−1 (Martin and Blossey 2013). We appraise nitrogen removal performed by P. australis at MAR at US$ 266–426 *ha−1*yr−1. This estimate underscores a potential value of P. australis to coastal systems in contrast to the cost of often unsuccessful eradication. This assessment aligns with other recent work focused on the role of invasive species in maintaining ecosystem services and function that can better inform management (Ramus et al. 2017; Hershner and Havens 2008; Davis et al. 2011).
This study highlights P. australis’ ability to foster higher rates of denitrification in underlying sediments in marine systems relative to predominant native species such as S. alterniflora and J. roemarianus. The evidence suggests that the presence of P. australis enhances the capacity of salt marshes to act as a sink for nitrogen, particularly during times of increased nutrient loading such as storm events. In contrast, nitrogen removal capacity was lower and not differentiated from native species at brackish sites; an important distinction when considering management. In light of the expense and poor success of eradication (Martin and Blossey 2013; Lombard et al. 2012), coastal managers should consider the role P. australis plays in different settings in providing ecosystem services such as nitrogen removal, shoreline stabilization, and increased vertical accretion (Rooth and Stevenson 2000; Rooth et al. 2003; Theuerkauf et al. 2017; Kiviat 2013; Soana et al. 2020; Sheng et al. 2021). Given this growing body of evidence, the net benefits of eradication efforts should receive further analysis from both ecological and economic perspectives.
References
Achenbach, L., F. Eller, L.X. Nguyen, and H. Brix. 2013. Differences in salinity tolerance of genetically distinct Phragmites australis clones. AoB PLANTS 5: plt019. https://doi.org/10.1093/aobpla/plt019
Adame, M.F., M.E. Roberts, D.P. Hamilton, C.E. Ndehedehe, V. Reis, J. Lu, M. Griffiths, G. Curwen, and M. Ronan. 2019. Tropical coastal wetlands ameliorate nitrogen export during floods. Frontiers in Marine Science 6: 671. https://doi.org/10.3389/fmars.2019.00671.
Alldred, M., S.B. Baines, and S. Findlay. 2016. Effects of invasive-plant management on nitrogen-removal services in freshwater tidal marshes. PLoS ONE 11 (2): e0149813. https://doi.org/10.1371/journal.pone.0149813.
Anderson, D.M. 2009. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean & Coastal Management 52: 342–347. https://doi.org/10.1016/j.ocecoaman.2009.04.006.
Anderson, D.M., P.M. Glibert, and J.M. Burkholder. 2002. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 25: 704–726. https://doi.org/10.1007/BF02804901.
Arkema, K.K., G. Guannel, G. Verutes, S.A. Wood, A. Guerry, M. Ruckelshaus, P. Kareiva, M. Lacayo, and J.M. Silver. 2013. Coastal habitats shield people and property from sea-level rise and storms. Nature Climate Change 3: 913–918. https://doi.org/10.1038/nclimate1944.
Asaeda, T., L.H. Nam, P. Hietz, N. Tanaka, and S. Karunaratne. 2002. Seasonal fluctuations in live and dead biomass of Phragmites australis as described by a growth and decomposition model: Implications of duration of aerobic conditions for litter mineralization and sedimentation. Aquatic Botany 73: 223–239. https://doi.org/10.1016/S0304-3770(02)00027-X.
Back, C.L., J.R. Holomuzki, D.M. Klarer, and R.S. Whyte. 2012. Herbiciding invasive reed: Indirect effects on habitat conditions and snail–algal assemblages one year post-application. Wetlands Ecology and Management 20: 419–431. https://doi.org/10.1007/s11273-012-9265-3.
Barry, A., S.K. Ooi, A.M. Helton, B. Steven, C.S. Elphick, and B.A. Lawrence. 2021. Vegetation zonation predicts soil carbon mineralization and microbial communities in southern New England salt marshes. Estuaries and Coasts 45: 168–180 (2022). https://doi.org/10.1007/s12237-021-00943-0.
Bart, D., D. Burdick, R. Chambers, and J.M. Hartman. 2006. Human facilitation of Phragmites australis invasions in tidal marshes: A review and synthesis. Wetlands Ecology and Management 14: 53–65. https://doi.org/10.1007/s11273-005-2566-z.
Bedford, A.P. 2005. Decomposition of Phragmites australis litter in seasonally flooded and exposed areas of a managed reedbed. Wetlands 25: 713–720. https://doi.org/10.1672/0277-5212(2005)025[0713:DOPALI]2.0.CO;2.
Boesch, D.F. 2002. Challenges and opportunities for science in reducing nutrient over-enrichment of coastal ecosystems. Estuaries 25: 886–900. https://doi.org/10.1007/BF02804914.
Bollmann, A., and R. Conrad. 1998. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biology 4: 387–396. https://doi.org/10.1046/j.1365-2486.1998.00161.x.
Burdick, D.M., and R.A. Konisky. 2003. Determinants of expansion for Phragmites australis, common reed, in natural and impacted coastal marshes. Estuaries 26: 407–416. https://doi.org/10.1007/BF02823717.
Burford, J.R., and J.M. Bremner. 1975. Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biology and Biochemistry 7: 389–394. https://doi.org/10.1016/0038-0717(75)90055-3.
Cao, Y., P.G. Green, and P.A. Holden. 2008. Microbial community composition and denitrifying enzyme activities in salt marsh sediments. Applied and Environmental Microbiology 74: 7585–7595. https://doi.org/10.1128/AEM.01221-08.
Chambers, R.M., L.A. Meyerson, and K. Saltonstall. 1999. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64: 261–273. https://doi.org/10.1016/S0304-3770(99)00055-8.
Conley, D.J., H.W. Paerl, R.W. Howarth, D.F. Boesch, S.P. Seitzinger, K.E. Havens, C. Lancelot, and G.E. Likens. 2009. Controlling eutrophication: Nitrogen and phosphorus. Science 323: 1014–1015. https://doi.org/10.1126/science.1167755.
Currin, C.A., S.C. Wainright, K.W. Able, M.P. Weinstein, and C.M. Fuller. 2003. Determination of food web support and trophic position of the mummichog, Fundulus heteroclitus, in New Jersey smooth cordgrass (Spartina alterniflora), common reed (Phragmites australis), and restored salt marshes. Estuaries 26: 495–510. https://doi.org/10.1007/BF02823726.
Dalsgaard, T., L.P. Nielsen, V. Brotas, P. Viaroli, J.C. Underwood, D.B. Nedwell. et al. (2000). “Sediment characteristics,” in Protocol Handbook for NICE: Nitrogen Cycling in Estuaries: A Project under the EU Research Programme: MARINE Science and Technology (MAST III), ed T. Dalsgaard (Silkeborg: National Environmental Research Institute), 53–54.
Davis, M.A., M.K. Chew, R.J. Hobbs, A.E. Lugo, J.J. Ewel, G.J. Vermeij, J.H. Brown, et al. 2011. Don’t judge species on their origins. Nature 474: 153–154. https://doi.org/10.1038/474153a.
de Groot, A.V., R.M. Veeneklaas, and J.P. Bakker. 2011. Sand in the salt marsh: Contribution of high-energy conditions to salt-marsh accretion. Marine Geology 282: 240–254. https://doi.org/10.1016/j.margeo.2011.03.002.
Deegan, L.A., D.S. Johnson, R.S. Warren, B.J. Peterson, J.W. Fleeger, S. Fagherazzi, and W.M. Wollheim. 2012. Coastal eutrophication as a driver of salt marsh loss. Nature 490: 388–392. https://doi.org/10.1038/nature11533.
Diaz, R.J., and R. Rosenberg. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929. https://doi.org/10.1126/science.1156401.
Elser, J.J., M.E.S. Bracken, E.E. Cleland, D.S. Gruner, W.S. Harpole, H. Hillebrand, J.T. Ngai, E.W. Seabloom, J.B. Shurin, and J.E. Smith. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x.
Eyre, B.D., D.T. Maher, and P. Squire. 2013. Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Global Biogeochemical Cycles 27: 1083–1095. https://doi.org/10.1002/2013GB004631.
Eyre, B., and A. Ferguson. 2002. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australian lagoons. Marine Ecology Progress Series 229: 43–59. https://doi.org/10.3354/meps229043.
Findlay, S., P. Groffman, and S. Dye. 2003. Effects of Phragmites australis removal on marsh nutrient cycling. Wetlands Ecology and Management 11: 157–165. https://doi.org/10.1023/A:1024255827418.
Fulweiler, R.W., and S.W. Nixon. 2009. Responses of benthic–pelagic coupling to climate change in a temperate estuary. Hydrobiologia 629: 147–156. https://doi.org/10.1007/s10750-009-9766-0.
Galloway, J.N., J.D. Aber, J.W. Erisman, S.P. Seitzinger, R.W. Howarth, E.B. Cowling, and B.J. Cosby. 2003. The nitrogen cascade. BioScience 53: 341. https://doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2.
Galloway, J.N., F.J. Dentener, D.G. Capone, E.W. Boyer, R.W. Howarth, S.P. Seitzinger, G.P. Asner, et al. 2004. Nitrogen cycles: Past, present, and future. Biogeochemistry 70: 153–226. https://doi.org/10.1007/s10533-004-0370-0.
Garner, M., and T. Allen. 2013. Synthesis of high and low marsh habitat mapping, vulnerability, and responses to sea-level rise in the Rachel Carson reserve. North Carolina Sea Grant. Final Report.
Granville, K.E., S.K. Ooi, L.E. Koenig, B.A. Lawrence, C.S. Elphick, and A.M. Helton. 2021. Seasonal patterns of denitrification and N2O production in a southern New England salt marsh. Wetlands 41: 7. https://doi.org/10.1007/s13157-021-01393-x.
Groffman, P.M., M.A. Altabet, J.K. Böhlke, K. Butterbach-Bahl, M.B. David, M.K. Firestone, A.E. Giblin, T.M. Kana, L.P. Nielsen, and M.A. Voytek. 2006. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecological Applications 16: 2091–2122. https://doi.org/10.1890/1051-0761(2006)016[2091:MFMDDA]2.0.CO;2.
Hazelton, E.L.G., T.J. Mozdzer, D.M. Burdick, K.M. Kettenring, and D.F. Whigham. 2014. Phragmites australis management in the United States: 40 years of methods and outcomes. AoB PLants 6. https://doi.org/10.1093/aobpla/plu001
Hershner, C., and K.J. Havens. 2008. Managing invasive aquatic plants in a changing system: Strategic consideration of ecosystem services. Conservation Biology 22: 544–550. https://doi.org/10.1111/j.1523-1739.2008.00957.x.
Howarth, R.W., and R. Marino. 2006. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnology and Oceanography 51: 364–376. https://doi.org/10.4319/lo.2006.51.1_part_2.0364.
Judd, K.E., and S.N. Francoeur. 2019. Short-term impacts of Phragmites management on nutrient budgets and plant communities in Great Lakes coastal freshwater marshes. Wetlands Ecology and Management 27: 55–74. https://doi.org/10.1007/s11273-018-9643-6.
Kana, T. M., C. Darkangelo, M. D. Hunt, J. B. Oldham, G. E. Bennett, and J.C. Cornwell. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry 66(23):4166–4170.
Kana, T.M., M.B. Sullivan, J.C. Cornwell, and K.M. Groxzkowski. 1998. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography 43: 334–339. https://doi.org/10.4319/lo.1998.43.2.0334.
Kaplan, W., I. Valiela, and J.M. Teal. 1979. Denitrification in a salt marsh ecosystem. Limnology and Oceanography 24: 726–734. https://doi.org/10.4319/lo.1979.24.4.0726.
Kiviat, E. 2013. Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB Plants 5: plt008. https://doi.org/10.1093/aobpla/plt008.
Knights, D., A.H. Sawyer, R.T. Barnes, C.T. Musial, and S. Bray. 2017. Tidal controls on riverbed denitrification along a tidal freshwater zone. Water Resources Research 53: 799–816. https://doi.org/10.1002/2016WR019405.
Kühl, H., P. Woitke, and J.-G. Kohl. 1997. Strategies of nitrogen cycling of Phragmites australis at two sites differing in nutrient availability. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 82: 57–66. https://doi.org/10.1002/iroh.19970820108.
Kuzyakov, Y., and X. Xu. 2013. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytologist 198: 656–669. https://doi.org/10.1111/nph.12235.
Li, N., M. Nie, B. Li, J. Wu, and J. Zhao. 2021. Contrasting effects of the aboveground litter of native Phragmites australis and invasive Spartina alterniflora on nitrification and denitrification. Science of the Total Environment 764: 144283. https://doi.org/10.1016/j.scitotenv.2020.144283.
Lissner, J., and H.-H. Schierup. 1997. Effects of salinity on the growth of Phragmites australis. Aquatic Botany 55: 247–260. https://doi.org/10.1016/S0304-3770(96)01085-6.
Lombard, K.B., D. Tomassi, and J. Ebersole. 2012. Long-term management of an invasive plant: Lessons from seven years of Phragmites australis control. Northeastern Naturalist 19: 181–193. https://doi.org/10.1656/045.019.s614.
Lorenzen, C.J. 1967. Determination of chlorophyll and pheopigments: Spectrophotometric equations. Limnology and Oceanography 12: 343–346. https://doi.org/10.4319/lo.1967.12.2.0343.
Mallin, M.A. 2000. Impacts of industrial animal production on rivers and estuaries. American Scientist 88: 26. https://doi.org/10.1511/2000.1.26.
Mallin, M.A., and C.A. Corbett. 2006. How hurricane attributes determine the extent of environmental effects: Multiple hurricanes and different coastal systems. Estuaries and Coasts 29: 1046–1061. https://doi.org/10.1007/BF02798667.
Mallin, M.A., M.H. Posey, G.C. Shank, M.R. McIver, S.H. Ensign, and T.D. Alphin. 1999. Hurricane effects on water quality and benthos in the Cape Fear watershed: Natural and anthropogenic impacts. Ecological Applications 9: 350–362. https://doi.org/10.1890/1051-0761(1999)009[0350:HEOWQA]2.0.CO;2.
Martin, L.J., and B. Blossey. 2013. The runaway weed: Costs and failures of Phragmites australis management in the USA. Estuaries and Coasts 36: 626–632. https://doi.org/10.1007/s12237-013-9593-4.
Marton, J.M., B.J. Roberts, A.E. Bernhard, and A.E. Giblin. 2015. Spatial and temporal variability of nitrification potential and ammonia-oxidizer abundances in Louisiana salt marshes. Estuaries and Coasts 38: 1824–1837. https://doi.org/10.1007/s12237-015-9943-5.
Mauchamp, A., and F. Mésleard. 2001. Salt tolerance in Phragmites australis populations from coastal Mediterranean marshes. Aquatic Botany 70: 39–52. https://doi.org/10.1016/S0304-3770(00)00140-6.
McClelland, J.W., and I. Valiela. 1998. Linking nitrogen in estuarine producers to land-derived sources. Limnology and Oceanography 43: 577–585. https://doi.org/10.4319/lo.1998.43.4.0577.
Mcleod, E., G.L. Chmura, S. Bouillon, R. Salm, M. Björk, C.M. Duarte, C.E. Lovelock, W.H. Schlesinger, and B.R. Silliman. 2011. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9: 552–560. https://doi.org/10.1890/110004.
Merrill, J.Z., and J.C. Cornwell. 2002. The role of oligohaline marshes in estuarine nutrient cycling. In Concepts and controversies in tidal marsh ecology, ed. M. P. Weinstein and D. A. Kreeger, 425–441. Dordrecht: Kluwer Academic Publishers. 425–441. https://doi.org/10.1007/0-306-47534-0_19.
Millennium Ecosystem Assessment (Program), ed. 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press.
Miller, W.D., L.W. Harding, and J.E. Adolf. 2006. Hurricane Isabel generated an unusual fall bloom in Chesapeake Bay. Geophysical Research Letters 33: L06612. https://doi.org/10.1029/2005GL025658.
Minchinton, T.E., and M.D. Bertness. 2003. Disturbance-mediated comepetition and the spread of Phragmites australis in a coastal marsh. Ecological Applications 13: 1400–1416. https://doi.org/10.1890/02-5136.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219. https://doi.org/10.1080/00785236.1995.10422044.
North Carolina Coastal Reserve, and J. Fear. 2009. Phragmites australis removal project at Currituck Banks. North Carolina Coastal Reserve. Final Report.
Paerl, H.W., J.R. Crosswell, B. Van Dam, N.S. Hall, K.L. Rossignol, C.L. Osburn, A.G. Hounshell, R.S. Sloup, and L.W. Harding. 2018. Two decades of tropical cyclone impacts on North Carolina’s estuarine carbon, nutrient and phytoplankton dynamics: Implications for biogeochemical cycling and water quality in a stormier world. Biogeochemistry 141: 307–332. https://doi.org/10.1007/s10533-018-0438-x.
Paerl, H.W., and T.G. Otten. 2013. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial Ecology 65: 995–1010. https://doi.org/10.1007/s00248-012-0159-y.
Paerl, R.W., R.E. Venezia, J.J. Sanchez, and H.W. Paerl. 2020. Picophytoplankton dynamics in a large temperate estuary and impacts of extreme storm events. Scientific Reports 10: 22026. https://doi.org/10.1038/s41598-020-79157-6.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2: art12. https://doi.org/10.1890/ES10-00082.1.
Quirion, B., Z. Simek, A. Dávalos, and B. Blossey. 2018. Management of invasive Phragmites australis in the Adirondacks: A cautionary tale about prospects of eradication. Biological Invasions 20: 59–73. https://doi.org/10.1007/s10530-017-1513-2.
Ramus, A.P., B.R. Silliman, M.S. Thomsen, and Z.T. Long. 2017. An invasive foundation species enhances multifunctionality in a coastal ecosystem. Proceedings of the National Academy of Sciences 114: 8580–8585. https://doi.org/10.1073/pnas.1700353114.
Rooth, J.E., and J.C. Stevenson. 2000. Sediment deposition patterns in Phragmites australis communities: Implications for coastal areas threatened by rising sea-level. Wetlands Ecology and Management 8: 173–183. https://doi.org/10.1023/A:1008444502859.
Rooth, J.E., J.C. Stevenson, and J.C. Cornwell. 2003. Increased sediment accretion rates following invasion by Phragmites australis: The role of litter. Estuaries 26: 475–483. https://doi.org/10.1007/BF02823724.
Sainsbury, N.C., M.J. Genner, G.R. Saville, J.K. Pinnegar, C.K. O’Neill, S.D. Simpson, and R.A. Turner. 2018. Changing storminess and global capture fisheries. Nature Climate Change 8: 655–659. https://doi.org/10.1038/s41558-018-0206-x.
Sala, O. E., F.S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sandwald, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. https://doi.org/10.1126/science.287.5459.1770.
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences 99: 2445–2449. https://doi.org/10.1073/pnas.032477999.
Seitzinger, S., J.A. Harrison, J.K. Böhlke, A.F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G.V. Drecht. 2006. Denitrification across landscapes and waterscapes: A synthesis. Ecological Applications 16: 2064–2090. https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2.
Sheng, Y.P., A.A. Rivera-Nieves, R. Zou, V.A. Paramygin, C. Angelini, and S.J. Sharp. 2021. Invasive Phragmites provides superior wave and surge damage protection relative to native plants during storms. Environmental Research Letters 16: 054008. https://doi.org/10.1088/1748-9326/abf288.
Silliman, B.R., and M.D. Bertness. 2004. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conservation Biology 18: 1424–1434. https://doi.org/10.1111/j.1523-1739.2004.00112.x.
Smith, C.S., B. Puckett, R.K. Gittman, and C.H. Peterson. 2018. Living shorelines enhanced the resilience of saltmarshes to Hurricane Matthew (2016). Ecological Applications 28: 871–877. https://doi.org/10.1002/eap.1722.
Smith, R.L., and J.H. Duff. 1988. Denitrification in a sand and gravel aquifer. Applied and Environmental Microbiology 54: 1071–1078. https://doi.org/10.1128/aem.54.5.1071-1078.1988.
Soana, E., A. Gavioli, F. Vincenzi, E.A. Fano, and G. Castaldelli. 2020. Nitrate availability affects denitrification in Phragmites australis sediments. Journal of Environmental Quality 49: 194–209. https://doi.org/10.1002/jeq2.20000.
Sullivan, M.J., and C.A. Currin. 2002. Community structure and functional dynamics of benthic microalgae in salt marshes. In Concepts and controversies in tidal marsh ecology, ed. Weinstein, M.P. and Kreeger, D.A., 81–106. Dordrecht: Kluwer Academic Publishers. https://doi.org/10.1007/0-306-47534-0_6.
Sundareshwar, P. V., J. T. Morris, E. K. Koepfler, and B. Fornwalt. 2003. Phosphorus limitation of coastal ecosystem processes. Science 29(5606):563–565. https://doi.org/10.1126/science.1079100.
Theuerkauf, S.J., B.J. Puckett, K.W. Theuerkauf, E.J. Theuerkauf, and D.B. Eggleston. 2017. Density-dependent role of an invasive marsh grass, Phragmites australis, on ecosystem service provision. PLoS ONE 12: e0173007. https://doi.org/10.1371/journal.pone.0173007.
USDA. 2012. North Carolina census publications. Retrieved from USDA Census of Agriculture Website. https://www.nass.usda.g.
Valiela, I., and M.L. Cole. 2002. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5: 92–102. https://doi.org/10.1007/s10021-001-0058-4.
Van de Broek, M., S. Temmerman, R. Merckx, and G. Govers. 2016. Controls on soil organic carbon stocks in tidal marshes along an estuarine salinity gradient. Biogeosciences 13: 6611–6624. https://doi.org/10.5194/bg-13-6611-2016.
Vasquez, E.A., E.P. Glenn, J.J. Brown, G.R. Guntenspergen, and S.G. Nelson. 2005. Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Marine Ecology Progress Series 298: 1–8. https://doi.org/10.3354/meps298001.
Vitousek, P.M., C.M. D’Antonio, L.L. Loope, and R. Westbrooks. 1996. Biological invasions as global environmental change. American scientist v. 84: 468–478. 61. PubAg.
Windham, L., and R.G. Lathrop. 1999. Effects of Phragmites australis (common reed) invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22: 927. https://doi.org/10.2307/1353072.
Windham, L., and L.A. Meyerson. 2003. Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes of the northeastern U.S. Estuaries 26: 452–464. https://doi.org/10.1007/BF02823722.
World Resources Institute. 2008. Eutrophication and hypoxia in coastal areas: A global assessment of the state of knowledge. Washington, DC: World Resources Institute.
Zhang, J.-Z., C.R. Kelble, C.J. Fischer, and L. Moore. 2009. Hurricane Katrina induced nutrient runoff from an agricultural area to coastal waters in Biscayne Bay, Florida. Estuarine, Coastal and Shelf Science 84: 209–218. https://doi.org/10.1016/j.ecss.2009.06.026.
Acknowledgements
This research was supported by funding from the North Carolina Sea Grant and the Albemarle Pamlico National Estuary Partnership (project R/MG-1711). We thank the following members of the Piehler Lab at the UNC Institute of Marine Sciences for field and laboratory assistance: O. Torano, A. Gold, P. Mullin, J. Gould, L. Arroyo and C. Brown. The authors would also like to thank C. Currin and three anonymous reviewers for their insightful comments that significantly enhanced this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Scott Warren
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. 1
Schematic of sampling design (EPS 608 KB)
Rights and permissions
About this article
Cite this article
Yacano, M.R., Thompson, S.P. & Piehler, M.F. Non-Native Marsh Grass (Phragmites australis) Enhances Both Storm and Ambient Nitrogen Removal Capacity in Marine Systems. Estuaries and Coasts 45, 2012–2025 (2022). https://doi.org/10.1007/s12237-022-01062-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01062-0