Abstract
Many applications of otolith chemistry use the ratios of strontium (Sr) and barium (Ba) to calcium (Ca) as indicators of salinity exposure, because typically, as salinity increases, Sr concentration increases and Ba concentration decreases. However, these relationships are nonlinear, can be confounded by temperature, and investigations of salinity and temperature effects on otolith chemistry produce varied results. To determine the relationships of temperature and salinity on Sr:Ca and Ba:Ca in otoliths, we used free ranging Gulf Killifish (Fundulus grandis) in the northern Gulf of Mexico. This species is ideal because it is euryhaline and exhibits limited movements. Otolith edge Sr:Ca and Ba:Ca ratios were related to the previous 30-day mean salinity and temperature experienced by fish. The best model to describe otolith Sr:Ca was one that included a positive asymptotic relationship for both salinity and temperature. However, the salinity asymptotic maximum was reached at 10 psu and changes in otolith Sr:Ca above 10 psu were indicative of temperature changes. Otolith Ba:Ca exhibited an exponential decreasing relationship with salinity, and an exponential increasing relationship with temperature, and these two models combined best explained otolith Ba:Ca. Above 10 psu, the modeled Ba:Ca ratio continued to decrease demonstrating that this ratio may be indicative of salinity changes beyond this value. Therefore, using both Sr:Ca and Ba:Ca could be beneficial in reconstructing fish environmental histories. Temperature effects on otolith element ratios could confound past salinity reconstructions as well and must be a result of endogenous processes, given that no relationship between temperature and water chemistry existed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Otolith microchemical analysis has become a widely used technique in fisheries science and has been used for a variety of applications including nursery habitat classification (Anstead et al. 2015; Fodrie and Herzka 2008; Vasconcelos et al. 2007), identification of population contingents of fishes (Kraus and Secor 2004a; Morissette et al. 2016; Nims and Walther 2014), and retrospective tracking of fish movements (Albuquerque et al. 2012; Gillanders et al. 2015). Otolith microchemistry is particularly effective when tracking fish movements and identifying population contingents of estuarine fishes, because strontium and barium commonly replace calcium in the otolith matrix (Campana 1999) and co-vary with salinity (Sturrock et al. 2012; Walther and Limburg 2012).
Strontium (Sr) concentrations and the Sr:Ca ratio in otoliths typically increase with increasing salinity (Farmer et al. 2013; Lowe et al. 2009; Walther and Limburg 2012). This relationship exists because seawater typically has a higher concentration of Sr:Ca than freshwater (de Villiers 1999), and water concentrations of Sr:Ca have been shown to have a direct relationship to otolith concentrations (Kraus and Secor 2004b; Walther and Thorrold 2006). However, this positive relationship of salinity and otolith Sr:Ca can be affected by temperature (Martin et al. 2004; Reis-Santos et al. 2013; Townsend et al. 1992), physiological processes (Kalish 1991), and may become insignificant or negative depending on the surrounding freshwater concentrations of Sr:Ca (Brown and Severin 2009; Kraus and Secor 2004b). This increasing relationship is also nonlinear, reaching an otolith Sr:Ca asymptote at low-salinity to mid-salinity values (Walther and Limburg 2012).
In contrast, barium (Ba) exhibits a negative relationship with salinity given that Ba enters estuaries adsorbed on suspended particulate matter, primarily clay in river outflows (Coffey et al. 1997; Hanor and Chan 1977). The decline in available Ba is due to the dilution of river water and the flocculation and settling of particulate matter as river waters mix with salt water. The relationship between dissolved Ba concentration and salinity is made more complex because at low salinities, Ba desorbs from clay increasing the amount of dissolved Ba in water (Coffey et al. 1997). Barium concentrations in estuaries are further complicated through benthic Ba input (Joung and Shiller 2014), which can be through groundwater discharge (Shaw et al. 1998), benthic dissolution of marine barite (Colbert and McManus 2005), and desorption from previously deposited riverine sediment (Carroll et al. 1993). In general, increasing salinity is negatively related to Ba:Ca in fish otoliths (Lowe et al. 2011; Nims and Walther 2014; Walther and Limburg 2012), although this relationship can be further complicated by temperature (DiMaria et al. 2010; Elsdon and Gillanders 2002; Miller 2009), physiological processes (Miller 2011), and surrounding water concentrations of Ba:Ca (Elsdon and Gillanders 2002; Reis-Santos et al. 2013; Walther and Thorrold 2006).
The effects of temperature and salinity on element incorporation into otoliths have been tested in the field and laboratory, although such studies can be inconclusive and contradictory, especially in regard to temperature. A positive relationship with temperature has been found for both Sr:Ca (Bath et al. 2000; Martin et al. 2004; Webb et al. 2012) and Ba:Ca (Miller 2009; Reis-Santos et al. 2013; Webb et al. 2012) incorporation into otoliths. However, negative relationships have also been found for Sr:Ca (DiMaria et al. 2010; Radtke et al. 1990; Townsend et al. 1992) and Ba:Ca (DiMaria et al. 2010), and non-significant temperature relationships for both elements have been found as well (Bath et al. 2000; López-Duarte et al. 2016; Martin and Thorrold 2005). Field investigations of salinity and temperature relationships with otolith chemistry are limited and typically confounded by the fact that temperature and salinity are highly correlated in estuarine systems. Furthermore, many fish are highly mobile, making it difficult to know the temperatures and salinities experienced by a fish at the time of otolith material deposition, and virtually impossible to disentangle the effects of temperature and salinity on otolith chemistry in the field. To prevent fish movement, many studies use caged fish placed in different areas of an estuary to test salinity effects on otoliths (Kraus and Secor 2004b; Rooker et al. 2004). Unfortunately, the relatively short duration of these cage experiments limits the ability to test the effect of temperature, given that temperatures do not vary sufficiently over the experimental period. Although lab studies and field caging experiments have proven useful to test the relationships of temperature and salinity on Sr and Ba incorporation into fish otoliths, an in situ field study using a non-caged fish would provide a better test of these relationships. Elements and their concentrations would vary naturally across the salinity gradient of the fish’s habitat, and a long-term study could be performed, allowing temperature to vary.
Given the importance of salinity and temperature effects on otolith Sr:Ca and Ba:Ca and the implications for environmental reconstructions based on otolith chemistry, we chose to investigate these effects in wild Gulf Killifish (Fundulus grandis) populations. Otolith edge signatures of Sr:Ca and Ba:Ca were related to the previous 30-day mean temperature and salinity experienced before fish collection. Water elemental:Ca ratios were also quantified at collection locations to determine the underlying relationships of temperature and salinity on water chemistry. Water chemical samples were then used to investigate similarities between otolith and water element:Ca ratios. Gulf Killifish is an ideal choice for this investigation, given that it is euryhaline, is one of the most abundant and ubiquitously distributed fishes in northern Gulf of Mexico marshes (Nelson et al. 2015; Rozas and Reed 1993; Rozas and Zimmerman 2000), and exhibits limited movements (Nelson et al. 2014). The use of this species overcomes the fish mobility problem without caging and allows for determination of field-based temperature and salinity effects on otoliths, providing insight for future habitat and movement reconstructions of fish based on otolith chemistry.
Methods
Field Collection and Lab Processing
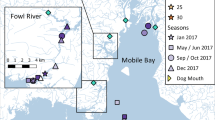
Gulf Killifish were collected at six salt marshes across the northern Gulf of Mexico (Fig. 1); one site in Baritaria Bay Louisiana (BB), one site near an oil refinery in Mississippi (RF), and four sites in Alabama. Two of the Alabama sites were located west of Mobile Bay, Fowl Bay A (FA) and Fowl Bay B (FB), and two sites were east of Mobile Bay, Perdido Pass (PD) and Wolf Bay (WF). Fish were collected in fall 2012, winter 2013, spring 2013, and summer 2013 from all sites using baited minnow traps. During each sampling, salinity (psu) and temperature (°C) were measured using a YSI model 30, and water samples were collected for elemental analyses. Water samples were filtered through a 0.45-μm filter, preserved with 1:1 nitric acid, stored in 125 ml acid-washed polypropylene bottles, and refrigerated until processing.
Sample sites, Barataria Bay (BB), Refinery (RF), Fowl Bay A (FA), Fowl Bay B (FB), Perdido Pass (PD), and Wolf Bay (WF) in the northern Gulf of Mexico. Boxes on the map in the top of the figure correspond to sample areas that are indicated by numbers in the lower four panels. Enlarged panels of each sample area are shown and denoted with the corresponding number and name of the area in the bottom right of each panel. Within each panel fish and water collection sites are denoted by a circle and the long-term abiotic monitoring stations used are denoted with an x inside a square. The long-term monitoring site in each panel is the one used for abiotic correlations, and in panel 4 predictions for WF were generated with the long-term site to the west, and PD used the adjacent long-term site
After collection, Gulf Killifish were transported back to the lab, weighed, and measured (total length [TL]). Fish were separated into five length classes (50 to 59, 60 to 69, 70 to 79, 80 to 89, > 90 mm), if present. One fish from each length class was selected for otolith edge analysis from each site and season (n = 30/season, total n = 120). If all length classes were not represented, then five fishes were chosen from the classes that were available. For each fish, a sagittal otolith was removed, cleaned using hydrogen peroxide, and polished to achieve a flat surface for microchemistry analysis.
Otolith and Water Chemistry
Otolith chemical analyses were conducted at the University of Windsor, Great Lakes Institute for Environmental Research (GLIER), Windsor, Ontario, Canada. Otolith concentrations of strontium 88, calcium 43, and barium 137 were quantified using a laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) system comprising a high energy, pulsed, ultrafast femtosecond laser (Integra-C® by Quantronix®, East Setauket, New York) and a Thermo-Electron® X Series-II® ICP-MS. Straight-line laser ablations were conducted using a 20-μm diameter spot size extending from one edge to the other across the otolith core to capture whole otolith elemental concentrations (see Nelson et al. 2015). In this study, the last 100 μm of each ablation was used as the otolith edge signature.
Two runs ablating borosilicate glass standard reference material (National Institute of Standards and Technology [NIST] 610) with known concentrations of Sr, Ba, and Ca were analyzed both before and after approximately every 16 otolith samples (~ 1 h) to calculate the limits of detection (LOD) for each element, to correct for instrumental drift, and to provide a known Ca standard for the internal calibration equation (Longerich et al. 1996). Ca was used as the internal calibration standard and was assigned a constant otolith concentration of 400,432 ppm (the concentration of Ca in aragonite [CaCO3]), to correct for differences in mass of ablated material and to convert raw isotope signal intensity measurements (icps) to concentration units (ppm) (Longerich et al. 1996). Strontium and barium concentrations (ppm) were converted to a molar ratio with Ca before statistical analyses. More detailed methods on otolith preparation, ICPMS operating parameters, and relative standard deviations (RSD) can be found in Nelson et al. (2015).
Water samples were analyzed using solution-ICPMS and a 100-fold dilution of all samples was performed before analysis using 1% HNO3. Prior to analysis, every sample was also spiked with internal standards Be, In, and Tl at concentrations of 10, 1, and 2 ppb, respectively, to correct for instrumental mass bias and drift, as well as for matrix effects on an individual sample basis.
Statistical Analysis
Long-Term Abiotic Monitoring
To relate elemental ratios in the otolith to the temperature and salinity experienced by a fish during otolith deposition, single-point salinity and temperature measurements during field collection of fish are not sufficient, given that a lag time ranging from 2 weeks to 30 days associated with changes in the environment and detectable Sr:Ca and Ba:Ca ratio changes in fish otoliths has been found (Elsdon and Gillanders 2005; Lowe et al. 2009; Macdonald and Crook 2010; Mohan et al. 2012). Therefore, the six sites chosen for this study were located near long-term abiotic monitoring stations (Fig. 1). These stations are maintained by the Mobile Bay National Estuary Program in Alabama, The Grand Bay National Estuarine Research Reserve in Mississippi, and the United States Geological Survey in Louisiana, and data are freely accessible online. The Alabama stations record a salinity and temperature measurement every 30 min, the Mississippi station records every 15 min, and the Louisiana station records every hour. Although located near one another, the fish collection sites and long-term monitoring sites did have spatial separation and experienced somewhat different abiotic conditions simultaneously. To account for this separation and the slight discrepancies in abiotic conditions, single-point observed salinity and temperature data during each collection were correlated with a single measurement from the respective long-term station data closest to the time of the abiotic data observation. The resulting models were used to predict the salinity and temperature experienced at each sampling site for 30 days prior to each fish collection. The 30 day predicted data (Alabama = 1440 data points, Mississippi = 2880 data points, Louisiana = 720 data points) prior to fish collections were averaged to obtain a single previous 30-day mean predicted salinity and temperature value for each site and collection event.
Salinity and Temperature Relationships
For both the observed values and the 30-day mean predicted values, a linear regression was performed to estimate the correlation of salinity with water temperature. One-way ANOVAs with Tukey post-hoc comparisons were performed for both observed and predicted values to determine if any site or seasonal patterns emerged for either salinity or temperature. Interactions between the two variables could not be tested because site was the replicate for season and season was the replicate for site.
Otolith edge Sr:Ca and Ba:Ca ratios were plotted against predicted 30-day mean salinity and temperature values and visually inspected to determine if any relationships were present. Relationships between Sr:Ca and Ba:Ca and predicted 30-day mean salinity and temperature were then modeled using the linear regression (lm) and nonlinear least squares (nls) functions in R, and the Akaike information criterion differences (Δ i AIC) were used to determine the best fitting model for each elemental ratio. The Δ i AIC values were calculated with, Δ i AIC = AIC i – AICmin, where model i with the smallest AIC (AICmin) had a Δ i AIC equal to zero and was considered the most parsimonious.
To investigate how elements in water were affected by temperature and salinity, plots were first generated to visualize relationships between water Sr:Ca and Ba:Ca ratios and observed salinity and temperature at time of collection. Elemental ratios that visually suggested relationships with salinity and temperature were modeled, and the best fitting model for each ratio was chosen by selecting the model with a Δ i AIC equal to zero. For all nonlinear models (otolith and water), 95% confidence intervals (95% CI) of parameter estimates were obtained using nonparametric bootstrapping with 10,000 iterations in the R package nlsBoot. One-way ANOVAs, with Tukey post-hoc comparisons, were also performed for both otolith and water Sr:Ca and Ba:Ca to determine if differences among sites in elemental ratios were present. Normality and homogeneity of variance were tested for all ANOVAs with the Shapiro Test and Levene’s test, respectively. If these tests failed assumptions of normality and variance, they were still used, given that these tests are robust to violations and have greater power than their nonparametric counterparts. This is especially true when the numbers of treatments and sample sizes are large, as is the case here (Underwood 1997; Brownie and Boos 1994; Kahn and Rayner 2003).
Results
Long-Term Abiotic Monitoring
The observed salinities and temperatures from all six sites were strongly correlated with values of the same parameters from the long-term monitoring stations (Table 1). The r 2 values for salinity ranged from 0.76 to 0.96 and those for temperature ranged from 0.91 to 1.00 (Table 1). Salinity was consistent over the periods averaged for each data point, given that the standard errors of these means ranged from 0.01–0.19, and were above 0.10 four times. The temperature standard errors were also consistent and ranged from 0.02–0.32 and were above 0.10 three times.
Salinity and Temperature
Each site experienced varying salinities and temperatures throughout the study, but salinity and temperature were not correlated in either observed (r 2 = 0.021, p = 0.497) or predicted values (r 2 < 0.001, p = 0.983). Salinity differed across sites for both observed (F 5,18 = 7.81, p < 0.001) and predicted (F 5,18 = 9.348, p < 0.001) values, with PD having the highest salinity, and BB having the lowest (Table 2, Fig. 2a). Salinity did not differ across seasons for either data set, further demonstrating no correlation between temperature and salinity. Water temperature did not differ across sites for either observed or predicted values. Seasonal patterns varied as expected for both data sets (observed F 3,20 = 21.12, p < 0.001, predicted F 3,20 = 42.24, p < 0.001); winter had the lowest temperatures and summer had the highest (Fig. 2b). All tests performed for salinity and temperature met variance and normality assumptions except the salinity across-sites tests for both data sets.
Otolith Chemistry Correlation with Salinity and Temperature
Otolith strontium (Sr:Ca) and barium (Ba:Ca) ratios exhibited relationships with both salinity and temperature; Sr:Ca exhibited a positive relationship with both salinity and temperature. Multiple models were fit to these relationships to capture the positive effect of temperature and salinity on Sr:Ca in the otolith (Table 3). The best fitting model contained two asymptotic functions that incorporated both temperature and salinity effects and is the following equation (Table 3, Fig. 3):
where Sr ∞ is the maximum mean predicted ratio of Sr:Ca in the otolith no matter how much salinity or temperature increases, k t is the rate that Sr:Ca approaches the asymptote (Sr∞) with increasing temperature, k s is the rate that Sr:Ca approaches the asymptote as salinity increases, and Sr:Ca is equal to the Sr:Ca ratio in the otolith at a given salinity (S) and temperature (T). This model predicted a Sr∞ of 6091.09 μmo lmol−1 (95% CI = 5047.36–8517.54, p < 0.001), a k t of 0.05 (95% CI = 0.03–0.07, p < 0.001), and a k s of 0.43 (95% CI = 0.34–0.58, p < 0.001) (Fig. 3). Based on this model, both salinity and temperature increases are positively correlated with Sr:Ca ratios. For instance, at 8 psu otolith Sr:Ca can range from 2319.81 μmol mol−1 at 10 °C to 4580.25 μmol mol−1 at 30 °C. If temperature is constrained at 20 °C, the Sr:Ca ratio can range from 2221.00 μmol mol−1 at 2 psu, to 3726.84 μmol mol−1 at 8 psu. Above 10 psu, Sr:Ca ratios reached an asymptote as a function of salinity (Fig. 3).
Sr:Ca ratios (μmol mol−1) of otoliths across predicted prior 30-day mean salinities and the best fitting model of Sr:Ca with salinity and temperature, represented with temperature lines ranging from 10 to 30 (°C) (a). Sr:Ca ratios (μmol mol−1) of otoliths across predicted prior 30-day mean temperatures and the best fitting model of Sr:Ca with salinity and temperature, represented with salinity lines ranging from 3 to 30 (psu) (b). Sr:Ca ratios (μmol mol−1) of otoliths plotted as a function of both predicted prior 30-day mean salinity and temperature (c). Data for this 3-D representation were smoothed using a running average (averages the values at neighboring data points) with a sampling proportion (fraction of a total number of data points used to compute each smoothed value) of 0.63 before plotting
Otolith Ba:Ca ratios exhibited an exponentially declining relationship with salinity and an exponentially increasing relationship with temperature. Combining these two correlations into one model resulted in the best fitting overall model (Table 4, Fig. 4). This combined model is the following equation:
where Ba0 is the otolith Ba:Ca ratio when both salinity and temperature equal 0, k s is the rate at which Ba:Ca ratios approach zero with respect to salinity, k t is the rate at which the Ba:Ca ratio increases with respect to temperature, and Ba:Ca is the Ba:Ca ratio in the otolith at a given salinity (S) and temperature (T). This model predicted a Ba0 of 3.04 μmol mol−1 (95% CI = 1.54–5.53, p = 0.003), a k s of 0.09 (95% CI = 0.07–0.12, p < 0.001), and a k t of 0.05 (95% CI = 0.03–0.08, p < 0.001). Based on this model, both salinity and temperature were correlated with Ba concentrations. For example, at 8 psu Ba:Ca ranged from 2.44 μmol mol−1 at 10 °C, to 6.63 μmol mol−1 at 30 °C. If temperature is constrained at 20 °C, Ba:Ca ranged from 6.90 μmol mol−1 at 2 psu, to 4.02 μmol mol−1 at 8 psu. However, unlike Sr:Ca, the modeled Ba:Ca ratio continues to decrease over the salinity range sampled (Fig. 4).
Ba:Ca ratios (μmol mol−1) of otoliths across predicted prior 30-day mean salinities and the best fitting model of Ba:Ca with salinity and temperature, represented with temperature lines ranging from 10 to 30 (°C) (a). Ba:Ca ratios (μmol mol−1) of otoliths across predicted prior 30-day mean temperatures and the best fitting model of Ba:Ca with salinity and temperature, represented with salinity lines ranging from 1 to 20 (psu) (b). Ba:Ca ratios (μmol mol−1) of otoliths plotted as a function of both predicted prior 30-day mean salinity and temperature (c). Data for this 3-D representation were smoothed using a running average (averages the values at neighboring data points) with a sampling proportion (fraction of a total number of data points used to compute each smoothed value) of 0.4 before plotting
Water Chemistry Correlation with Salinity and Temperature
The ratio of Sr:Ca in water exhibited a positive asymptotic relationship with salinity, similar to the relationship of otolith Sr:Ca with salinity, and had no relationship with temperature (Table 5, Fig. 5(a, b)). The model that best fit the Sr:Ca relationship with salinity is the following equation:
where Sr∞ is the mean maximum Sr:Ca ratio that can occur in water no matter how much salinity (S) increases, k s is the rate at which it approaches the asymptote (Sr∞), and S 0 is the salinity at which Sr:Ca would theoretically equal zero. This model predicted a Sr∞ of 7.51 mmol mol−1 (95% CI = 7.04–8.51, p < 0.001), a k s of 0.30 (95% CI = 0.11–2.12, p = 0.070), and S 0 = −1.78 (95% CI = − 7.20–0.10, p = 0.240).
The ratio of Ba:Ca in water exhibited an exponential decline with increasing salinity, similar to the relationship of otolith Ba:Ca with salinity. This correlation, also had no relationship with temperature (Table 5, Fig. 5(c, d)), and the best fitting model is the following equation:
where Ba0 is the ratio of Ba:Ca in water where salinity is zero, and k is the rate at which Ba:Ca approaches 0 with respect to salinity (S). This model predicted a Ba0 of 0.58 mmol mol−1 (95% CI = 0.48–0.71, p < 0.001) and a k of 0.15 (95% CI = 0.11–0.19, p < 0.001).
Otolith and Water Chemistry Among Sites
Sr:Ca values in otoliths were similar across all sites, however BB had significantly less Sr:Ca than all others (F 5,114 = 4.255, p = 0.001, Table 2, Fig. 6a) and Sr:Ca in water showed no significant difference among sites (F 5,12 = 2.951, p = 0.058, Table 2, Fig. 6b). Ba:Ca values in otoliths were also similar across sites except BB had higher Ba:Ca than all other sites (F 5,114 = 111.53, p < 0.001, Table 2, Fig. 6c). Patterns of Ba:Ca in water were similar to otolith Ba:Ca patterns; however, FA was not significantly different than BB (F 5,12 = 10.623, p < 0.001, Table 2, Fig. 6d). Not all tests met assumptions of normality, and Ba:Ca across sites failed homogeneity of variance; however, given that these tests are robust to these violations, they were still used.
Mean (± 1 SE) otolith (a, c, μmol mol−1) and water (b, d, mmol mol−1) Sr:Ca (a, b) and Ba:Ca (c, d) ratios among sites. Bars with different letters indicate significant differences among sites based on Tukey pairwise comparisons. In panel b, no significant differences are present, therefore no letters are used
Discussion
This study used field populations of Gulf Killifish to test the relationships of both temperature and salinity on otolith ratios of Sr:Ca and Ba:Ca and demonstrated that both temperature and salinity were correlated with otolith elemental concentrations. Otolith Sr:Ca and Ba:Ca were positively related to temperature, and each ratio exhibited expected patterns with salinity, Sr:Ca increased with increasing salinity in both otoliths and water, while Ba:Ca decreased in both cases. Fish were collected across a wide range of temperatures and salinities without these two variables being correlated with one another. Fish did not require caging as in other studies (Kraus and Secor 2004b; Mohan et al. 2012; Rooker et al. 2004) given that Gulf Killifish exhibits limited movement (Nelson et al. 2014) providing confidence that the individuals experienced the temperature and salinities measured where they were collected. This provides a unique test of temperature and salinity effects on otolith elemental ratios in a way that has not been done previously.
The previous 30-day mean predicted abiotic values provide a good representation of conditions experienced by a fish during otolith edge deposition, given that the lag times for otolith chemical changes in response to abiotic changes has been shown to be anywhere from 2 weeks (Mohan et al. 2012) to around 30 days (Elsdon and Gillanders 2005; Lowe et al. 2009; Macdonald and Crook 2010). In addition, the salinities and temperatures at these sites expressed minimal variation during the periods that were averaged to achieve each mean value so that otolith edge chemistries should be indicative of recent environmental conditions experienced by fish (Campana 1999; Mohan et al. 2012).
A recent study on Gulf Killifish found that otolith elemental incorporation was correlated with salinity and followed expected patterns of increasing Sr:Ca and decreasing Ba:Ca with increasing salinity; however, no temperature effect was found (López-Duarte et al. 2016). There are two reasons for the temperature discrepancy between the two studies that need to be highlighted. First, in López-Duarte et al. (2016), fish were only collected during warm months May, June, August, and early September, and therefore temperatures during collection were all above 20 °C. Second, no salinity and temperature readings were taken at the time of fish collection; rather everything was based on long-term monitoring stations. These stations were likely not always close enough to experience the same abiotic conditions as the fish collection sites, given the heterogeneity in abiotic conditions in northern Gulf of Mexico estuaries (Schroeder et al. 1990). Perhaps if fish had been collected year-round, and location discrepancies were accounted for, the same temperature patterns would have emerged; however, this was not the objective of their study.
Strontium
Strontium in the otolith was positively related to both salinity and temperature and the highest Sr:Ca ratios in the otolith were observed when temperatures and salinities were both high. Functionally, both temperature and salinity cause Sr:Ca in otoliths to increase to asymptotic maxima. The maximum Sr:Ca due to increasing salinity was reached at around 10 psu and Sr:Ca never reached its asymptote due to the effect of temperature. This mid-saline maximum of Sr:Ca was also present in water Sr:Ca ratios and has consistently been found in other studies (Farmer et al. 2013; Lowe et al. 2009, 2011). This suggests that at salinities above 10 psu otolith Sr:Ca is no longer a good indicator of salinity in northern Gulf of Mexico estuaries and changes above this salinity may be more indicative of temperature.
The positive asymptotic relationship of otolith Sr:Ca with salinity in Gulf Killifish otoliths is a result of the similar underlying positive asymptotic relationship of water Sr:Ca with salinity. Given that water concentrations of Sr:Ca have been shown to be the main determinant of otolith Sr:Ca at least when confounding variables such as temperature are controlled for (Kraus and Secor 2004b; Walther and Thorrold 2006). The relationship of Sr:Ca found here is also what is generally expected with fresh water having lower Sr:Ca than salt water. However, this is not always the case; some coastal rivers and creeks in the United states can have Sr:Ca ranging from 10 to > 19 mmol mol−1 (Brown and Severin 2009; Kraus and Secor 2004b), well above the global average of 8.5 mmol mol−1 in seawater (de Villiers 1999). Brown and Severin (2009) also demonstrated that in the St. Johns river in Florida, the relationship of Sr:Ca with salinity is an exponential decline with fresh water endmembers having roughly 13.5 mmol mol−1 Sr:Ca concentrations decreasing to the global average of salt water. Their study continues to shed light on the complex relationships of Sr:Ca, given that the water concentrations of Sr:Ca were the main determinants of otolith Sr:Ca in freshwater and diadromous species, but not in marine species (Brown and Severin 2009). These results are in agreement with what was found for Gulf Killifish in our study, where differences in otolith Sr:Ca were present between low and mid-salinity, but above the 10 psu asymptote the temperature relationship was more important. The ubiquitous distribution of Sr:Ca in marine and many mid to high saline estuarine waters is the root cause, and all variation in otolith Sr:Ca must be due to other variables such as physiological influences and/or temperature when water concentrations are constant.
The positive relationship between temperature and Sr:Ca has been demonstrated previously in fish otoliths (Barnes and Gillanders 2013; Bath et al. 2000; Martin et al. 2004; Webb et al. 2012); however, other studies have shown no significant relationship (Campana 1999; Chesney et al. 1998; Secor et al. 1995) and even a negative relationship between temperature and Sr:Ca has been shown in fish otoliths (DiMaria et al. 2010; Radtke et al. 1990; Townsend et al. 1992; Townsend et al. 1995) and coral aragonite (de Villiers et al. 1994; Smith et al. 1979). These negative relationships in otoliths all occurred in northern species and it is thought that increased Sr:Ca is due to stress effects at low temperatures (Kalish 1992; Townsend et al. 1992). Overall though, the majority of studies are in agreement with this Gulf Killifish study and demonstrate a positive relationship between temperature and otolith Sr:Ca (Sturrock et al. 2012).
The positive correlation between otolith Sr:Ca and temperature must be driven by increased uptake of Sr into the fish, increased bioavailability of Sr in the blood and/or endolymph, thermodynamic effects on otolith precipitation and calcification rate, and/or an increased incorporation rate into the otolith, given that this study and others (Bath et al. 2000; Martin et al. 2004) have demonstrated that Sr:Ca in water does not increase with increasing temperature. One possibility for increased bioavailability would be that concentrations of Sr in the blood plasma and endolymph change as a result of changes in physiological processes such as reproduction, growth rate, and stress across temperatures (Kalish 1989, 1991, 1992; Walther et al. 2010).
An explanation of increased incorporation relies on the fact that fish growth is positively related to temperature until an optimum thermal maximum is reached (Brett 1979) and otoliths lay down new material at a rate proportional to that of fish growth (Campana and Neilson 1985). Therefore, as temperature increases, fish growth rate increases, as well as otolith growth rate and the resulting rate of new material (CaCO3) deposition to the otolith until the optimal thermal maximum. Perhaps at this increased rate of otolith calcification more Sr is incorporated into the otolith increasing the Sr:Ca ratio. Increased calcification rate has been shown to lead to increased impurities in inorganic CaCO3, increasing element:Ca ratios (Gaetani and Cohen 2006). However, Bath et al. (2000) suggested that increased temperature resulting in increased fish and otolith growth is not the case. Bath et al. (2000) showed no correlation between otolith mass and Sr:Ca or temperature and otolith mass.
Barium
Ba:Ca in the otolith exhibited an exponentially declining relationship with salinity and an exponential increase with temperature and combining these two models best fit the data. The variability at low salinities was accounted for by variation in temperature, and the highest Ba:Ca ratios were present at low salinities and high temperatures. This interactive effect of a positive relationship with temperature and a negative relationship with salinity on otolith Ba:Ca has been found in other estuarine species (Barnes and Gillanders 2013; Webb et al. 2012). However, no significant relationship of otolith Ba:Ca with temperature was found for Spot (Leiostomus xanthurus) (Martin and Thorrold 2005), and both positive (Miller 2009) and negative (DiMaria et al. 2010) relationships with temperature have been documented. Assuming that Ba behaves similarly to Sr in the otolith, the positive effect of temperature on otolith Ba:Ca is likely a result of one of the mechanisms mentioned for Sr:Ca, given that water Ba:Ca was also not correlated with temperature. However, Martin and Thorrold (2005) demonstrated that otolith growth rate also had no significant effect on otolith Ba:Ca ratios.
Water Ba:Ca concentrations are likely driving the negative relationship of otolith Ba:Ca with salinity, given that Ba:Ca in the otolith has been shown to be directly related to Ba:Ca in water (Miller 2009; Walther and Thorrold 2006), and Ba:Ca is generally negatively correlated with salinity (Mohan and Walther 2014; Walther and Limburg 2012; Walther and Nims 2014). The BB site in Louisiana had the highest water Ba:Ca ratios, the highest otolith Ba:Ca ratios, and experienced the lowest salinities; however, Ba:Ca in water was not significantly different than the FA site. The confounding effects of water Ba:Ca, salinity, and temperature on Ba:Ca otolith ratios cannot be disentangled here, given that BB exhibited some of the highest temperatures and lowest salinities. An increase of Ba water concentration has been suggested around 5–10 psu because at these salinities Ba desorption occurs rapidly from the clay particles and enters the dissolved phase (Coffey et al. 1997; Walther and Limburg 2012). The Ba:Ca in otolith vs. salinity plot suggests that this mid-saline peak, around 5 psu might be occurring; however, Ba:Ca in water does not show this pattern. This discrepancy could be driven by the fact that water sampling was not frequent enough to capture Ba:Ca pulses into the estuary as a result of increased river flow (Coffey et al. 1997; Hanor and Chan 1977). Another explanation could be that when these intermediate salinities were occurring, temperatures were high leading to increased Ba:Ca in the otolith without having increased Ba:Ca in the water.
Inputs of Ba besides increased river flow, could be present at the Baritaria Bay (BB) site potentially decoupling the salinity and Ba:Ca relationships. López-Duarte et al. (2016) demonstrated elevated Ba in otoliths from Grand Isle, Louisiana following the Deepwater Horizon oil spill, and attributed this to increased freshwater input following the spill. However, Sr increased concurrently with Ba during this time period, pointing to a potential decoupling of the Ba:Ca and salinity relationship. Other Ba sources that may have been present in their study and at the BB site are groundwater input (Shaw et al. 1998), seawater weathering of previously deposited riverine sediments (Carroll et al. 1993), and marine derived barite contributions (Colbert and McManus 2005). However, given the relationship between Ba:Ca in water and salinity demonstrated in our study, the general pattern of increasing salinity with decreasing Ba:Ca holds true, regardless of where the Ba is derived from.
Conclusions
The results of this study have implications for use of strontium and barium to reconstruct past salinities experienced by a fish. Based on our models, Sr:Ca in fish otoliths could be a useful indicator of temperature variation when salinity is above 10 psu or remains constant, as is the case for marine fishes. Sr:Ca is likely still useful as a salinity indicator at salinities less than 10 psu or for determining a shift from a fresh water to a saline habitat. Barium also exhibited a positive relationship with temperature; however, changes in Ba:Ca ratios should be indicative of salinity changes beyond 10 psu. Nims and Walther (2014) suggested that Ba:Ca was a better indicator of salinity variation in Texas estuaries instead of Sr:Ca, and according to our model this is likely the case in northern Gulf of Mexico estuaries as well.
Based on these results, a combination approach using both Sr:Ca and Ba:Ca would be best when trying to identify salinity use or movements of fishes determined through otolith chemistry, as has been suggested before (Nims and Walther 2014; Walther and Limburg 2012). For instance, if Ba:Ca is exhibiting a peak at low salinities, then Sr:Ca may be able to help refine salinity estimates. Once salinity increases above 10 psu and Sr:Ca is no longer viable as a salinity indicator, Ba:Ca could be more useful as an indicator element. Otolith chemical relationships with salinity are a function of the underlying water chemical gradients, and are only present if the water elemental ratios follow the typical patterns of increasing Sr:Ca and decreasing Ba:Ca with increasing salinity. However, these underlying chemical gradients can deviate from expected patterns (Brown and Severin 2009; Kraus and Secor 2004b). Therefore, investigation of the water chemistry across salinities in the system of interest is imperative. Species-specific Sr:Ca and Ba:Ca ratios should also be investigated across various salinities and temperatures of fish occurrence before inferences on movement or past salinity use are made.
Although there were confounding effects of temperature for both Sr:Ca and Ba:Ca, it is likely that the difference in otoliths observed between purely fresh water and salt water, would be greater than those as a result of temperature. Therefore, these ratios would still prove useful for characterizing diadromous fish migrations or large salinity shifts of euryhaline species, as has been done before (Kerr et al. 2009; Nims and Walther 2014; Walther and Limburg 2012). However, given that the majority of our sampling occurred at 5 psu and greater, and Gulf Killifish are resident, this was impossible to test here.
When investigating salinity shifts of fishes across multiple years, temperature effects may become evident as clear seasonal patterns that can be correlated with expected annual temperature cycles. It may then be possible to account for this variation and the remaining ratio shifts may be attributable to salinity, baring other confounding variables. Caution should be used with this approach however, especially in within-year salinity reconstructions when only 1 year of otolith data is available, given that it would be difficult to separate the effects of salinity and temperature. The correlation of temperature with increased otolith Sr:Ca and Ba:Ca must be a result of endogenous processes within the fish, and the driving mechanisms of this relationship in Gulf Killifish and other species are still not well understood. Future experiments are needed to elucidate whether these mechanisms involve growth, stress, and/or other physiological controls. The Gulf Killifish would provide an ideal organism for these experiments, given that they are easily collectable, tolerant to a wide range of abiotic conditions, and should prove amenable to experimental tanks and mesocosms.
References
Albuquerque, C.Q., N. Miekeley, J.H. Muelbert, B.D. Walther, and A.J. Jaureguizar. 2012. Estuarine dependency in a marine fish evaluated with otolith chemistry. Marine Biology 159 (10): 2229–2239. https://doi.org/10.1007/s00227-012-2007-5.
Anstead, K.A., J.J. Schaffler, and C.M. Jones. 2015. Coastwide otolith signatures of juvenile Atlantic menhaden, 2009–2011. Transactions of the American Fisheries Society 144 (1): 96–106.
Barnes, T.C., and B.M. Gillanders. 2013. Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions. Canadian Journal of Fisheries and Aquatic Sciences 70 (8): 1159–1166. https://doi.org/10.1139/cjfas-2012-0442.
Bath, G.E., S.R. Thorrold, C.M. Jones, S.E. Campana, J.W. McLaren, and J.W.H. Lam. 2000. Strontium and barium uptake in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 64 (10): 1705–1714. https://doi.org/10.1016/s0016-7037(99)00419-6.
Brett, J.R. 1979. Environmental factors and growth. In Fish physiology, volume 8: Bioenergetics and growth, ed. W.S. Hoar, D.J. Randall, and J.R. Brett, 599–675. New York: Academic Press.
Brown, R.J., and K.P. Severin. 2009. Otolith chemistry analyses indicate that water Sr: Ca is the primary factor influencing otolith Sr: Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Sciences 66 (10): 1790–1808. https://doi.org/10.1139/F09-112.
Brownie, C., and D.D. Boos. 1994. Type I error robustness of ANOVA and ANOVA on ranks when the number of treatments is large. Biometrics 50 (2): 542–549. https://doi.org/10.2307/2533399.
Campana, S.E. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188: 263–297. https://doi.org/10.3354/meps188263.
Campana, S.E., and J.D. Neilson. 1985. Microstructure of fish otoliths. Canadian Journal of Fisheries and Aquatic Sciences 42 (5): 1014–1032. https://doi.org/10.1139/f85-127.
Carroll, J., K.K. Falkner, E.T. Brown, and W.S. Moore. 1993. The role of the Ganges-Brahmaputra mixing zone in supplying barium and 226Ra to the bay of Bengal. Geochimica et Cosmochimica Acta 57 (13): 2981–2990. https://doi.org/10.1016/0016-7037(93)90287-7.
Chesney, E.J., B.M. McKee, T. Blanchard, and L.H. Chan. 1998. Chemistry of otoliths from juvenile menhaden Brevoortia patronus: evaluating strontium, strontium: calcium and strontium isotope ratios as environmental indicators. Marine Ecology Progress Series 171: 261–273.
Coffey, M., F. Dehairs, O. Collette, G. Luther, T. Church, and T. Jickells. 1997. The behaviour of dissolved barium in estuaries. Estuarine, Coastal and Shelf Science 45 (1): 113–121. https://doi.org/10.1006/ecss.1996.0157.
Colbert, D., and J. McManus. 2005. Importance of seasonal variability and coastal processes on estuarine manganese and barium cycling in a Pacific northwest estuary. Continental Shelf Research 25 (11): 1395–1414. https://doi.org/10.1016/j.csr.2005.02.003.
de Villiers, S. 1999. Seawater strontium and Sr/Ca variability in the Atlantic and Pacific oceans. Earth and Planetary Science Letters 171 (4): 623–634. https://doi.org/10.1016/s0012-821x(99)00174-0.
de Villiers, S., G.T. Shen, and B.K. Nelson. 1994. The Sr/Ca-temperature relationship in coralline aragonite: Influence of variability in (Sr/Ca)seawater and skeletal growth parameters. Geochimica et Cosmochimica Acta 58 (1): 197–208.
DiMaria, R.A., J.A. Miller, and T.P. Hurst. 2010. Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus Macrocephalus. Environmental Biology of Fishes 89 (3): 453–462. https://doi.org/10.1007/s10641-010-9665-2.
Elsdon, T.S., and B.M. Gillanders. 2002. Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59 (11): 1796–1808. https://doi.org/10.1139/f02-154.
Elsdon, T.S., and B.M. Gillanders. 2005. Strontium incorporation into calcified structures: separating the effects of ambient water concentration and exposure time. Marine Ecology Progress Series 285: 233–243.
Farmer, T.M., D.R. DeVries, R.A. Wright, and J.E. Gagnon. 2013. Using seasonal variation in otolith microchemical composition to indicate largemouth bass and southern flounder residency patterns across an estuarine salinity gradient. Transactions of the American Fisheries Society 142 (5): 1415–1429. https://doi.org/10.1080/00028487.2013.806348.
Fodrie, F.J., and S.Z. Herzka. 2008. Tracking juvenile fish movement and nursery contribution within arid coastal embayments via otolith microchemistry. Marine Ecology - Progress Series 361: 253–265. https://doi.org/10.3354/meps07390.
Gaetani, G.A., and A.L. Cohen. 2006. Element partitioning during precipitation of aragonite from seawater: a framework for understanding paleoproxies. Geochimica et Cosmochimica Acta 70 (18): 4617–4634. https://doi.org/10.1016/j.gca.2006.07.008.
Gillanders, B.M., C. Izzo, Z.A. Doubleday, and Q. Ye. 2015. Partial migration: growth varies between resident and migratory fish. Biology Letters 11 (3): 20140850.
Hanor, J.S., and L.H. Chan. 1977. Non-conservative behavior of barium during mixing of Mississippi River and Gulf of Mexico waters. Earth and Planetary Science Letters 37 (2): 242–250. https://doi.org/10.1016/0012-821x(77)90169-8.
Joung, D., and A.M. Shiller. 2014. Dissolved barium behavior in Louisiana shelf waters affected by the Mississippi/Atchafalaya River mixing zone. Geochimica et Cosmochimica Acta 141: 303–313.
Kahn, A., and G.D. Rayner. 2003. Robustness to non-normality of common tests for the many-sample location problem. Journal of Applied Mathematics and Decision Sciences 7: 187–206.
Kalish, J.M. 1989. Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. Journal of Experimental Marine Biology and Ecology 132 (3): 151–178.
Kalish, J.M. 1991. Determinants of otolith chemistry: seasonal variation in the composition of blood plasma, endolymph and otoliths of bearded rock cod Pseudophycis barbatus. Marine Ecology-Progress Series 74 (2–3): 137–159. https://doi.org/10.3354/meps074137.
Kalish, J.M. 1992. Formation of a stress-induced chemical check in fish otoliths. Journal of Experimental Marine Biology and Ecology 162 (2): 265–277. https://doi.org/10.1016/0022-0981(92)90206-P.
Kerr, L.A., D.H. Secor, and P.M. Piccoli. 2009. Partial migration of fishes as exemplified by the estuarine-dependent white perch. Fisheries 34 (3): 114–123.
Kraus, R.T., and D.H. Secor. 2004a. Dynamics of white perch Morone Americana population contingents in the Patuxent River estuary, Maryland, USA. Marine Ecology Progress Series 279: 247–259.
Kraus, R.T., and D.H. Secor. 2004b. Incorporation of strontium into otoliths of an estuarine fish. Journal of Experimental Marine Biology and Ecology 302 (1): 85–106. https://doi.org/10.1016/j.jembe.2003.10.004.
Longerich, H.P., S.E. Jackson, and D. Gunther. 1996. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. Journal of Analytical Atomic Spectrometry 11 (9): 899–904. https://doi.org/10.1039/ja9961100899.
López-Duarte, P.C., F.J. Fodrie, O.P. Jensen, A. Whitehead, F. Galvez, B. Dubansky, and K.W. Able. 2016. Is exposure to Macondo oil reflected in the Otolith chemistry of marsh-resident fish? PLoS One 11 (9): e0162699. https://doi.org/10.1371/journal.pone.0162699.
Lowe, M.R., D.R. DeVries, R.A. Wright, S.A. Ludsin, and B.J. Fryer. 2009. Coastal largemouth bass (Micropterus salmoides) movement in response to changing salinity. Canadian Journal of Fisheries and Aquatic Sciences 66 (12): 2174–2188. https://doi.org/10.1139/f09-152.
Lowe, M.R., D.R. DeVries, R.A. Wright, S.A. Ludsin, and B.J. Fryer. 2011. Otolith microchemistry reveals substantial use of freshwater by southern flounder in the northern Gulf of Mexico. Estuaries and Coasts 34 (3): 630–639. https://doi.org/10.1007/s12237-010-9335-9.
Macdonald, J.I., and D.A. Crook. 2010. Variability in Sr: Ca and Ba: Ca ratios in water and fish otoliths across an estuarine salinity gradient. Marine Ecology Progress Series 413: 147–161.
Martin, G.B., and S.R. Thorrold. 2005. Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Marine Ecology Progress Series 293: 223–232.
Martin, G.B., S.R. Thorrold, and C.M. Jones. 2004. Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Canadian Journal of Fisheries and Aquatic Sciences 61 (1): 34–42. https://doi.org/10.1139/f03-143.
Miller, J.A. 2009. The effects of temperature and water concentration on the otolith incorporation of barium and manganese in black rockfish Sebastes Melanops. Journal of Fish Biology 75 (1): 39–60. https://doi.org/10.1111/j.1095-8649.2009.02262.x.
Miller, J.A. 2011. Effects of water temperature and barium concentration on otolith composition along a salinity gradient: implications for migratory reconstructions. Journal of Experimental Marine Biology and Ecology 405 (1): 42–52. https://doi.org/10.1016/j.jembe.2011.05.017.
Mohan, J.A., and B.D. Walther. 2014. Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: II. Regional, local, and seasonal salinity-element relationships. Estuaries and Coasts 38 (3): 769–781.
Mohan, J.A., R.A. Rulifson, D.R. Corbett, and N.M. Halden. 2012. Validation of Oligohaline elemental Otolith signatures of striped bass by use of in situ caging experiments and water chemistry. Marine and Coastal Fisheries 4 (1): 57–70. https://doi.org/10.1080/19425120.2012.656533.
Morissette, O., F. Lecomte, G. Verreault, M. Legault, and P. Sirois. 2016. Fully equipped to succeed: migratory contingents seen as an intrinsic potential for striped bass to exploit a heterogeneous environment early in life. Estuaries and Coasts 39 (2): 571–582. https://doi.org/10.1007/s12237-015-0015-7.
Nelson, T.R., D. Sutton, and D.R. DeVries. 2014. Summer movements of the Gulf killifish (Fundulus Grandis) in a northern Gulf of Mexico salt marsh. Estuaries and Coasts 37 (5): 1295–1300. https://doi.org/10.1007/s12237-013-9762-5.
Nelson, T.R., D.R. DeVries, R.A. Wright, and J.E. Gagnon. 2015. Fundulus grandis otolith microchemistry as a metric of estuarine discrimination and oil exposure. Estuaries and Coasts 38 (6): 2044–2058. https://doi.org/10.1007/s12237-014-9934-y.
Nims, M.K., and B.D. Walther. 2014. Contingents of southern flounder from subtropical estuaries revealed by otolith chemistry. Transactions of the American Fisheries Society 143 (3): 721–731.
Radtke, R.L., D.W. Townsend, S.D. Folsom, and M.A. Morrison. 1990. Strontium: calcium concentration ratios in otoliths of herring larvae as indicators of environmental histories. Environmental Biology of Fishes 27 (1): 51–61. https://doi.org/10.1007/BF00004904.
Reis-Santos, P., S.E. Tanner, T.S. Elsdon, H.N. Cabral, and B.M. Gillanders. 2013. Effects of temperature, salinity and water composition on otolith elemental incorporation of Dicentrarchus labrax. Journal of Experimental Marine Biology and Ecology 446: 245–252. https://doi.org/10.1016/j.jembe.2013.05.027.
Rooker, J.R., R.T. Kraus, and D.H. Secor. 2004. Dispersive behaviors of black drum and red drum: Is otolith Sr : Ca a reliable indicator of salinity history? Estuaries 27 (2): 334–341. https://doi.org/10.1007/bf02803389.
Rozas, L.P., and D.J. Reed. 1993. Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence. Marine Ecology-Progress Series 96 (2): 147–157. https://doi.org/10.3354/meps096147.
Rozas, L.P., and R.J. Zimmerman. 2000. Small-scale patterns of nekton use among marsh and adjacent shallow nonvegetated areas of the Galveston Bay Estuary, Texas (USA). Marine Ecology-Progress Series 193: 217–239. https://doi.org/10.3354/meps193217.
Schroeder, W.W., S.P. Dinnel, and W.J. Wiseman. 1990. Salinity stratification in a river-dominated estuary. Estuaries 13 (2): 145–154.
Secor, D.H., A. Hendersonarzapalo, and P.M. Piccoli. 1995. Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes. Journal of Experimental Marine Biology and Ecology 192 (1): 15–33. https://doi.org/10.1016/0022-0981(95)00054-u.
Shaw, T.J., W.S. Moore, J. Kloepfer, and M.A. Sochaski. 1998. The flux of barium to the coastal waters of the southeastern USA: the importance of submarine groundwater discharge. Geochimica et Cosmochimica Acta 62 (18): 3047–3054. https://doi.org/10.1016/s0016-7037(98)00218-x.
Smith, S.V., R.W. Buddemeier, R.C. Redalje, and J.E. Houck. 1979. Strontium-calcium thermometry in coral skeletons. Science 204 (4391): 404–407. https://doi.org/10.1126/science.204.4391.404.
Sturrock, A.M., C.N. Trueman, A.M. Darnaude, and E. Hunter. 2012. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81 (2): 766–795. https://doi.org/10.1111/j.1095-8649.2012.03372.x.
Townsend, D.W., R.L. Radtke, S. Corwin, and D.A. Libby. 1992. Strontium: calcium ratios in juvenile Atlantic herring Clupea harengus L. otoliths as a function of water temperature. Journal of Experimental Marine Biology and Ecology 160 (1): 131–140. https://doi.org/10.1016/0022-0981(92)90115-Q.
Townsend, D.W., R.L. Radtke, D.P. Malone, and J.P. Wallinga. 1995. Use of otolith strontium:calcium ratios for hindcasting larval cod Gadus morhua distributions relative to water masses on Georges Bank. Marine Ecology Progress Series 119 (1/3): 37–44.
Underwood, A.J. 1997. Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge: Cambridge University Press.
Vasconcelos, R.P., P. Reis-Santos, S. Tanner, V. Fonseca, C. Latkoczy, D. Gunther, M.J. Costa, and H. Cabral. 2007. Discriminating estuarine nurseries for five fish species through otolith elemental fingerprints. Marine Ecology-Progress Series 350: 117–126. https://doi.org/10.3354/meps07109.
Walther, B.D., and K.E. Limburg. 2012. The use of otolith chemistry to characterize diadromous migrations. Journal of Fish Biology 81 (2): 796–825. https://doi.org/10.1111/j.1095-8649.2012.03371.x.
Walther, B.D., and M.K. Nims. 2014. Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: I. Freshwater endmembers and mixing curves. Estuaries and Coasts 38 (3): 754–768.
Walther, B.D., and S.R. Thorrold. 2006. Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Marine Ecology-Progress Series 311: 125–130. https://doi.org/10.3354/meps311125.
Walther, B.D., M.J. Kingsford, M.D. O’Callaghan, and M.T. McCulloch. 2010. Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environmental Biology of Fishes 89 (3): 441–451. https://doi.org/10.1007/s10641-010-9661-6.
Webb, S.D., S.H. Woodcock, and B.M. Gillanders. 2012. Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Marine Ecology Progress Series 453: 189–199. https://doi.org/10.3354/meps09653.
Acknowledgements
This project would not have been possible without field help and we would like to thank Adrian Stanfill and all other Ireland Center staff and students who helped collect samples, as well as Dr. Ash Bullard for help with sample site selection and protocol. Special thanks to Tammy DeVries for pulling miniscule otoliths and dealing with strong H2O2 for cleaning purposes. Two reviewers greatly improved the manuscript and we thank them for their comments.Finally, we would like to thank Dr. Joel E. Gagnon and Dr. Mohamed Shaheen of the University of Windsor GLIER for the help with the LA-ICP-MS.
Funding
Funding for this research was provided by British Petroleum. This manuscript was supported by the Alabama Agricultural Experiment Station and the Hatch program of the National Institute of Food and Agriculture, U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S. Peterson
Rights and permissions
About this article
Cite this article
Nelson, T.R., DeVries, D.R. & Wright, R.A. Salinity and Temperature Effects on Element Incorporation of Gulf Killifish Fundulus grandis Otoliths. Estuaries and Coasts 41, 1164–1177 (2018). https://doi.org/10.1007/s12237-017-0341-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0341-z