Abstract
Anaerobic ammonium oxidation (anammox) is an important pathway for the removal of fixed nitrogen from aquatic and terrestrial ecosystems. Previous studies on anammox were focused on the surface sediments in estuaries, but the activity and community composition of anammox bacteria in the estuarine subsurface sediments remained unknown. In this study, we used high-throughput sequencing of 16S rRNA gene combined with 15N isotope tracing method to investigate the activity, diversity, and spatio-temporal distribution of anammox bacteria in sediment cores of the Pearl River Estuary (PRE). Our results indicated that anammox in the subsurface sediments has significant potential activity, contributing to approximately 17.49% of the total microbial nitrogen loss. A variety of anammox bacteria, including Candidatus Scalindua, Ca. Brocadia, Ca. Jettenia, and Ca. Kuenenia, were all detected in the subsurface sediments. Moreover, the anammox bacterial community had a significant specific geographic distribution but no obvious difference along the sediment depth. Multiple environmental factors including salinity, and NH4+ and NO3− contents, synergistically shaped the diversity and distribution of anammox bacteria in PRE sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Pearl River Estuary (PRE) is one of the most complex estuarine systems in the world, forming a transition zone between the Pearl River and the South China Sea. Recently, rapid industrialization and urbanization of the Pearl River Delta region has accelerated the discharges of anthropogenic pollutants, such as domestic industrial wastewater discharge, fisheries, agricultural activities, and so on, leading to a high load of dissolved inorganic nitrogen (DIN) and phosphate (PO43−) in PRE (Cai et al. 2004; Chai et al. 2006; Hong and Shen 2012; Huang et al. 2003). Increasing nutrients loading broke the ecology balance of biogeochemical cycle and caused a series of serious environmental problems, such as widespread eutrophication, algal blooms, and hypoxia (Liu et al. 2014; Dai et al. 2006).
Over the past several decades, heterotrophic denitrification has been known as the only pathway for nitrogen removal (Zumft 1997). Discovery of anaerobic ammonium oxidation (anammox) in natural ecosystems as a pathway for the permanent removal of reactive nitrogen and reduction of greenhouse gases (N2O and CO2), leading to the production of dinitrogen gas (N2), has extended our understanding of the microbial diversity in the global nitrogen cycle (Dalsgaard et al. 2003; Dalsgaard et al. 2005; Kuypers et al. 2003; Mulder et al. 1995; Thamdrup and Dalsgaard 2002). Anammox bacteria has a wide occurrence in oxygen-depleted estuarine and marine sediments (Penton et al. 2006; Risgaard-Petersen 2004; Thamdrup and Dalsgaard 2002; Wu et al. 2019; Zhang et al. 2007), marine oxygen minimum zones (Dalsgaard et al. 2003; Kuypers et al. 2003; Lam et al. 2007), wastewater (Kartal et al. 2008; Quan et al. 2008; Schmid et al. 2003), multiyear sea ice (Rysgaard et al. 2004), agricultural soils (Zhu et al. 2011; Shen et al. 2015; Wu et al. 2018), and in geothermal subterranean oil reservoirs (Li et al. 2010) and black smokers (Byrne et al. 2009). They contribute significantly to total N2 production in various environments, for example 50% in ocean (Arrigo 2005; Brandes et al. 2010) and ranging from 0 to 80% in estuarine and marine sediments (Engström et al. 2005; Engström et al. 2009; Thamdrup and Dalsgaard 2002). At present, known anammox bacteria include 5 genera and 16 species (belong to phylum Planctomycetes) (Sonthiphand et al. 2014) with the dominant species in different ecosystems being variable. Extensive research has confirmed that the Ca. Scalindua dominates in marine and pristine environments while Ca. Brocadia possesses better adaptability in terrestrial ecosystems (Cao et al. 2011; Dale et al. 2009; Kartal et al. 2008; Li et al. 2011; Schmid et al. 2007; Wu et al. 2018).
Estuaries are the ecotones between riverine and marine ecosystems and are characterized by complex natural and anthropogenic environments (Dai et al. 2006), providing habitats for a large number of organisms and playing a significant role in regulating biogeochemical cycles and ecological processes. The PRE receives excessive anthropogenic nitrogen input, which has strengthened the microbial nitrogen removal process in this area (Hong et al. 2015, 2019). We hypothesize that anammox plays an important role in estuarine nitrogen loss in the subsurface sediments. However, previous studies have identified a diverse community of anammox bacteria in estuarine waters through constructing clone libraries of 16S rRNA and hzo genes and quantitative PCR (qPCR) technology (Fu et al. 2015; Liu et al. 2014). Similarly, Ye et al. (2016) investigated the microbial diversity, distribution, and community structure in the surface sediments of the Changjiang Estuary. These studies mainly focused on the estuarine surface sediments, and thus, knowledge of the activity, and community structure of anammox bacteria in the subsurface estuarine sediments remained limited. Herein, we present the activity and spatio-temporal distribution of anammox bacteria in sediment cores collected from upstream to downstream of PRE. The objective of this study was (1) to measure potential activity of anammox and denitrification in the sediments with 15NO3− incubation; (2) to investigate the abundance and community diversity of anammox bacteria in the sediment cores of the estuary with high-throughput sequencing analysis; and (3) to analyze the relationship between the diversity of anammox bacteria and environmental factors.
Materia and Methods
Site Description and Sediment Sample Collection
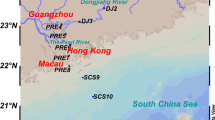
PRE is a typical subtropical estuary (113.5 °E–114 °E, 22 °N–23 °N), bound by the South China Sea (Dai et al. 2013). It is formed by the confluence of the Beijiang, Xijiang, and Dongjiang rivers. These rivers discharge an average of 2.6 million gallons (10 million liters) of water per second into the South China Sea (Guo et al. 2009). It drains water from some of the most densely populated areas of China, and therefore is severely polluted with sewage and industrial waste. About 617 million tons (560 million metric tons) of domestic waste and 2.2 billion tons (2 billion metric tons) of industrial effluents enter the estuary each year (Guo et al. 2009). There is a strong seasonal shift in physicochemical processes in the PRE due to monsoon in summer-winter alternation and significant seasonal variations in river discharge (Lu and Gan 2015). Therefore, high nitrogen loading resulting from anthropogenic activities allows for a comprehensive evaluation of the nitrogen cycle in the sediments of the PRE.
Sediment samples were collected using a gravity stainless steel sediment core sampler with a polyvinyl chloride (PVC) tube (KC-Denmark), at five sites from PRE-1 to PRE-18 (Fig. 1), during a summer and a winter cruise in August 2016 and January 2017, respectively. The lengths of the five sediment cores were 60, 52, 96, 56, and 64 cm, respectively. These were sliced at 4-cm intervals from the surface to the bottom, and a 4-cm layer in the surface, middle, and bottom cores were selected for diversity analysis of the anammox bacteria. One fraction of each sample was immediately placed in sealed polyethylene bags and stored at − 20 °C for DNA extraction and PCR amplification. The other fraction was placed at 4 °C for slurry experiments to determine potential nitrogen loss rates. The sediment pore waters were extracted by centrifugation at 5,000 rpm for 20 min (Eppendorf 5804R), followed by filtration with a 0.22-μm membrane for the analysis of physicochemical parameters (Wu et al. 2019).
Analysis of Physicochemical Parameters
Physiochemical parameters of estuarine water, including dissolved oxygen (DO), salinity, pH, depth, and temperature, were determined in situ with a multi-parameter water quality analyzer (YSI 6600, USA). The oxidation-reduction potential (ORP) of sediments was measured in situ with an ORP meter (Mettler-Toledo, Switzerland). Concentrations of NH4+, NO2−, and NO3− in pore water were determined by flow injection analysis and spectrometric detection (Seal QuAAtro Analyzer, Germany). Total nitrogen (Ntot) and organic carbon (Corg) analyses were conducted with an elemental analyzer (Flash 2000, Thermal Fisher Scientific). The samples for Corg were acidified with 1.5 mol L−1 HCl to remove inorganic carbon, and Ntot was freeze-dried and homogenized without acidification, to avoid the loss of organic nitrogen (Norg). Here, Norg was estimated to be the difference between Ntot and the sum of dissolved inorganic nitrogen (Nex), using the formula Norg = Ntot−Nex.
Isotope Tracer Experiments
15N-isotope tracing method was used to measure the spatial and temporal activity of denitrification and anammox in the sediments from PRE, following protocols in previous studies (Brin et al. 2014; Hou et al. 2013; Lin et al. 2017a, b). In short, slurry was made at a sediment/benthic water ratio of 1:7 (w/v) in a 500-mL plastic bottle (Nalgene). The mixed slurry was then transferred into a 12.5-ml vial (Labco) and was sealed with rubber stoppers after purging with helium for 25 min and uniform stirring. To consume the residual NO3−, the vials were pre-incubated in the dark for 36 h at a temperature close to the in situ temperature. Subsequently, these vials with slurries were divided into three groups, which were spiked through the septum with helium-purged stock solutions of (1) 15NH4+ (15N at 99.6%), (2) 15NH4++14NO3−, and (3) 15NO3− (15N at 99%), respectively. The final concentration of 15N in the vial was about 100 μmol L−1. After incubation for 8 h, the mixture was poisoned by injecting 200 μL of 50% ZnCl2 solution into each vial to stop the biological activity and subsequently kept at room temperature in the dark until detection with a Membrane Inlet Mass Spectrometer (MIMS, HPR-40, US) (Hou et al. 2013). The potential rates of anammox and denitrification can be calculated from the quantities of 29N2 and 30N2 detected by MIMS, according to the method previously described (Hou et al. 2012; Risgaard-Petersen et al. 2003; Xiao et al. 2018).
DNA Extraction, PCR Amplification, Illumina-Based 16S rRNA Sequencing and q-PCR Analysis
About 0.3 g (wet weight) sediment from each sample was used for DNA extraction using the PowerSoil DNA Isolation Kit (MO BIO Laboratories, Inc., USA). Purified DNA was dissolved in 50 μL ddH2O and stored at − 20 °C. Anammox bacterial 16S rRNA was amplified using primers 438F (5′-GTCRGGAGTTADGAAATG-3′)/684R (5′-ACCAGAAGTTCCACTCTC-3′), which performed good coverage for the community structure of anammox bacteria from different ecosystems (Humbert et al. 2012; Wu et al. 2019). Barcode and adaptor sequences were designed and ligated to the 5′-end of the sequencing primers for multiplexing of the samples in the pyrosequencing runs. The PCR reaction mixtures contained the following components: 12.5 μL PCR mix (Promega), 1 μL primers, 1 μL DNA, and 9.5 μL H2O. The PCR protocol was as follows: initial denaturation at 95 °C for 3 min, 35 cycles at 94 °C for 45 s, and 49 °C for 45 s, and a final extension at 72 °C for 10 min. The PCR products were purified using the TaKaRa MiniBEST Agarose Gel DNA Extraction Kit (TaKaRa) following the manufacturer’s instructions, and concentration was measured using the NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Willing Minton, USA). The PCR products were subjected to Illumina MiSeq sequencing. All the anammox-specific 16S rRNA gene sequences were analyzed using Mothur (V.1.35.1), referring to standard operating procedures (https://mothur.org/wiki/MiSeq_SOP) (Schloss et al. 2009). A detailed description of the Mothur software procedure is provided in the Supplementary Information. The selected sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity level. Twenty-one OTUs were selected as the dominant OTUs (> 1% of total sequences in each community, covering 64.83% of the sequences). The phylogenetic tree of the main OTUs was built with the representative sequences of the main OTUs combined with the reference sequences of identified anammox bacteria, using MEGA 7.0 neighbor-joining method.
To quantify anammox bacteria in sediments, q-PCR assays were performed on a LightCycler 480 II Real-Time PCR system (Roche) using SYBR Green as a fluorescent dye (Promega, Madison, Wisconsin, USA) with set A438f/A684r targeting 16S rRNA gene of anammox bacteria as description in previous study (Wu et al. 2019) .
Statistical Analyses
To determine the composition of anammox bacterial assemblages, principal coordinate analysis (PCA) was performed with SigmaPlot (v12.5). Relationships between anammox bacterial community and environmental factors were explored by canonical correspondence analysis (CCA), using CANOCO (v5.0). In addition, one-way analysis of variance, followed by Tukey’s HSD test, was also conducted to compare the physicochemical properties and microbial characteristics.
Results
Physicochemical Characteristic of Sediments
The ORP, salinity, Ntotal, NO3−, NO2−, NH4+, Norg, Corg, and Norg:Corg values in sediment samples are shown in Table 1. Obvious salinity gradient characteristics were observed from upstream to downstream of PRE, ranging from 0 to approximately 30 psu. ORP remained negative throughout the sediment cores, reflecting the anaerobic status of the sediments. The Ntotal was the highest in the surface sediments for all of the cores except PRE-13 and decreased sharply in the middle part of PRE-1 (from 1169.16 to 962.13 mg·kg−1), PRE-3 (from 1042.43 to 875.92 mg·kg−1), and decreased slightly at PRE-7 and PRE-18. The distribution trend of Norg and Corg was similar to that of Ntotal. However, there were some differences in Norg:Corg between different samples, ranging from 10.88 to 14.13, with the highest value in the bottom sample of PRE-18 (14.05). The ratio in surface samples was lower than that in the bottom sample for all of the cores except PRE-7. The exchangeable nitrogen (NO3−, NO2−, and NH4+) values were significantly lower than the Ntotal (p < 0.01) and gradually decreased from upstream to downstream in an overall trend. The concentrations of NO2− and NO3− were relatively higher in the surface sediment than in the deep zones, but the concentrations of NH4+ showed a reverse trend. Samples from PRE-1 were significantly different from other samples as the chemical parameters fluctuated significantly. The Ntotal concentration was extremely high in the bottom at 1809.25 mg kg−1. NO3− was 34.76 mg kg−1 in the surface, 27.23 mg kg−1 in the middle, and declined to 2.36 mg kg−1 in the bottom. NH4+ increased with depth, reaching 134.91 mg kg−1 at the bottom, which is much higher than that in other samples (13.97 ± 9.82 mg kg−1, n = 14).
Potential Activity of Anammox and Denitrification in the Sediments
15N-isotope tracing incubation method showed remarkable spatio-temporal variation of the N loss rates in the sediment cores by anammox and denitrification (Fig. 2). We found denitrification exceeded anammox and dominated the nitrogen loss processes. Furthermore, the denitrification rate in each site was higher in summer than in winter, except in PRE-18. In summer, anammox and denitrification activity ranged from 0 to 1.10 ± 0.03 μmol N kg−1 h−1 and 0.57 ± 0.03 to 7.14 ± 0.20 μmol N kg−1 h−1, respectively (Fig. 2a). In winter, anammox rates could be detected in most samples and ranged from 0 to 0.73 ± 0.04 μmol N kg−1 h−1, with denitrification rates decreasing sharply in PRE-13 and PRE-18 (Fig. 2b). Notably, anammox rates were higher in the winter (0.37 μmol N kg−1 h−1) than in summer (0.17 μmol N kg−1 h−1). Based on the denitrification and anammox rates, the relative contributions of these two processes to nitrogen loss were estimated. The contribution of anammox to nitrogen loss ranged from 0 to 65.82% and 0 to 72.52% in summer and winter, respectively. In addition, the anammox contributions to N2 production in surface, middle, and bottom sediments were 18.17 ± 21.59% (n = 10), 15.44 ± 22.69% (n = 10), and 18.85 ± 21.08% (n = 10), respectively (Fig. 2c).
Vertical distribution of denitrification and anammox characteristics in sediment cores of PRE. Potential rates (red and blue bars) and N loss contribution (green dot) of denitrification and anammox in the sediments during summer (a) and winter (b). Samples collected in the summer are labeled “S” and “W” in the winter. The letters “S,” “M,” and “B” after numbers represented the samples collected from surface, middle, and bottom sediments, respectively. c The N loss contribution of anammox in different layers of sediment cores (surface, middle, or bottom sediments). In the box plot, the middle line represents the median value, and the vertical lines represent the maximum and minimum values. Vertical bars denote standard error of triplicate samples
The Abundance of Anammox Bacteria in the Sediments
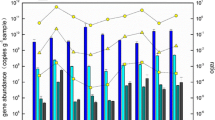
As shown in Fig. 3, the abundances of anammox bacteria in sediments had significant spatial and temporal variation (p < 0.05). In spatial, the abundances in the sediments of upper and middle stream were higher than those in downstream. At stations PRE1, PRE3, and PRE7, the average abundance in summer was 1.27 × 106 copies g−1, and the average abundance was 4.01 × 105 copies g−1 in winter. So, the abundance of anammox bacteria in summer was significantly higher than that in sediments of winter in the stations PRE 1, PRE 3, and PRE 7 (p < 0.01). However, in the PRE 13 and PRE 18 of downstream, the average abundance of anammox bacteria in summer was 2.17 × 105 copies g−1, and in winter it is 3.03 × 105 copies g-1. The abundance in winter was slightly higher than that in summer at downstream. The abundance of anammox bacteria varied insignificantly along the sediment depth.
The abundance of anammox-specific 16S rRNA gene in sediments of PRE. a The samples were collected in summer. b The samples were collected in winter. Samples collected in the summer are labeled “S” and “W” in the winter. The letters “S,” “M,” and “B” after numbers represented the samples collected from surface, middle, and bottom sediments, respectively. Vertical bars denote standard error of triplicate samples
Diversity of Anammox Bacteria in the Sediments
A total of 123,258 reads were obtained from the 30 samples collected from sediments of PRE. High-quality sequences were obtained ranging from 1,565 to 4,579 sequences per sample (at an average of 4,108). The sequences were clustered at 97% similarity by Mothur and OTUs generated, ranging from 57 to 197 in different samples (Table 2). Higher than 96% coverage implied that the OTUs of each anammox bacterial library had been well captured.
To estimate the community diversity and richness of anammox bacteria in the sediment of PRE, the alpha diversity indices, viz. Chao1, ACE, Shannon, Simpson, and Coverage indices, were calculated (Table 2). The ACE and Chao1 diversity estimators for anammox bacteria ranged from 75.55 to 311.06 and 71.87 to 219.50, respectively. ACE estimators were remarkably higher than that of Chao1 in most samples, suggesting greater species richness in the sediments. This estimation was verified by rarefaction curves, showing no plateaus in most samples (Fig. S1), implying much higher diversity than measured in the anammox bacteria 16S rRNA gene reads. However, there was no obvious change in both Chao1 and ACE indices between summer and winter, suggesting that the diversity of anammox bacteria in PRE had no remarkable seasonal shift. The Shannon richness ranged from 0.4 to 3.56. The evenness of anammox bacteria fluctuated around 0.5, suggesting that anammox bacterial community was evenly distributed in the sediments.
Community Composition and Spatial Distribution of Anammox Bacteria
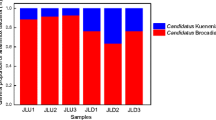
Dominant OTUs accounting for 64.83% of the reads in the samples belonged to Ca. Scalindua (27.15%), Ca. Brocadia (19.60%), Ca. Kuenenia (8.11%), Ca. Jettenia (0.01%), Ca. Anammoxoglobus (0.01%), and unclassified genera (45.12%) (Fig. 4). Along the sediment depth, the community composition of anammox bacteria showed no significant difference in summer or winter. On the contrary, the significant differences of the anammox bacterial composition were observed from upstream to downstream. Ca. Brocadia was dominant in the upstream sediments and decreased gradually from upstream to downstream. Ca. Brocadia cannot be detected in the sediments of PRE-18. Contrarily, Ca. Scalindua was the major group in the downstream sediments and decreased gradually from downstream to upstream. Ca. Brocadia, Ca. Kuenenia, and unclassified genera were dominant in the sediments of PRE-1. However, in winter, Ca. Kuenenia had a relatively higher abundance than in summer. In the sediment samples of PRE-7, Ca. Scalindua accounted for a higher proportion in winter (more than 50%) than in summer.
A heat map based on OTU level was present to show the composition of anammox bacteria (Fig. 5). There was a slight variation between summer and winter but a significant spatial shift was observed from upstream to downstream. Phylogenetic relationship among dominant OTUs of anammox-specific 16S rRNA gene is shown in Fig. S2. OTU1, OTU15, OTU7, OTU19, OTU3, and OTU20 affiliated to the Ca. Brocadia, which were mainly distributed in the up- and middle stream. Only OTU6 belonged to the Ca. Kuenenia, which was also mainly distributed in the up- and middle stream. OTU14, closely related to Ca. Jettenia, had relatively high abundance in the sediments of PRE-1 from winter. Other dominant OTUs were affiliated to Ca. Scalindua and appeared in the middle- and downstream. Therefore, the richest diversity of anammox bacteria was in the mixed zone of freshwater and seawater at middle stream.
PCA analysis present conclusive evidence for geographically specific distribution of anammox bacteria in the sediment cores of PRE (Fig. 6). The plots of the first two principal coordinate axes (P1 and P2) showed 44.41% variability in anammox bacterial community, among all the samples. These results showed that anammox bacterial assemblage could be classified into two groups in summer. PRE-1, PRE-3, and PRE-7 could be grouped together, while PRE-13 and PRE-18 belonged to the other group. In winter, anammox bacterial assemblage could be classified into four groups, PRE-13 and PRE-18 being grouped and other three stations separated from each other.
Correlation Between Anammox Bacterial Diversity and Chemical Variables
The correlation between anammox bacterial diversity and chemical parameters in sediment cores of PRE was revealed by CCA analysis (Fig. S3). The first two CCA axes showed 43.83% anammox bacterial variation. Of all the chemical parameters, exchangeable ammonium (Nex-NH4+, P = 0.012, F = 2.11, 1000 Monte Carlo permutations) and nitrate (Nex-NO3−, P = 0.026, F = 1.34, 1000 Monte Carlo permutations) were the key factors in shaping the community structure of anammox bacteria. The signs of blue triangular (OTU3, OTU5, OTU9, OTU10, OTU11, OTU13, and OTU18) were close related to Ca. Scalindua that was rich in the downstream (PRE-13 and PRE-18). The signs of green triangular (OTU1, OTU3, OTU7, OTU15, OTU19, and OTU20) were affiliated to Ca. Brocadia and the sign of purple triangular (OTU6) belonged to Ca. Kuenenia.
Discussion
Contribution to Nitrogen Loss by Anammox in the Estuarine Sediment Cores
Many studies so far have estimated the potential activities of anammox in the estuarine and coastal sediments (Engström et al. 2005; Hou et al. 2013; Teixeira et al. 2012; Thamdrup and Dalsgaard 2002). However, these mainly focused on surface sediments, with knowledge about anammox in the subsurface sediments remaining largely unknown.
The physicochemical parameters in the sediment cores of PRE were investigated. According to the chemical analysis, the environment characteristic of the subsurface sediments was sufficiently anaerobic for the anammox process, with the ORP values being below − 69.80 mV. Furthermore, the dissolved inorganic nitrogen (NO3−, NO2−, and NH4+) can provide substrates like NH4+ that act as an electron donor, with NO2− or NO3− acting as electron acceptors for their catabolism, respectively (Kartal et al. 2013; Walter et al. 1999). Moreover, active denitrification in the sediments would utilize organic matter to generate NO2− through reduction of NO3−, providing more substrates for the anammox bacteria (Yang et al. 2015).
In order to provide insights into the activity of anammox in the subsurface estuarine sediments, we determined the denitrification and anammox rates simultaneously with 15N isotopic tracer methods. Our results showed that the potential denitrification was much higher than anammox. The anammox contributed 8.62% and 26.35% to N loss in summer and winter, respectively. In line with previous reports, denitrification was the main process for nitrogen loss in many ecosystems (Engström et al. 2005; Engström et al. 2009; Lin et al. 2017a, b; Shen et al. 2014; Teixeira et al. 2012; Yang et al. 2015; Zhu et al. 2013; Zhu et al. 2011). Although the proportional contribution of anammox is much less relative to denitrification, the annual flux of nitrogen loss for anammox makes a significant contribution from a macro perspective. Wang et al. (2012) found that N loss via anammox was about 3.0 and 42.3 t N year−1 in surface sediments of Pearl River Estuary in summer and winter, respectively. Anammox possess a high potential to remove the buried or transported nitrogen that cannot be fully removed in the surface sediments, maintaining a low level of DIN in the bottom sediment. These results suggested that anammox in the subsurface sediments played an important role in the nitrogen loss and should not be neglected to estimate the total flux of nitrogen sink.
The anammox rates in the sediment cores of PRE were in line with the values reported in the surface sediments of Yangtze estuary (Zheng et al. 2016) and New River Estuary (Lisa et al. 2014). Furthermore, we found that there was remarkably higher activity of anammox in the subsurface sediments compared to that in the surface (Fig. 2c). This may be regulated by the organic matter as denitrification is a heterotrophic process dependent on consumption of organic nutrients, while anammox is an autotrophic process (Strous et al. 2006; Zumft 1997). In the sediment cores, Norg was the dominant component of Ntot, accounting for more than 80%. Rates of sedimentation and organic carbon decomposition were extremely high in the estuary (Hong et al. 2019), resulting in an anoxic environment below a depth of a few centimeters or less. Labile organic matter would be rich in the surface sediments and the deposited content in the sediment should comprise of recalcitrant humic substances. Thus, lack of labile organic matter would weaken denitrification and strengthen anammox. In line with the spatiotemporal variations of anammox rates in riparian sediments of PRE, the activities and contribution of anammox were higher than that in summer in sediment cores of PRE (Wang et al. 2012). The hydrological condition and background nutrients of the two seasons are different. Anammox bacteria process better adaptability to temperature variety (Rysgaard and Glud 2004). Compared with temperature, the concentration of DIN was an important factor in affecting the anammox process (Hou et al. 2013; Meyer et al. 2005; Naeher et al. 2015; Trimmer et al. 2005). Higher DIN contents of PRE in winter were also evidenced by Wang et al. (2012). Lower river runoff and inadequate hydrodynamic mixing leads to longer retention time in winter, and more amounts of DIN can provide substrates like NH4+ and NOx− for anammox bacterial catabolism and enhance the anammox rates (Hong et al. 2015).
The potential anammox rates in sediments were measured as described in Risgaard-Petersen et al. (2003). Following addition of 15NO3−, anammox process will only produce two isotopic N2 species (28N2 and 29N2), while denitrification will produce three isotopic N2 species (28N2, 29N2, and 30N2). The potential rate and contribution to N2 production of anammox and denitrification were calculated using the produced 29N2 and 30N2, as described by Thamdrup and Dalsgaard (2002). Up to now, this isotope pairing technique (15NO3− as the tracer) has been used as the widely accepted method to estimate the contribution of anammox and denitrification processes in various environmental samples where anammox and denitrification coexist, such as marine sediments and anoxic water (Dalsgaard et al. 2003, 2012; Thamdrup and Dalsgaard 2002), estuarine and coastal sediments (Wang et al. 2012; Zheng et al. 2016; Song et al. 2013; Lin et al. 2017a, b), river network (Lin et al. 2017a, b; Zheng et al. 2019), soils (Zhu et al. 2011; Shan et al. 2016; Zhu et al. 2019), and constructed wetland (Tan et al. 2017; Wang et al. 2018). It is noted that the potential anammox rates are probably underestimated because using 15NO3− as the tracer may give denitrifiers an advantage (as anammox prefer 15NO2−). NO3− reduction in anammox is not very efficient, and anammox may therefore rely on the first step of denitrification to provide NO2− as a substrate (Lam et al. 2007). So using 15NO2− as the tracer was also employed to estimate the anammox rates in previous studies (Kim et al. 2016; Plummer et al. 2015; Shen et al. 2014). However, the final injection concentrations of 15NO2− (25–200 μM) are significantly higher than those in natural estuarine environments (Table 1), which will overestimate the anammox rates. Shen et al. (2014) found that 15NO2− labeled method will overestimate the rates of the anammox in the layers with low NOx− concentration. Song and Tobias (2011) suggested that all substrate amendment techniques (15NO3−/15NO2− + 14NH4+) will enhance or inhibit rates of anammox and denitrification. So rates using this isotopic approach should be considered “potential rate,” a measure of metabolic readiness for activity under favorable conditions.
Community Structure of Anammox Bacteria in the Sediment Cores of PRE
In this study, we investigated the anammox bacteria in the sediments along the depth, presenting a comprehensive spatio-temporal distribution in sediment core of PRE. Our results indicate that anammox bacteria exist in deep estuarine sediments and contribute to estuarine nitrogen loss.
To study the diversity and community structure of anammox bacteria in the sediment cores, we used high-throughput sequencing of the 16S rRNA gene. Anammox bacteria have been detected in multiple types of environments in previous studies, providing information regarding community structure and distribution in different ecosystems (Caporaso et al. 2012; Lee et al. 2011; Qian et al. 2010; Shen et al. 2016; Vila-Costa et al. 2013; Zhao et al. 2013). However, previous studies were mostly performed on the basis of clone library analysis with some specific primers (Fu et al. 2015; Schmid et al. 2000), which limited deep analysis of diversity. Fu et al. (2015) found that dominant genus of anammox bacteria shifted from Ca. Brocadia or Ca. Anammoxoglobus to Ca. Scalindua along the salinity gradient in surface sediments of PRE. In this study, 317 OTUs were found in 30 samples, affiliated to Ca. Scalindua (27.15%), Ca. Brocadia (19.60%), Ca. Kuenenia (8.11%), Ca. Jettenia (0.01%), Ca. Anammoxoglobus (0.01%), and unclassified genera (45.12%) (Fig. 4). Our results showed that Ca. Brocadia was the most abundant phylum in near-shore (PRE-1 and PRE-3), and Ca. Scalindua was rich in off-shore (PRE-13 and PRE-18). Community structures in the PRE sediment cores present typically mixed characteristics of terrestrial and marine environments, consistent with the understanding that Ca. Scalindua was dominant in marine environments (Schmid et al. 2007) and Ca. Kuenenia, Ca. Brocadia, Ca. Anammoxoglobus, and Ca. Jettenia co-occurred in terrestrial environments of wetland soils, porous aquifer, estuary sediment, and soil environments (Humbert et al. 2010; Shen et al. 2015; Thrush et al. 2012; Zhu et al. 2013). Moreover, a distinct geographically specific distribution of anammox bacteria was evidently presented by PCA analysis (Fig. 6). This result reaffirmed previous findings of the dominant genus shift from Ca. Brocadia to Ca. Scalindua, from freshwater to open sea, with salinity being the main factor (Dale et al. 2009; Fu et al. 2015; Hou et al. 2013). Ca. Brocadia has been reported to harbor diverse metabolic processes. Therefore, they can effectively adapt in terrestrial ecosystems (Gori et al. 2011) and the presence of Ca. Brocadia indicates more eutrophication in the upstream and middle stream of PRE (Naeher et al. 2015). Contrary to Ca. Brocadia, the presence of Ca. Scalindua suggests that the examined sediments have not been significantly exposed to pollution and urbanization (Byrne et al. 2009; Cao et al. 2011; Li et al. 2011; Schmid et al. 2007).
We also found that Ca. Brocadia have adaptability to high-nutrient environments and Ca. Scalindua prefers high Corg:Norg environments. Compared with other anammox genera, Ca. Brocadia possesses diverse metabolic pathways and better adaptability to higher substrate concentration (Gori et al. 2011; Strous 1999; Gao et al. 2018). The environmental parameters, NO2− concentration, Corg:Norg, and median sediment grain size were regarded as influential in determining the composition and distribution of the sediment anammox bacterial community (Dang et al. 2010). Ca. Jettenia, Ca. Brocadia, Ca. Anammoxoglobus, and Ca. Kuenenia are usually associated with sewage treatment system, freshwater, or diverse terrestrial environments (Humbert et al. 2010; Jetten et al. 2009, 2005; Zhang et al. 2007), which might explain the resistance of Ca. Brocadia to stressful and adverse environmental changes. Therefore, it dominated at near-shore regions which are affected by significant human activity. Nevertheless, the heat map showed that there was not distinct change between summer and winter, suggesting that temperature was not an important factor in shaping the anammox bacterial community distribution (Fig. 5). Hou et al. (2015) and Yang et al. (2017) found that the distribution and diversity of anammox bacterial community exhibited a latitudinal gradient along the coastal wetland of China. Temperature is a key environmental factor shaping the distribution and diversity of anammox bacteria with majority of researchers finding significant difference between summer and winter (Bao et al. 2016). But the temperature variation of subtropical Pearl River Estuary (15–30 °C) cannot shape the anammox bacterial community. And anammox bacteria appear at a broader temperature range with ranged from − 2.5 to 100 °C (Rysgaard and Glud 2004; Byrne et al. 2009). In addition, there was no significant variation in the vertical zonation of anammox bacteria in the sediment cores (Fig. 4). In line with the results found in intertidal marshes (Zheng et al. 2016), the anammox community in the sediment cores remained stable. It suggested that vertical variation of physicochemical parameters (TN and DIN) in sediment core (1-m depth) cannot shape the anammox bacterial community.
In conclusion, our results provide a comprehensive understanding of the activity and spatio-temporal distribution of anammox bacteria in the sediment cores of PRE. Anammox bacteria exist in the deep sediment and contribute to the estuarine nitrogen loss. Although there were no obvious differences in the composition of anammox bacterial community in summer and winter, a distinct geography-specific distribution of anammox bacteria was evident, with salinity, NH4+, and NO3− contents being significant drivers of the rates and community composition of anammox bacteria.
References
Arrigo, K.R. 2005. Marine microorganisms and global nutrient cycles. Nature 437 (7057): 349–355.
Bao, L.L., X.Y. Wang, Y.J. Chen, and L.R. Zhang. 2016. Diversity, abundance and distribution of nirS-type denitrifiers and Anammox bacteria in sediments of Beiyun River. China Environmental Science 36 (05): 1520–1529.
Brandes, J.A., A.H. Devol, and D. Curtis. 2010. New developments in the marine nitrogen cycle. Chemical Reviews 38:no–no.
Brin, L.D., A.E. Giblin, and J.J. Rich. 2014. Environmental controls of anammox and denitrification in southern New England estuarine and shelf sediments. Limnology and Oceanography 59: 851–860.
Byrne, N., M. Strous, V. Crepeau, B. Kartal, J.L. Birrien, M. Schmid, F. Lesongeur, S. Schouten, A. Jaeschke, M. Jetten, D. Prieur, and A. Godfroy. 2009. Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents. ISME J 3 (1): 117–123.
Cai, W.-J., M. Dai, Y. Wang, W. Zhai, T. Huang, S. Chen, F. Zhang, Z. Chen, and Z. Wang. 2004. The biogeochemistry of inorganic carbon and nutrients in the Pearl River estuary and the adjacent Northern South China Sea. Continental Shelf Research 24: 1301–1319.
Cao, H., Y. Hong, M. Li, and J.D. Gu. 2011. Diversity and abundance of ammonia-oxidizing prokaryotes in sediments from the coastal Pearl River estuary to the South China Sea. Antonie Van Leeuwenhoek 100 (4): 545–556.
Caporaso, J.G., C.L. Lauber, W.A. Walters, D. Berg-Lyons, J. Huntley, N. Fierer, S.M. Owens, J. Betley, L. Fraser, M. Bauer, N. Gormley, J.A. Gilbert, G. Smith, and R. Knight. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6 (8): 1621–1624.
Chai, C., Z. Yu, X. Song, and X. Cao. 2006. The status and characteristics of eutrophication in the Yangtze River (Changjiang) Estuary and the adjacent East China Sea, China. Hydrobiologia 563: 313–328.
Dai, M., X. Guo, W. Zhai, L. Yuan, B. Wang, L. Wang, P. Cai, T. Tang, and W.-J. Cai. 2006. Oxygen depletion in the upper reach of the Pearl River estuary during a winter drought. Marine Chemistry 102: 159–169.
Dai, M., Z. Cao, X. Guo, W. Zhai, Z. Liu, Z. Yin, Y. Xu, J. Gan, J. Hu, and C. Du. 2013. Why are some marginal seas sources of atmospheric CO2? Geophysical Research Letters 40: 2154–2158.
Dale, O.R., C.R. Tobias, and B. Song. 2009. Biogeographical distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in Cape Fear River Estuary. Environ Microbiol 11 (5): 1194–1207.
Dalsgaard, T., D.E. Canfield, J. Petersen, B. Thamdrup, and J. Acuna-Gonzalez. 2003. N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature 422 (6932): 606–608.
Dalsgaard, T., B. Thamdrup, and D.E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156 (4): 457–464.
Dalsgaard, Tage, Bo Thamdrup, Laura Farías, and Niels Peter Revsbech. 2012. Anammox and denitrification in the oxygen minimum zone of the eastern South Pacific. Limnol Oceanogr 57: 1331–1346.
Dang, H., R. Chen, L. Wang, L. Guo, P. Chen, Z. Tang, F. Tian, S. Li, and M.G. Klotz. 2010. Environmental factors shape sediment anammox bacterial communities in hypernutrified Jiaozhou Bay, China. Appl Environ Microbiol 76 (21): 7036–7047.
Engström, P., T. Dalsgaard, S. Hulth, and R.C. Aller. 2005. Anaerobic ammonium oxidation by nitrite (anammox): Implications for N2 production in coastal marine sediments. Geochimica et Cosmochimica Acta 69: 2057–2065.
Engström, P., C.R. Penton, and A.H. Devol. 2009. Anaerobic ammonium oxidation in deep-sea sediments off the Washington margin. Limnology & Oceanography 54: 1643–1652.
Fu, B., J. Liu, H. Yang, T.C. Hsu, B. He, M. Dai, S.J. Kao, M. Zhao, and X.-H. Zhang. 2015. Shift of anammox bacterial community structure along the Pearl Estuary and the impact of environmental factors. Journal of Geophysical Research: Oceans 120: 2869–2883.
Gao, D., X. Wang, H. Liang, Q. Wei, Y. Dou, and L. Li. 2018. Anaerobic ammonia oxidizing bacteria: Ecological distribution, metabolism, and microbial interactions. Frontiers of Environmental Science & Engineering 12 (3): 10–15. https://doi.org/10.1007/s11783-018-1035-x.
Gori, F., S.G. Tringe, B. Kartal, E. Marchiori, and M.S. Jetten. 2011. The metagenomic basis of anammox metabolism in Candidatus ‘Brocadia fulgida’. Biochemical Society Transactions 39 (6): 1799–1804.
Guo, X., M. Dai, W. Zhai, W.-J. Cai, and B. Chen. 2009. CO2 flux and seasonal variability in a large subtropical estuarine system, the Pearl River Estuary, China. Journal of Geophysical Research: Biogeosciences 114.
Hong, B., and J. Shen. 2012. Responses of estuarine salinity and transport processes to potential future sea-level rise in the Chesapeake Bay. Estuarine, Coastal and Shelf Science 104-105: 33–45.
Hong, Y.G., S.L. Wang, X.R. Xu, J.P. Wu, L. Liu, W.Z. Yue, M.L. Wu, and Y.S. Wang. 2015. Developing a salinity-based approach for the evaluation of DIN removal rate in estuarine ecosystems. Ecotoxicology 24 (7-8): 1611–1620.
Hong, Y.G., J.P. Wu, F.J. Guan, W.Z. Yue, and A.M. Long. 2019. Nitrogen removal in the sediments of the Pearl River Estuary, China: Evidence from the distribution and forms of nitrogen in the sediment cores. Marine Pollution Bulletin 138: 115–124.
Hou, L., M. Liu, S.A. Carini, and W.S. Gardner. 2012. Transformation and fate of nitrate near the sediment–water interface of Copano Bay. Continental Shelf Research 35: 86–94.
Hou, L., Y. Zheng, M. Liu, J. Gong, X. Zhang, G. Yin, and L. You. 2013. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. Journal of Geophysical Research: Biogeosciences 118: 1237–1246.
Hou, L., Y. Zheng, M. Liu, X. Li, X. Lin, G. Yin, J. Gao, F. Deng, F. Chen, and X. Jiang. 2015. Anaerobic ammonium oxidation and its contribution to nitrogen removal in China’s coastal wetlands. Scientific Reports 5.
Huang, X.P., L.M. Huang, and W.Z. Yue. 2003. The characteristics of nutrients and eutrophication in the Pearl River estuary, South China. Marine Pollution Bulletin 47 (1-6): 30–36.
Humbert, S., S. Tarnawski, N. Fromin, M.P. Mallet, M. Aragno, and J. Zopfi. 2010. Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4 (3): 450–454.
Humbert, S., J. Zopfi, S.E. Tarnawski, et al. 2012. Abundance of anammox bacteria in different wetland soils. Environmental Microbiology Reports 4: 484–490.
Jetten, M.S.M., I. Cirpus, B. Kartal, L. Van Niftrik, K.T. Van De Pas-Schoonen, O. Sliekers, S. Haaijer, W. Van Der Star, M. Schmid, and J. Van De Vossenberg. 2005. 1994-2004: 10 years of research on the anaerobic oxidation of ammonium. Biochemical Society Transactions 33 (Pt 1): 119–123.
Jetten, M.S., L. Niftrik, M. Strous, B. Kartal, J.T. Keltjens, and H.J. Op den Camp. 2009. Biochemistry and molecular biology of anammox bacteria. Critical Reviews in Biochemistry and Molecular Biology 44 (2-3): 65–84.
Kartal, B., L. Van Niftrik, J. Rattray, J.L.C.M. Van De Vossenberg, M.C. Schmid, J. Sinninghe Damsté, M.S. Jetten, and M. Strous. 2008. Candidatus 'Brocadia fulgida': an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiology Ecology 63 (1): 46–55.
Kartal, B., N.M. de Almeida, W.J. Maalcke, H.J. Op den Camp, M.S. Jetten, and J.T. Keltjens. 2013. How to make a living from anaerobic ammonium oxidation. FEMS Microbiology Reviews 37 (3): 428–461.
Kim, H., H.S. Bae, K.R. Reddy, and A. Ogram. 2016. Distributions, abundances and activities of microbes associated with the nitrogen cycle in riparian and stream sediments of a river tributary[J]. Water Research 106: 51–61.
Kuypers, M.M., A.O. Sliekers, G. Lavik, M. Schmid, B.B. Jørgensen, J.G. Kuenen, J.S. Sinninghe Damsté, M. Strous, and M.S. Jetten. 2003. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422: 608.
Lam, P., M.M. Jensen, G. Lavik, D.F. McGinnis, B. Müller, C.J. Schubert, R. Amann, B. Thamdrup, and M.l M. M. Kuypers. 2007. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proceedings of the National Academy of Sciences 104: 7104–7109.
Lee, O.O., Y. Wang, J. Yang, F.F. Lafi, A. Al-Suwailem, and P.Y. Qian. 2011. Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea. ISME J 5 (4): 650–664.
Li, H., S. Chen, B.Z. Mu, and J.D. Gu. 2010. Molecular detection of anaerobic ammonium-oxidizing (Anammox) bacteria in high-temperature petroleum reservoirs. Microbial Ecology 60 (4): 771–783.
Li, M., Y.G. Hong, H.L. Cao, and J.D. Gu. 2011. Mangrove trees affect the community structure and distribution of anammox bacteria at an anthropogenic-polluted mangrove in the Pearl River Delta reflected by 16S rRNA and hydrazine oxidoreductase (HZO) encoding gene analyses. Ecotoxicology 20 (8): 1780–1790.
Lin, X., M. Liu, L. Hou, D. Gao, X. Li, K. Lu, and J. Gao. 2017a. Nitrogen losses in sediments of the East China Sea: Spatiotemporal variations, controlling factors, and environmental implications. Journal of Geophysical Research: Biogeosciences 122: 2699–2715.
Lin, X., Li, X., Gao, D., Liu, M., & Cheng, L. 2017b. Ammonium production and removal in the sediments of Shanghai river networks: Spatiotemporal variations, controlling factors, and environmental implications. Journal of Geophysical Research: Biogeosciences 122. https://doi.org/10.1002/2017JG003769
Lisa, J.A., B. Song, C.R. Tobias, and K.A. Duernberger. 2014. Impacts of freshwater flushing on anammox community structure and activities in the New River Estuary, USA. Aquatic Microbial Ecology 72 (72): 17–31.
Liu, J., S. Yu, M. Zhao, B. He, and X.H. Zhang. 2014. Shifts in archaeaplankton community structure along ecological gradients of Pearl Estuary. FEMS Microbiology Ecology 90 (2): 424–435.
Lu, Z., and J. Gan. 2015. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep Sea Research Part II Topical Studies in Oceanography 117: 86–96.
Meyer, J.L., M.J. Paul, and W.K. Taulbee. 2005. Stream ecosystem function in urbanizing landscapes. Journal of the North American Benthological Society 24 (3): 602–612.
Mulder, A., A.A.V.D. Graaf, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. Fems Microbiology Ecology 16: 177–184.
Naeher, S., A. Huguet, C.L. Roose-Amsaleg, A.M. Laverman, C. Fosse, M.F. Lehmann, S. Derenne, and J. Zopfi. 2015. Molecular and geochemical constraints on anaerobic ammonium oxidation (anammox) in a riparian zone of the Seine Estuary (France). Biogeochemistry 123: 237–250.
Penton, C.R., A.H. Devol, and J.M. Tiedje. 2006. Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Applied and Environmental Microbiology 72 (10): 6829–6832.
Plummer, P., C. Tobias, and D. Cady. 2015. Nitrogen reduction pathways in estuarine sediments: Influences of organic carbon and sulfide. Journal of Geophysical Research: Biogeosciences 120: 1958–1972. https://doi.org/10.1002/2015JG003057.
Qian, P.-Y., Y. Wang, O.O. Lee, S.C.K. Lau, J. Yang, F.F. Lafi, A. Al-Suwailem, and T.Y.H. Wong. 2010. Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing. The ISME Journal 5: 507–518.
Quan, Z.X., S.K. Rhee, J.E. Zuo, Y. Yang, J.W. Bae, J.R. Park, S.T. Lee, and Y.H. Park. 2008. Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environmental Microbiology 10 (11): 3130–3139.
Risgaard-Petersen, N. 2004. Anaerobic ammonium oxidation in an estuarine sediment. Aquatic Microbial Ecology 36: 293–304.
Risgaard-Petersen, N., L.P. Nielsen, S. Rysgaard, T. Dalsgaard, and R.L. Meyer. 2003. Erratum: Application of the isotope pairing technique in sediments where anammox and denitrification co-exist. Limnology & Oceanography Methods 1: 63–73.
Rysgaard, S., and R.N. Glud. 2004. Anaerobic N2 production in Arctic sea ice. Limnology & Oceanography 49 (1): 86–94.
Rysgaard S., Glud R.N., Risgaard-Petersen N, and Dalsgaard T. 2004. Denitrification and anammox activity in Arctic marine sediments. Limnology and Oceanography 49(5): 1493–1502. https://doi.org/10.4319/lo.2004.49.5.1493.
Schloss, P.D., S.L. Westcott, T. Ryabin, J.R. Hall, M. Hartmann, E.B. Hollister, R.A. Lesniewski, B.B. Oakley, D.H. Parks, and C.J. Robinson. 2009. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied & Environmental Microbiology 75: 7537.
Schmid, M., U. Twachtmann, M. Klein, M. Strous, S. Juretschko, M. Jetten, J.W. Metzger, K.-H. Schleifer, and M. Wagner. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Systematic and Applied Microbiology 23 (1): 93–106.
Schmid, M., K. Walsh, R. Webb, W.I. Rijpstra, K. van de Pas-Schoonen, M.J. Verbruggen, T. Hill, B. Moffett, J. Fuerst, S. Schouten, J.S. Sinninghe Damsté, J. Harris, P. Shaw, M. Jetten, and M. Strous. 2003. Candidatus “Scalindua brodae”, sp. nov., Candidatus “Scalindua wagneri”, sp. nov., Two New Species of Anaerobic Ammonium Oxidizing Bacteria. Systematic and Applied Microbiology 26 (4): 529–538.
Schmid, M.C., N. Risgaard-Petersen, J. van de Vossenberg, M.M. Kuypers, G. Lavik, J. Petersen, S. Hulth, B. Thamdrup, D. Canfield, T. Dalsgaard, S. Rysgaard, M.K. Sejr, M. Strous, H.J. den Camp, and M.S. Jetten. 2007. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology 9 (6): 1476–1484.
Shan J , Zhao X , Sheng R , et al. 2016. Dissimilatory nitrate reduction processes in typical Chinese paddy soils: Rates, relative contributions and influencing factors. Environmental Science & Technology, acs.est.6b01765.
Shen, L.-D., S. Liu, Q. Huang, X. Lian, Z.-F. He, S. Geng, R.-C. Jin, Y.-F. He, L.-P. Lou, X.-Y. Xu, P. Zheng, B.-L. Hu, and C.R. Lovell. 2014. Evidence for the cooccurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in a flooded paddy field. Applied and Environmental Microbiology 80 (24): 7611–7619.
Shen, L.D., H.S. Wu, Z.Q. Gao, X.H. Xu, T.X. Chen, S. Liu, and H.X. Cheng. 2015. Occurrence and importance of anaerobic ammonium-oxidising bacteria in vegetable soils. Applied Microbiology and Biotechnology 99 (13): 5709–5718.
Shen, L.D., H.S. Wu, Z.Q. Gao, H.X. Cheng, J. Li, X. Liu, and Q.Q. Ren. 2016. Distribution and activity of anaerobic ammonium-oxidising bacteria in natural freshwater wetland soils. Applied Microbiology and Biotechnology 100 (7): 3291–3300.
Song, B., and C.R. Tobias. 2011. Molecular and stable isotope methods to detect and measure anaerobic ammonium oxidation (anammox) in aquatic ecosystems. Methods in Enzymology 496: 63–89.
Song, G. D., Liu, S. M., Marchant, H., Kuypers, M. M. M., and Lavik, G. 2013. Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences 10: 6851–6864.
Sonthiphand, P., M.W. Hall, and J.D. Neufeld. 2014. Biogeography of anaerobic ammonia-oxidizing (anammox) bacteria. Frontiers in Microbiology 5: 399.
Strous, M. 1999. Key physiology of anaerobic ammonium oxidation. Applied and Environmental Microbiology 65 (7): 3248–3250.
Strous, M., E. Pelletier, S. Mangenot, T. Rattei, A. Lehner, M.W. Taylor, M. Horn, H. Daims, D. Bartol-Mavel, P. Wincker, V. Barbe, N. Fonknechten, D. Vallenet, B. Segurens, C. Schenowitz-Truong, C. Medigue, A. Collingro, B. Snel, B.E. Dutilh, H.J. Op den Camp, C. van der Drift, I. Cirpus, K.T. van de Pas-Schoonen, H.R. Harhangi, L. van Niftrik, M. Schmid, J. Keltjens, J. van de Vossenberg, B. Kartal, H. Meier, D. Frishman, M.A. Huynen, H.W. Mewes, J. Weissenbach, M.S. Jetten, M. Wagner, and D. Le Paslier. 2006. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440 (7085): 790–794.
Tan, E., T.C. Hsu, X. Huang, H.J. Lin, and S.J. Kao. 2017. Nitrogen transformations and removal efficiency enhancement of a constructed wetland in subtropical Taiwan. Science of The Total Environment 601-602: 1378–1388.
Teixeira, C., C. Magalhães, S.B. Joye, and A.A. Bordalo. 2012. Potential rates and environmental controls of anaerobic ammonium oxidation in estuarine sediments. Aquatic Microbial Ecology 66: 23–32.
Thamdrup, B., and T. Dalsgaard. 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology 68 (3): 1312–1318.
Thrush, S., B. Hu, L. Shen, P. Du, P. Zheng, X. Xu, and J. Zeng. 2012. The influence of intense chemical pollution on the community composition, diversity and abundance of anammox bacteria in the Jiaojiang Estuary (China). PLoS ONE 7.
Trimmer, M., J. Petersen, D.B. Sivyer, C. Mills, E. Young, and E.R. Parker. 2005. Impact of long-term benthic trawl disturbance on sediment sorting and biogeochemistry in the southern North Sea. Marine Ecology Progress 298: 79–94.
Vila-Costa, M., A. Barberan, J.C. Auguet, S. Sharma, M.A. Moran, and E.O. Casamayor. 2013. Bacterial and archaeal community structure in the surface microlayer of high mountain lakes examined under two atmospheric aerosol loading scenarios. FEMS Microbiology Ecology 84 (2): 387–397.
Walter, J., S. Melzak, J. Okello, and B. Efraime. 1999. Key physiology of anaerobic ammonium oxidation. Applied and Environmental Microbiology 65: 3248–3250.
Wang, S., G. Zhu, Y. Peng, M.S. Jetten, and C. Yin. 2012. Anammox bacterial abundance, activity, and contribution in riparian sediments of the Pearl River estuary. Applied and Environmental Microbiology 46 (16): 8834–8842.
Wang, S., W. Wang, L. Liu, L. Zhuang, S. Zhao, Y. Su, Y. Li, M. Wang, C. Wang, L. Xu, and G. Zhu. 2018. Microbial nitrogen cycle hotspots in the plant-bed/ditch system of a constructed wetland with N2O mitigation. Environmental Science & Technology 52 (11): 6226–6236.
Wu, Y. Hong, X. He, L. Jiao, X. Wen, S. Chen, G. Chen, Y. Li, T. Huang, Y. Hu, and X. Liu. 2018. Anaerobic ammonium oxidation in acidic red soils. Frontiers in Microbiology 9.
Wu, J., Y. Hong, X. Chang, L. Jiao, Y. Li, X. Liu, H. Xie, and J.-D. Gu. 2019. Unexpectedly high diversity of anammox bacteria detected in deep-sea surface sediments of the South China Sea. FEMS Microbiology Ecology 95.
Xiao, K., J. Wu, H. Li, Y. Hong, A.M. Wilson, J.J. Jiao, and M. Shananan. 2018. Nitrogen fate in a subtropical mangrove swamp: Potential association with seawater-groundwater exchange. Science of the Total Environment 635: 586–597.
Yang, X.-R., H. Li, S.-A. Nie, J.-Q. Su, B.-S. Weng, G.-B. Zhu, H.-Y. Yao, J.A. Gilbert, Y.-G. Zhu, and H. Nojiri. 2015. Potential contribution of anammox to nitrogen loss from paddy soils in Southern China. Applied and Environmental Microbiology 81: 938–947.
Yang, X.-R., B.-S. Weng, H. Li, C.W. Marshall, H. Li, Y.-S. Chen, S. Yu, G.-B. Zhu, and Y.-G. Zhu. 2017. An overlooked nitrogen loss linked to anaerobic ammonium oxidation in estuarine sediments in China. Journal of Soils and Sediments 17: 2537–2546.
Ye, Q., Y. Wu, Z. Zhu, X. Wang, Z. Li, and J. Zhang. 2016. Bacterial diversity in the surface sediments of the hypoxic zone near the Changjiang Estuary and in the East China Sea. Microbiologyopen 5 (2): 323–339.
Zhang, Y., X.H. Ruan, H.J. Op den Camp, T.J. Smits, M.S. Jetten, and M.C. Schmid. 2007. Diversity and abundance of aerobic and anaerobic ammonium-oxidizing bacteria in freshwater sediments of the Xinyi River (China). Environmental Microbiology 9 (9): 2375–2382.
Zhao, Y., Y. Xia, T.M. Kana, Y. Wu, X. Li, and X. Yan. 2013. Seasonal variation and controlling factors of anaerobic ammonium oxidation in freshwater river sediments in the Taihu Lake region of China. Chemosphere 93 (9): 2124–2131.
Zheng, Y., X. Jiang, L. Hou, M. Liu, X. Lin, J. Gao, X. Li, G. Yin, C. Yu, and R. Wang. 2016. Shifts in the community structure and activity of anaerobic ammonium oxidation bacteria along an estuarine salinity gradient. Journal of Geophysical Research: Biogeosciences 121: 1632–1645. https://doi.org/10.1002/2015JG003300.
Zheng, Y., L. Hou, M. Liu, Z. Liu, X.i Li, X. Lin, G. Yin, J. Gao, C. Yu, R. Wang, and Xi. Jiang. 2016. Tidal pumping facilitates dissimilatory nitrate reduction in intertidal marshes. Scientific Reports 6: 21338.
Zheng, Y., L. Hou, M. Liu, and G. Yin. 2019. Dynamics and environmental importance of anaerobic ammonium oxidation (anammox) bacteria in urban river networks. Environmental Pollution. https://doi.org/10.1016/j.envpol.2019.112998.
Zhu, G., S. Wang, Y. Wang, C. Wang, N. Risgaard-Petersen, M.S. Jetten, and C. Yin. 2011. Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J 5 (12): 1905–1912.
Zhu, G., S. Wang, W. Wang, Y. Wang, L. Zhou, B. Jiang, H.J.M. Op den Camp, N. Risgaard-Petersen, L. Schwark, Y. Peng, M.M. Hefting, M.S.M. Jetten, and C. Yin. 2013. Hotspots of anaerobic ammonium oxidation at land–freshwater interfaces. Nature Geoscience 6: 103–107.
Zhu, G., Wang, S, Wang C, et al. 2019. Resuscitation of anammox bacteria after >10,000 years of dormancy. The ISME Journal. https://doi.org/10.1038/s41396-018-0316-5
Zumft, W.G. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61 (4): 533–616.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31870100, 91851111, 41576123, and 41977153), and the Research Foundation for Talented Scholars of Guangzhou University (Grant No. GU2017001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Zhanfei Liu
Electronic supplementary material
ESM 1
(DOCX 1485 kb)
Rights and permissions
About this article
Cite this article
Wu, J., Hong, Y., Wen, X. et al. Activity, Abundance, and Community Composition of Anaerobic Ammonia–Oxidizing (Anammox) Bacteria in Sediment Cores of the Pearl River Estuary. Estuaries and Coasts 43, 73–85 (2020). https://doi.org/10.1007/s12237-019-00668-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-019-00668-1