Abstract
We compared the responses of two estuarine phytoplankton communities, one from a temperate (Chubut River estuary (CH), Argentina) and one from a sub-tropical site (Babitonga Bay (BB), Brazil), in a scenario of nutrient enrichment under solar ultraviolet radiation (UVR) exposure. Seawater samples were exposed in microcosms to two nutrients, ambient vs. enriched, and two radiation conditions, with and without UVR, and exposed to solar radiation for 4 days. We evaluated the short- (PSII photochemistry, during 90 min light and 90 min dark cycles, before and after the 4 days of acclimation) and mid-term effects (growth and taxonomic changes) of the phytoplankton communities. Before acclimation, short-term UVR effects were dominant in CH, whereas in BB, nutrient effects prevailed. Such differences were related to the previous light history of the cells and to the ambient nutrient status. After acclimation, an overall improvement of the photosynthetic performance was observed at both sites, either by reducing the relative inhibition or by increasing the recovery of the effective photochemical quantum yield. Interactive effects of UVR and nutrients on growth at CH were antagonistic, while at BB, no differences were observed between the interactive and the sum of effects. Part of the differences in the mid-term observed responses can be attributed to taxonomic changes, with the CH community dominated by diatoms throughout the experiment, but with a shift from a diatom to a flagellate-dominated community in BB. Temperature differences between both sites might have favored higher growth rates and flagellates dominance in BB under the nutrient enriched conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in several abiotic variables, either due to the natural variability or due to anthropogenic activities, collectively named as “global change,” are profoundly affecting the metabolism and performance of aquatic organisms as well as ecosystems’ goods and services (Chapin et al. 2000; de Groot et al. 2002; IPCC 2013). The release of large amounts of greenhouse gases and chlorofluorocarbons into the atmosphere has been translated into increases of surface water temperature (IPCC 2013) and of solar radiation levels (particularly of ultraviolet radiation (UVR) 280–400 nm) due to increasing stratification of the water column (Häder et al. 2014). In addition, coastal ecosystems and particularly estuaries will continue to receive increased amounts of nutrients due to a combination of urban-derived activities, agriculture (Rabalais et al. 2009), and global change issues that also result in higher amounts of dissolved organic matter (DOM) and sediments (due to river runoff) into the system (Cloern et al. 2014; Häder et al. 2014; IPCC 2013). This situation will not only enhance the eutrophication (Cloern 2001; Rabalais et al. 2009) but will also change the penetration of solar radiation in coastal environments as compared to that from pelagic areas (Häder et al. 2014). Due to this relatively dark underwater light environment and thus a previous light history of low irradiances, coastal phytoplanktons are more sensitive to solar radiation (Litchman and Neale 2005) and/or because they generally have lower amounts of photoprotective compounds (Ayoub et al. 2012).
Some different latitudinal trends of the effects of global change variables on marine organisms have been observed (Tittensor et al. 2010; Thomas et al. 2012). For example, when considering only the effects of solar UVR upon phytoplankton primary productivity, tropical species are more resistant than polar ones, due to previous acclimation to high levels of solar radiation (Helbling et al. 1992). Similarly, the effects of increased temperature are generally more beneficial in temperate species than in tropical ones, because the latter may be already at their upper tolerance limit (Banaszak and Lesser 2009; Thomas et al. 2012). Thus, latitudinal comparisons are very useful, as they provide information on a range of responses that can occur when different communities are exposed in a similar manner to variables or stressors affected by global change.
Photosystem II (PSII) is one of the main targets of UVR which negatively affects the maximum quantum yield and electron transport rates (ETR) of phytoplankton (Liang et al. 2006). However, the interaction between variables can potentiate or mitigate the individual effects of them. It is well documented that nutrient addition generally counteracts the negative effects of solar UVR on phytoplankton photosynthesis (Beardall et al. 2009; Häder et al. 2011); thus, the combined effects of both variables are generally antagonistic (Marcoval et al. 2007; Harrison and Smith 2013), although synergistic effects can also occur (Carrillo et al. 2008). Previous studies have shown that the interactive effects of global change variables are rather ubiquitous on plankton communities (Christensen et al. 2006; Crain et al. 2008); however, the type of interaction, as well as their extent, varies in spatial and temporal sense, making global predictions rather difficult to obtain. These responses are also complicated by the fact that the communities tend to acclimate to the experimental variables; thus, the responses generally differ over short-, mid-, and long-term periods (Litchman and Neale 2005; Sobrino et al. 2008).
The aim of this study is to compare and to obtain a range of photosynthetic responses when two estuarine phytoplankton communities (one from a sub-tropical site and one from a temperate site) are exposed in a similar manner to solar ultraviolet radiation under predicted conditions, for coastal areas, of high input of nutrients (e.g., IPCC 2013). We further considered how the potential acclimation of phytoplankton over a 4-day period would affect the growth and species composition of these communities exposed to solar UVR and increased nutrients conditions. Our working hypotheses are as follows: (1) at both sites, short-term nutrient enrichment does not affect the PSII photochemistry, as both areas normally receive high amounts of nutrients carried by their rivers; and (2) solar ultraviolet radiation has a higher impact on growth in the temperate community as compared to the sub-tropical one due to the previous light history to high radiation levels at lower latitudes.
Materials and Methods
Study Sites
This study was carried out in two estuaries of the southern Hemisphere: the Chubut River estuary, a temperate site (Chubut Province, Argentina, 43° 20.5′ S, 65° 02.0′ W), hereafter CH, and the Babitonga Bay estuary, a sub-tropical site (Santa Catarina State, Brazil, 26° 09′ S; 48° 33.5′ W), hereafter BB, both on the Atlantic coast of South America (Fig. 1). The general characteristics of the study sites are the following:
-
1.
Chubut River estuary: It is a coastal plain meso-tidal estuary (Piccolo and Perillo 1999), characterized by a wide range of physical, chemical, and biological conditions due to the interaction between the river and the sea (Helbling et al. 2010; Villafañe et al. 2004). The site has a characteristic seasonal succession, with a microplankton diatom bloom that usually occurs during June–August (winter time; Barbieri et al. 2002; Villafañe et al. 2004; Villafañe et al. 2013; Helbling et al. 2005). Phytoplankton from CH have gained much attention due to their outmost importance as the base of a highly productive aquatic food web (Pájaro et al. 2005; Skewgar et al. 2007). Also, in the context of global change, these natural phytoplankton communities have been the focus of several studies that evaluated the joint effects of solar radiation and of other abiotic variables on their physiology and ecology (Barbieri et al. 2002; Villafañe et al. 2013; Villafañe et al. 2015; Helbling et al. 2015).
-
2.
Babitonga Bay estuary: It is the largest estuary of the Santa Catarina State (area: 1567 km2), with several rivers discharging into the bay. This is an area of economic and social relevance, as activities such as intense fishing are carried out in its surroundings, with two large ports located within the bay. Some areas of the bay are severely contaminated due to sewage disposal from nearby cities (Martins et al. 2014). Several phytoplankton studies have been performed in BB, mainly focusing on the distribution and the temporal variability of communities, with a microplankton diatom bloom occurring during spring/early summer (Pereira Camacho and Souza-Conceição 2007; Parizzi 2014), as well as on the taxonomy of species (Brandini et al. 2006; Fernandes and Pereira Brandini 2010; Parizzi 2013, 2014). Recently, one study carried out by our research group has addressed the responses to global change variables of phytoplankton from the inlet waters of this estuary (Villafañe et al. 2014).
Sampling and Experimentation
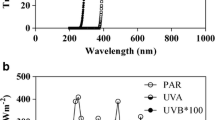
The experiments were carried out in CH during September 9–12 (Julian days 252–255), 2014, whereas those performed in BB were done during March 16–19 (Julian days 77–80), 2011. These dates were chosen in order to conduct the experiments with post-bloom phytoplankton communities from both sites. In addition, both phytoplankton communities had similar light histories, and during the exposure to solar radiation, they received comparable energy ratios PAR/UV-A/UV-B (see below).
The experimental setup was identical for both sites: Samples for experimentation were collected in a site close to the mouth of the estuaries, at their seawater end (Fig. 1). Surface (i.e., from the upper 0.5 m) water samples were collected late in the afternoon on the day before the experiment started, using acid-clean (HCl 1 N) dark containers and immediately transported to the laboratory (~15–30 min away from the sampling sites). At each site, samples were pre-screened through a 200 μm Nitex mesh to eliminate large zooplankton and put into 12 10 l (microcosms) UV-transparent bags (Alpax Trade Lab, São Paulo, Brazil; 72 % transmission at 280 nm). A 2 × 2 experimental matrix, using triplicates, was implemented, with solar radiation and nutrients as factors as follows: Six microcosms were maintained with nutrients at their ambient concentration at the sampling time, whereas in the other six microcosms, macronutrients were added (i.e., 60 μmol of NO3 −, 50 μmol of PO4 3−,, and 20 μmol of SiO3 2− per liter of sample). The 12 microcosms containing the samples were put in tanks with circulating water to keep the in situ temperature and exposed to full solar radiation under two radiation treatments: (1) with UVR (+UVR), samples receiving PAR + UV-A + UV-B, uncovered microcosms; (2) without UVR (−UVR), samples receiving only PAR (>400 nm), microcosms wrapped with Ultraphan UV Opak 395 Digefra film. By using this set up (which mimicked the worst case scenario for solar radiation, as the cells were under a few centimeters of water), we obtained the four different nutrients and radiation treatments of our experimental matrix, and throughout the text they will be referred as (1) Namb−UVR, samples under ambient nutrient concentration without UVR; (2) Namb+UVR, samples under ambient nutrient concentration receiving UVR; (3) Nenr−UVR, samples under increased nutrient concentration without UVR; and (4) Nenr+UVR, samples under increased nutrient concentration receiving UVR. The microcosms were filled the night previous to the exposure to solar radiation, and the samples were subjected to the different experimental conditions for 4 days.
To determine the short-term photosynthesis responses (PSII photochemistry) to the different nutrient/radiation conditions, samples were taken at day 1 and at day 4 (i.e., end of the acclimation period), placed in 50-ml quartz tubes, and exposed to full solar radiation under two conditions, PAB (>280 nm—as above) and P (>400 nm—as above), for 90 min (“light” period i.e., from 10:30-11:00 to 12-12:30 h), followed by 90 min recovering under dim PAR light (“dark” period, under a PAR level of ca. 50 μmol photon m−2 s−1).
At day 1, we used sub-samples (i.e., Namb and Nenr) from the initial water that did not have any previous acclimation to the new solar radiation conditions (sampled at ca. 9:30 a.m.). So, for day 1, we had a total of 12 tubes, i.e., triplicates for Namb−UVR, Namb+UVR, Nenr−UVR, and Nenr+UVR. However, since at day 4, we had samples coming from the 12 microcosms and we exposed sub-samples of them to 2 radiation treatments, the total number of tubes in this set up was 24 for each sampling site. The whole setup of quartz tubes was put in a water bath for temperature control, with samples kept at their respective in situ temperature that were 10 ± 1 °C (in CH) and 25 ± 1 °C (in BB) and exposed to solar radiation around local noon. Mid-term effects, i.e., growth and taxonomic changes, were also evaluated over the experimental time frame.
Analyses and Measurements
Fluorescence Parameters
Sub-samples (3 ml) were taken at the moment of the daily sampling (ca. 9:30 a.m.) and then from the quartz tubes during the short-term exposures to measure every 15–20 min in vivo chlorophyll a (Chl-a) fluorescence parameters of the photosystem II, using a portable pulse amplitude modulated (PAM) fluorometer (Walz, model Water-ED PAM). The samples were measured six times immediately after sampling, with each measurement lasting 10 s; therefore, the total time for measuring each sample was 1 min, without any dark adaptation period. The photochemical effective quantum yield (ΦPSII) was calculated using the equations of Genty et al. (1990) and Weis and Berry (1987) as
where F′m is the maximum fluorescence in the light-exposed cells induced by a saturating light pulse (ca. 5300 μmol photons m−2 s−1 in 0.8 s) and Ft the current steady state fluorescence induced by an actinic light (492 μmol photons m−2 s−1—peak at 660 nm) in light-adapted cells.
The non-photochemical quenching (NPQ) of Chl-a fluorescence, used as a proxy of the dissipation of the excess energy as heat, was determined by measuring Fm, the maximum fluorescence in the original sample, and F′m during the short-term exposures. For the determination of the maximum fluorescence, the initial sample (collected late in the afternoon before the experiment started) was left overnight in darkness at the in situ temperature and measured early the following morning; the obtained value was stored by the PAM software. NPQ was calculated following the equation of Schreiber et al. (1994) as
Chlorophyll-a
Chl-a concentration was measured on the initial samples as well as on a daily basis up to the end of the experiment by filtering 100–500 ml of sample (depending on the site and on the time of collection during each experiment) onto Munktell MG-F (CH) or Whatman GF/F (BB) glass fiber filters (25 mm) and extracting the photosynthetic pigments in absolute methanol (Holm-Hansen and Riemann 1978). A scan between 250 and 750 nm was done using a spectrophotometer (Hewlett Packard model HP 8453E in CH, Shimadzu UV-1601PC in BB), and Chl-a concentration was calculated using the equations of Porra (2002).
Counting and Identification of Phytoplankton Cells
Samples for the identification and counting of phytoplankton cells (>2 μm, i.e., nano- and microplankton) were collected at day 1 and at day 4. The samples were placed in brown glass bottles and fixed with buffered formalin (final concentration 0.4 % of formaldehyde in the sample). Sub-samples of 10–25 ml were allowed to settle for 24 h in a Utermöhl chamber (Hydro-Bios GmbH), and species were identified and enumerated using an inverted microscope (Leica model DM IL) following the technique described by Villafañe and Reid (1995).
The specific growth rates (μ) in each treatment were calculated using both the Chl-a concentration and cell abundances; no differences between the two methods used were observed. Since the daily measurements of Chl-a demonstrated that the samples were in the exponential growth phase, the initial and final data points of cell abundances were used to obtain information on the growth rates of the different taxonomic groups (diatoms, flagellates, and dinoflagellates) as
where N 0 and N 1 represent the initial and final cell concentration of cells at the initial time (t 0) and at the end (t 1) of the experimental period.
Solar Radiation
Solar radiation was continuously monitored using an European Light Dosimeter Network broadband filter radiometer (ELDONET, Real-Time Computers) that measures UV-B (280–315 nm), UV-A (315–400 nm), and PAR (400–700 nm) every second, averages the data over a 1-min interval, and stores them in a computer. This radiometer is routinely calibrated (once a year) using a solar calibration procedure. For this calibration, the irradiance data during a clear sky condition was compared with the output of radiation transfer models such as STAR (Ruggaber et al. 1994) and Daylight (Björn and Murphy 1985). This same procedure was used over a decade for radiometers of the ELDONET network distributed around the World (Häder et al. 2007).
Physical, Optical, and Chemical Characteristics of the Water Column
Vertical profiles of temperature, conductivity, dissolved oxygen, and pH were obtained using a multiparameter probe (Yellow Spring Instruments, model 600XLM in CH; HANNA HI 9828 with HI 769828 sensor in BB) at noon on the sampling day. Chemical characteristics of seawater were also measured, i.e., phosphate, silicate, nitrate + nitrite by standard techniques (Strickland and Parsons 1972) using a spectrophotometer (Hewlett Packard model HP 8453E) in CH, and kit reagents and a spectrophotometer (Hach Odyssey DR 2500) in BB. The underwater radiation field was measured with a spectroradiometer (Ocean Optics, HR 2000CG UV-NIR) at both sites.
Data Treatment and Statistics
The microcosms were set up in triplicates, so during each short-term exposure triplicates were used for each radiation/nutrient condition. Thus, mean values and standard deviations are reported in the figures.
Since the phytoplankton communities at both sites were different, and consequently the fluorescence responses (i.e., ΦPSII) were different as well, we calculated the inhibition (%) and recovery (%) as compared to the initial effective photochemical quantum yield (ΦPSII) within each sampling site community as
where ΦPSIIto and ΦPSIItL are the effective photochemical quantum yield of PSII at the initial time and at the end of the exposure period (i.e., after 90 min of exposure), respectively, while ΦPSIItD is the effective photochemical quantum yield of PSII at the end of the dark period (i.e., after 90 min under dim light).
We calculated the single effects of UVR and nutrient enrichment on the specific growth rates as:
where the control represents samples at ambient nutrients without UVR (i.e., Namb−UVR) in all cases, and variablesingle represents (i) samples under ambient nutrients with UVR (Namb+UVR) for UVR effects and (ii) samples under enriched nutrients without UVR (Nenr−UVR) for nutrients enrichment effects. Error propagation was used to calculate the variance of UVR and nutrient enrichment (%).
The interactive effects of nutrient enrichment × UVR on specific growth rates were calculated as
where the control represents samples at ambient nutrients without UVR (Namb−UVR), and the variablemultiple represents samples under enriched nutrients with UVR (Nenr+UVR). Error propagation was also used to calculate the variance of the interactive effects (%).
Three-way ANOVAs were used to determine differences in inhibition and recovery as a function of the nutrient and radiation treatments for the initial samples, while four-way ANOVAs were used for the data obtained at the end of the experimental period, with nutrients, light acclimation history, radiation exposure treatments, and processes (i.e., inhibition–recovery) as factors. Three-way ANOVAs were also used to determine differences in the specific growth rates as a function of nutrients, light acclimation history, and taxonomic group. Two-way ANOVAs were used to determine differences in the effects (i.e., single variables, interactive, and sum) on growth rates of the main taxonomic groups between sites. When significant differences were determined, post hoc Tukey’s tests were performed. For all statistical analysis, normality and homoscedasticity of the data set were tested using the Kolmogorov–Smirnov and Levene tests, respectively. Significance between the samples exposed to different treatments was established using a 95 % confidence limit (Zar 1999).
Results
General Characteristics of the Study Sites
The mean initial characteristics of surface waters of the two estuaries sampled are shown in Table 1. Surface waters collected in CH were colder, more saline, and with higher Chl-a concentration than those from BB. Nutrients also displayed some differences among sites: CH had ca. three times higher phosphate concentration as compared to that in BB; however, silicate concentrations were higher (ca. three times) in BB than in CH. On the other hand, similar concentrations of nitrate + nitrite were found in both study sites. Also, waters from CH were more turbid than those from BB, with PAR and UVR attenuation coefficients (kPAR and kUVR, respectively) that were ~2 and 1.5 times higher in CH, respectively. The mean daily irradiances received by the cells during the experiments carried out at BB were slightly higher than those at CH; however, the ratios PAR/UV-A/UV-B were rather comparable (i.e., 1/0.14/0.0026 for CH and 1/0.14/0.0038 for BB) although with higher relative UV-B in the sub-tropical site, as expected due to different solar zenith angles in the two study sites.
Short-Term Responses: ΦPSII Dynamics
Chubut River Estuary
The ΦPSII decreased as soon as the exposure started and remained low throughout the light period; partial or almost complete recovery was observed once the stress was removed (Fig. 2). In the initial condition (Fig. 2a), there were clear differences among radiation treatments, with samples under the P treatment having higher ΦPSII than those under PAB treatment, regardless of the nutrient conditions. These differences, however, were reduced after acclimation with UVR (+UVR; Fig. 2b) or without UVR (−UVR; Fig. 2c). A fine detail of the inhibition and recovery dynamics (expressed as inhibition and recovery percentages at the end of the exposure and recovery periods, respectively; see Eqs. 4 and 5) during these short-term experiments (Fig. 3) showed that at the initial time (Fig. 3a), samples incubated under the PAB had significantly higher inhibition than those under the P treatment, within any nutrient conditions; similarly, recovery was higher in the PAB than in the P treatment. Moreover, all inhibition values were significantly higher than the recovery within any radiation treatment and nutrient condition (Fig. 3a), indicating that the recovery was not complete. Exposure in the +UVR (Fig. 3b) or in the −UVR (Fig. 3c) reduced, in most cases, the differences between inhibition and recovery, particularly under ambient nutrients (Namb), so that almost complete recovery was achieved after the dim light period.
Mean effective photochemical quantum yield (ΦPSII) of phytoplankton communities from the Chubut River estuary during 90 min exposure under solar radiation (horizontal white bar on top) and 90 min recovery under dim light (horizontal gray bar on top) at the beginning (a) and at the end of the experiment, i.e., after acclimation (b, c). Samples were acclimated to two radiation treatments, with UVR (+UVR) (b) and without UVR (−UVR) (c), and two nutrient conditions, ambient (Namb, white symbols) and enriched (Nenr, black symbols), and exposed to two radiation treatments, PAB and P (circles and squares, respectively). The vertical lines around the symbols indicate the standard deviation. The initial measurement of ΦPSII is plotted at time 0 for simplicity but it was measured ca. 1 h before the starting of the short-term incubation
Inhibition (slashed bars) and recovery (black bars) percentage of phytoplankton communities from the Chubut River estuary after the 90 min exposure under solar radiation and 90 min recovery under dim light at the beginning (a) and at the end of the experiment, i.e., after acclimation (b, c). Samples were acclimated to two radiation treatments, with UVR (+UVR) (b) and without UVR (−UVR) (c), and two nutrient conditions, ambient (Namb, white symbols) and enriched (Nenr, black symbols), and exposed to two radiation treatments, PAB and P (circles and squares, respectively). The bars with the same letters (a) are not significantly different (p > 0.05); the different numbers on top of the bars (b, c) identify the treatments, whereas the lines at the bottom connect treatments (numbers) that are not significantly different
Babitonga Bay Estuary
The ΦPSII values of the initial sample (Fig. 4a) were higher under nutrient enrichment (Nenr) than under ambient nutrient conditions (Namb), independently of the radiation treatment. However, the general patterns of ΦPSII throughout this short-term experiment were rather similar under both nutrient conditions, with ΦPSII values decreasing during the exposure period, and partially recovering under dim light. After acclimation in the +UVR treatment (Fig. 4b), the ΦPSII response had a similar pattern regardless the treatment imposed to the samples. However, samples acclimated to −UVR (Fig. 4c) had also similar pattern of responses in all treatments, but with higher ΦPSII values under Nenr as compared to Namb conditions. The inhibition in the initial samples (Fig. 5a) was significantly higher than recovery for all treatments, regardless of the nutrient conditions. Samples under the P treatment, however, had significantly higher recovery than those exposed to full solar radiation, in both nutrient conditions. At the end of the experimental period, samples under +UVR (Fig. 5b) had relatively similar inhibition and recovery percentages in all treatments; thus, any inhibition/damage that occurred during the exposure period was, in general, completely reversed under dim light. Samples under −UVR (Fig. 5c), however, had significantly higher inhibition in the PAB treatment under the Namb condition than in all others; recovery was similar to inhibition within each treatment, with the exception of samples exposed to the P treatment under Nenr conditions that had higher recovery than inhibition.
Mean effective photochemical quantum yield (ΦPSII) of phytoplankton communities from Babitonga Bay during 90 min exposure under solar radiation (horizontal white bar on top) and 90 min recovery under dim light (horizontal gray bar on top) at the beginning (a) and at the end of the experiment, i.e., after acclimation (b, c). Samples were acclimated to two radiation treatments, with UVR (+UVR) (b) and without UVR (−UVR) (c), and two nutrient conditions, ambient (Namb, white symbols) and enriched (Nenr, black symbols), and exposed to two radiation treatments, PAB and P (circles and squares, respectively). The vertical lines around the symbols indicate the standard deviation. The initial measurement of ΦPSII is plotted at time 0 for simplicity, but it was measured ca. 1 h before the starting of the short-term incubation
Inhibition (slashed bars) and recovery (black bars) percentage of phytoplankton communities from Babitonga Bay after the 90 min exposure under solar radiation and 90 min recovery under dim light at the beginning (a) and at the end of the experiment, i.e., after acclimation (b, c). Samples were acclimated to two radiation treatments, with UVR (+UVR) (b) and without UVR (−UVR) (c), and two nutrient conditions, ambient (Namb, white symbols) and enriched (Nenr, black symbols), and exposed to two radiation treatments, PAB and P (circles and squares, respectively). The bars with the same letters (a) are not significantly different (p > 0.05); the different numbers on top of the bars (b, c) identify the treatments, whereas the lines at the bottom connect treatments (numbers) that are not significantly different
Dissipation of Excess Energy
The general pattern of the dissipation of excess energy (estimated through measurements of non-photochemical quenching (NPQ)) at both sites and in all treatments (Fig. 6) was of increasing NPQ values during the exposure period and decreasing under dim light to reach values similar to those at the beginning of the short-term exposure. In the initial community at the CH site (Fig. 6a), NPQ reached values ca. 2.5 in Nenr samples under the PAB treatment, whereas in the rest of the treatments, the highest values were ~1.5. After exposure under +UVR and −UVR (Fig. 6b, c), the range of NPQ values was comparable to those at the initial sample; however, samples under Namb conditions had higher NPQ values than in the Nenr conditions. This was especially evident in samples acclimated to −UVR (Fig. 6c). In the BB community, NPQ reached high values (~7) in the initial sample under Namb conditions; on the other hand, in Nenr samples, NPQ values were <0.5 (Fig. 6d). Exposure under +UVR and −UVR resulted in low NPQ that had maximal values of ~0.8 and 0.4, respectively, under the PAB treatments (Fig. 6e, f). Samples in the Nenr condition had NPQ values that were very low or even null, regardless the light acclimation condition.
Mean non-photochemical quenching (NPQ) of phytoplankton communities from the Chubut River estuary (a–c) and Babitonga Bay (d–f) during 90 min exposure under solar radiation (horizontal white bar on top) and 90 min recovery under dim light (horizontal gray bar on top) at the beginning (a, d) and at the end of the experiments. Samples were acclimated to two radiation treatments, with UVR (+UVR) (b–e) and without UVR (−UVR) (c–f), and two nutrient conditions, ambient (Namb, white symbols) and enriched (Nenr, black symbols), and exposed to two radiation treatments, PAB and P (circles and squares, respectively). The vertical lines around the symbols indicate the standard deviation. Note the different scales in the y-axes
Mid-Term Responses: Taxonomic Changes and Specific Growth Rates
The phytoplankton taxonomic composition and abundance, as well as the growth rates throughout the experiments, were different at the two sites. At the beginning of the experiment in CH (Fig. 7a), the phytoplankton community was dominated by diatoms, i.e., ~900 cells ml−1, which accounted for ca. 70 % of the total abundance (typically Thalassiosira sp. forming chains (10–20 μm in diameter) and to a lesser extent, Odontella aurita), whereas the rest of the community was characterized by unidentified flagellates (i.e., ~500 cells ml−1). The abundance of dinoflagellates was negligible (<1 % of total abundance) at the beginning as well as throughout the experiment and for all treatments (data not shown); no small grazers were observed in any of the samples. The proportion and abundances of diatoms and flagellates were rather maintained throughout the duration of the experiment in samples under Namb conditions. On the other hand, in Nenr condition, the abundance of diatoms rose to ~2400 and 4500 cells ml−1 (under +UVR and −UVR, respectively) which represented >85 % of total abundance of the community; the most conspicuous diatom genus was small Thalassiosira sp. forming chains. The abundance of flagellates at the end of the experiment in samples from both nutrients conditions and exposed to +UVR was similar to that at the beginning, but it changed in samples exposed to −UVR, resulting in significant differences in the specific growth rates (μ) (Fig. 7b). Diatoms under Nenr conditions had significantly higher μ than those in flagellates (Fig. 7b). In samples exposed to −UVR under Namb, however, their abundance decreased (and so, μ had a negative value) as compared to the initial condition.
Mean abundance of cells (in cells ml−1 × 103) (a, c) and specific growth rates (μ, in day−1) (b, d) of the different taxonomic groups, diatoms (white bars) and flagellates (black bars), of phytoplankton communities from the Chubut River estuary (a, b) and Babitonga Bay estuary (c, d) at the initial sampling time (t 0) and at the end of experiment, after samples had been acclimated to two radiation treatments with (+UVR) and without (−UVR) and two nutrient conditions, ambient (Namb) and enriched (Nenr). The letters on top of the bars indicate significant differences (p < 0.05) among taxonomic groups. Note the differences in the y-axis in a and c. Abundance and growth rates of dinoflagellates are not represented, as abundance was <1 % in all cases
In BB (Fig. 7c), the community was initially dominated by diatoms which accounted for ca. 61 % of the total abundance (~5000 cells ml−1) with Leptocylindrus spp., small Thalassiosira species (10–20 μm in diameter) and Skeletonema costatum being the most representative species. The rest of the community was characterized by flagellates and dinoflagellates (data not shown for this later group which accounted for <1 % of the total). At the end of the experiment, an important change in the taxonomic composition was observed with respect to the initial sample, with higher abundance of flagellates (mostly chlorophtes and prymnesiophytes), as compared to that of diatoms in all treatments. Particularly, and in samples under Namb conditions, the abundance of flagellates was similar under both light acclimations (~3000 cells ml−1) and higher than that of diatoms (~750 cells ml−1). In the Nenr condition, the abundance of flagellates was also similar among light acclimations; however, these values (~21,000 cells ml−1) were higher than those in the Namb condition. With the exception of samples under Nenr exposed to −UVR, the abundance of diatoms decreased as compared to the initial conditions; therefore, in these treatments, diatoms had negative values of μ (Fig. 7d). The diatoms that characterized the communities at the end of the experiment were Thalassiosira spp. and S. costatum; eventually, small pennate diatoms and Asterionellopsis glacialis contributed to an important share of the total abundance. The increase observed in the abundances of flagellates was translated in their highest μ under Nenr conditions (Fig. 7d).
There were significant differences between the two sites in relation to the single and the interactive effects (Eqs. 6 and 7) of UVR and nutrients on growth (Fig. 8). In general, there were positive single effects of the variables tested, as well as their interaction, with the only exception being the effect of UVR in BB that was negatively decreasing μ of both diatoms (Fig. 8a) and flagellates (Fig. 8b). The single effects of UVR and nutrients (N), as well as their interaction, resulted in higher growth of diatoms in CH as compared to BB (Fig. 8a). The interactive effect of the two variables on diatom growth, however, was lower than the sum of their individual effects in CH, but there were no significant differences in BB. In the case of flagellates (Fig. 8b), the single effect of UVR resulted in higher growth in CH as compared to BB, where it caused an inhibition of growth. Nevertheless, the single effect of nutrients as well as its interaction with UVR resulted in significant higher growth of flagellates at BB than at CH. The interactive effect of UVR and N on the growth of flagellates was lower than the sum of the individual effects in CH, while there were no differences in BB (Fig. 8b).
Mean (±SD) UVR and nutrients effects as single, interactive, and arithmetic sum of effects on specific growth rates of the main taxonomic groups, diatoms (a) and flagellates (b) for the Chubut River estuary and Babitonga Bay. The lines on top of the bars are the standard deviation. The numbers (a) and letters (b) on top of the bars indicate significant differences (p < 0.05) among the different effects at each site
Discussion
Many studies have addressed the single effects of UVR and of nutrients enrichment on phytoplankton communities within the context of global change (Bricker et al. 2008; Beardall et al. 2009; Häder et al. 2014). Experimental studies assessing the combined impact of both variables on these organisms are less abundant and have shown contrasting responses (Bergmann et al. 2002; Marcoval et al. 2007; Ogbebo and Ochs 2008). In the present study, we went a further step in the evaluation of the joint effects of UVR and nutrient enrichment in two phytoplankton communities by considering also the influence of the potential acclimation to those variables. As expected, there was an important range of responses between the two communities studied which hints for a combination of several intrinsic and extrinsic factors acting to produce such differences in photosynthesis and growth. In the following paragraphs, we will discuss some of the facts that could be responsible for such differences in the responses observed at both sites.
The communities used in this study were characteristics of the post-bloom period in both a temperate (Chubut River estuary, Argentina) and a sub-tropical site (Babitonga Bay, southern Brazil). These post-bloom situations follow typical blooms that occur at different times of the year in the two sites: Historical data show that blooms occur in winter in CH (Villafañe et al. 2004, Villafañe et al. 2013) and in spring–early summer in BB (Parizzi 2014). Although the two communities were dominated by diatoms (Fig. 7), their photosynthetic responses were different: At the beginning, radiation effects were especially evident in CH (Fig. 2a and Fig. 3a) but not so in BB, where mainly nutrients played a role in affecting inhibition and/or recovery of ΦPSII (Fig. 4a and Fig. 5a). These findings agree with previous reports that show that organisms from lower latitudes are less affected by UVR exposure than those from mid or high latitudes, due to its previous light history to high radiation levels (Helbling et al. 1992; Buma et al. 2003). In fact, the CH community inhabited a more dimmed environment than the BB one (Table 1), as they were coming from a relatively deep upper mixed layer, and thus the mean irradiance received by the phytoplankton was reduced (Helbling et al. 2010). Hence, it was expected that phytoplankton from CH would be more sensitive than the one in BB to the extreme radiation exposure that we imposed to the samples (i.e., worst-case scenario), partially explaining the differential short-term responses among them. This is consistent with the findings of Harrison et al. (2015) in six Canadian lakes that showed that phytoplankton communities from clear environments (kPAR ~0.35–0.37) exhibited greater capacity for ΦPSII and NPQ than communities from dimmer environments (kPAR ~1.20–2.40). The differential ΦPSII values observed at the initial time (especially at the start of the short-term exposures) in BB (Fig. 4a) hint for nutrient limited conditions, as also observed in similar experiments assessing the effects of nutrient enrichment (Parkhill et al. 2001, Harrison and Smith 2013). These differential nutrient responses in BB occur in spite of the heavy load of nutrients input reported for this site (Cremer et al. 2006). In fact, nutrient limitation seems to be a common feature of tropical coastal waters of Brazil (Aidar et al. 1993, Longhi et al. 2006). Furthermore, our results obtained with the community sampled at the mouth of Babitonga Bay also agree with previous studies carried out by our group in inlet waters (Villafañe et al. 2014), in which it was found that phytoplankton are not exposed to very high levels of nutrients and instead, the heavy load of nutrients from the nearby human activities might remain in the sediment.
After the acclimation period, UVR did not have, in general, a significant short-term impact (i.e., the differences between inhibition and recovery within any treatment were reduced as compared to the initial time) at both sites (Figs. 2, 3, 4, and 5), although some effects were still observed in samples with a light history without UVR (−UVR) (Fig. 3c and Fig. 5c). This decrease in the UVR impact after the acclimation period also agrees with previous reports for natural assemblages (Hernando et al. 2006; Villafañe et al. 2014), as well as for monospecific cultures (Marcoval et al. 2007) that indicated that the acclimation to new radiation and nutrient conditions takes days. The relative inhibition was lower in BB (30–50 %; Fig. 5) than in CH (50–70 %; Fig. 3), although the effective photochemical quantum yield was relatively low at both sites at the end of the exposure period (ca. 0.2). Part of the differences in the relative inhibition among sites could be related to the different in situ temperatures and/or to the strategies of photoacclimation observed during the short-term exposures (Fig. 6). At CH, the lower temperature (i.e., 10 °C in CH and 25 °C in BB) limited the metabolism, and thus the excitation pressure on PSII (Maxwell et al. 1995; Hüner et al. 1998) was higher than that at BB, in spite of the similar PAR values received during the experiments. The differences in the excitation pressure were also evident in the higher NPQ values determined at CH than at BB after the acclimation period (Fig. 6). In the case of the CH community, the energy dissipation as heat increased during exposures (both at the beginning and after exposure under +UVR and −UVR); however, the BB community showed a strong downregulation of NPQ toward the end of the experimentation (from ca. ~8 at the beginning to ~1 after exposure under +UVR and −UVR) getting even no detectable induction of NPQ under nutrient enriched conditions at all times. Part of these variations seems also to be related to the observed taxonomic changes (Fig. 7) as diatoms dominated in CH, and they are known to have higher NPQ and to better cope with excitation pressure as compared to other groups (Kulk et al. 2011). In addition, the adaptation of estuarine diatoms to a variable light regime is such that NPQ can be standing by and be triggered only if the PSII redox state is very close to saturation (Ruban et al. 2004). Moreover, the NPQ of estuarine diatoms seems to be induced at higher light intensities than the one at which they are grown (ca. 3–5 times, Lavaud et al. 2007), and this agrees with our worst-case scenario at CH of cells exposed to maximum solar radiation. Previous studies also indicated a high degree of change in the NPQ regulation (Goss and Jakob 2010; van de Poll et al. 2010) depending on the growth phase (Dimier et al. 2009) or nutrient status (Staehr et al. 2002) considered. The lower relative inhibition (or higher UVR tolerance) observed in BB as compared to the CH community could have also been enhanced by mechanisms other than NPQ, including increases in antioxidants, reactive oxygen species quenching, as well as production of carotenoids (Häder et al. 2015). We considered the presence of UV-absorbing compounds, i.e., mycosporine amino acids (MAAs) which could help organisms to cope with UVR, but we did not detect significant amounts of them neither in CH nor in BB at any step of the experimental period (data not shown).
Despite the differential taxonomic composition, both dominant groups (i.e., diatoms at CH; flagellates at BB) at the end of the experiment exhibited an increase in their abundances (CH, 2.5–4.5-fold; BB, ca. 10-fold) and growth rates (CH, 5–8-fold; BB, 6-fold) (Fig. 7) under nutrient-enriched conditions, matching with previous results reported in studies carried out with post-bloom communities in both areas (Marcoval et al. 2007; Villafañe et al. 2014). Part of the differences in growth rates and changes in taxonomic composition could be due to the differences in temperature among sites, as temperature influences growth rates (Eppley 1972), and chlorophytes are favored at higher temperatures than diatoms (Paerl and Otten 2013); so in our case, the flagellates (chlorophytes) at BB took advantage of the higher in situ temperature, having higher growth rates under the nutrient enhanced condition. In contrast, at CH, lower temperature was optimal for diatoms that continue their dominance at the end of the experiment in the nutrient enriched conditions (Fig. 7). The analysis of the individual and interactive effects of the studied variables on the specific growth rates indicates that both UVR and nutrient enrichment, either individually or in combination, were beneficial for diatoms and flagellates in CH (Fig. 8a), thus rejecting our initial hypotheses for this site. In contrast, in the BB community flagellates took advantage over the diatoms in most of the conditions, except when considering the single effect of UVR (Fig. 8b), thus partially rejecting the hypothesis for this study site.
Overall, and in the view of the differential responses obtained, as a consequence of the intrinsic characteristics of the communities and/or the sites, this study highlights the need to be cautious with predictions and extrapolations about the phytoplankton responses to the combination of multiple variables even under similar environmental/experimental conditions. Moreover, this study strengthens and expands the necessity to take into account both the acclimation capacity, as well as the seasonal dynamics of phytoplankton communities, particularly when considering the potential impacts of global change variables on highly productive and dynamic ecosystems, such as estuarine and coastal areas.
References
Aidar, E., S.A. Gaeta, S.M.F. Gianesella-Galvão, M.B.B. Kutner, and C. Teixeira. 1993. Ecossistema costeiro subtropical: nutrients dissolvidos, fitoplâncton e clorofila-a e suas relações com as condições oceanográficas na região de Ubatuba, SP. Publicação Especial do Instituto Oceanografico Sao Paulo 10: 9–43.
Ayoub, L.M., P. Hallock, P.G. Coble, and S.S. Bell. 2012. MAA-like absorbing substances in Florida Keys phytoplankton vary with distance from shore and CDOM: Implications for coral reefs. Journal of Experimental Marine Biology and Ecology 420-421: 91–98.
Banaszak, A.T., and M.P. Lesser. 2009. Effects of solar ultraviolet radiation on coral reef organisms. Photochemical and Photobiological Sciences 8: 1276–1294.
Barbieri, E.S., V.E. Villafañe, and E.W. Helbling. 2002. Experimental assessment of UV effects upon temperate marine phytoplankton when exposed to variable radiation regimes. Limnology and Oceanography 47: 1648–1655.
Beardall, J., C. Sobrino, and S. Stojkovic. 2009. Interactions between the impacts of ultraviolet radiation, elevated CO2, and nutrient limitation on marine primary producers. Photochemical and Photobiological Sciences 8: 1257–1265.
Bergmann, T., T.L. Richardson, H.W. Paerl, J.L. Pinckney, and O. Schofield. 2002. Synergy of light and nutrients on the photosynthetic efficiency of phytoplankton populations from the Neuse River Estuary, North Carolina. Journal of Plankton Research 24: 923–933.
Björn, L.O., and T.M. Murphy. 1985. Computer calculation of solar ultraviolet radiation at ground level. Physiologie Vegetale 23: 555–561.
Brandini, F.P., F. Alquini, R.B. Pereira, and R.L. Leite. 2006. Abundância e estrutura populacional da comunidade planctônica na Baía da Babitonga: Subsídios para a avaliação de impactos ambientais. In Diagnóstico Ambiental da Baía da Babitonga, ed. M.J. Cremer, P.R.D. Moralo, and T.M.N. Oliveira, 112–134. Joinville: Univille.
Bricker, S.B., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2008. Effects of nutrient enrichment in the nation's estuaries: A decade of change. Harmful Algae 8: 21–32.
Buma, A.G.J., P. Boelen, and W.H. Jeffrey. 2003. UVR-induced DNA damage in aquatic organisms. In UV effects in aquatic organisms and ecosystems, ed. E.W. Helbling, and H.E. Zagarese, 291–327. Cambridge: The Royal Society of Chemistry.
Carrillo, P., J.A. Delgado-Molina, J.M. Medina-Sánchez, F.J. Bullejos, and M. Villar-Argaiz. 2008. Phosphorus inputs unmask negative effects of ultraviolet radiation on algae in a high mountain lake. Global Change Biology 14: 423–439.
Chapin, F.S. III, E.S. Zavaleta, V.T. Eviner, R.L. Naylor, P.M. Vitousek, H.L. Reynolds, D.U. Hooper, S. Lavorel, O.E. Sala, S.E. Hobbie, M.C. Mack, and S. Díaz. 2000. Consequences of changing biodiversity. Nature 405: 234–242.
Christensen, M.R., M.D. Graham, R.D. Vinebrooke, D.L. Findlay, M.J. Paterson, and M.A. Turner. 2006. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Global Change Biology 12: 2316–2322.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Cloern, J.E., S.Q. Foster, and A.E. Fleckner. 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11: 2477–2501.
Crain, C.M., K. Kroeker, and B.S. Halpern. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11: 1304–1315.
Cremer, M.J., T.M.N. Oliveira, and P.R.D. Morales. 2006. Diagnóstico ambiental da Baía da Babitonga. Joinville: Editora da UNIVILLE.
de Groot, R., M.A. Wilson, and R.M.J. Boumans. 2002. A typology for the classification, description and evaluation of ecosystem functions, goods and services. Ecological Economics 41: 393–408.
Dimier, C., S. Giovanni, T. Ferdinando, and C. Brunet. 2009. Comparative ecophysiology of the xanthophyll cycle in six marine phytoplankton species. Protist 160: 397–411.
Eppley, R.W. 1972. Temperature and phytoplankton growth in the sea. Fishery Bulletin 70: 1063–1085.
Fernandes, L.F., and F. Pereira Brandini. 2010. The potentially toxic diatom Pseudo-nitzschia H. Peragallo in the Paraná and Santa Catarina States, Southern Brazil. Iheringia Ser.Bot. 65: 47–62.
Genty, B.E., J. Harbinson, and N.R. Baker. 1990. Relative quantum efficiencies of the two photosystems of leaves in photorespiratory and non-photorespiratory conditions. Plant Physiology and Biochemistry 28: 1–10.
Goss, R., and T. Jakob. 2010. Regulation and function of xantophyll cycle-dependent photoprotection in algae. Photosynthesis Research 106: 103–122.
Häder, D.-P., M. Lebert, M. Schuster, L. del Ciampo, E.W. Helbling, and R. McKenzie. 2007. ELDONET—A decade of monitoring solar radiation on five continents. Photochemistry and Photobiology 83: 1348–1357.
Häder, D.-P., E.W. Helbling, C.E. Williamson, and R.C. Worrest. 2011. Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochemical and Photobiological Sciences 10: 242–260.
Häder, D.P., V.E. Villafañe, and E.W. Helbling. 2014. Productivity of aquatic primary producers under global climate change. Photochemical and Photobiological Sciences 13: 1370–1392.
Häder, D.-P., C.E. Williamson, S.-A. Wängberg, M. Rautio, K.C. Rose, K. Gao, E.W. Helbling, R.P. Sinha, and R. Worrest. 2015. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochemical and Photobiological Sciences 14: 108–126.
Harrison, J.W., and R.E.H. Smith. 2013. Effects of nutrients and irradiance on PSII variable fluorescence of lake phytoplankton assemblages. Aquatic Sciences 75: 399–411.
Harrison, J.W., G.M. Silsbe, and R.H. Smith. 2015. Photophysiology and its response to visible and ultraviolet radiation in freshwater phytoplankton from contrasting light regimes. Journal of Plankton Research 37: 472–488.
Helbling, E.W., V.E. Villafañe, M.E. Ferrario, and O. Holm-Hansen. 1992. Impact of natural ultraviolet radiation on rates of photosynthesis and on specific marine phytoplankton species. Marine Ecology Progress Series 80: 89–100.
Helbling, E.W., E.S. Barbieri, M.A. Marcoval, R.J. Gonçalves, and V.E. Villafañe. 2005. Impact of solar ultraviolet radiation on marine phytoplankton of Patagonia, Argentina. Photochemistry and Photobiology 81: 807–818.
Helbling, E.W., D.E. Pérez, C.D. Medina, M.G. Lagunas, and V.E. Villafañe. 2010. Phytoplankton distribution and photosynthesis dynamics in the Chubut River estuary (Patagonia, Argentina) throughout tidal cycles. Limnology and Oceanography 55: 55–65.
Helbling, E.W., A.T. Banaszak, and V.E. Villafañe. 2015. Differential responses of two phytoplankton communities from the Chubut river estuary (Patagonia, Argentina) to the combination of UVR and elevated temperature. Estuaries and Coasts 38: 1134–1146.
Hernando, M., I. Schloss, S. Roy, and G. Ferreyra. 2006. Photoacclimation to long-term ultraviolet radiation exposure of natural sub-Antarctic phytoplankton communities: Fixed surface incubations versus mixed mesocosms. Photochemistry and Photobiology 82: 923–935.
Holm-Hansen, O., and B. Riemann. 1978. Chlorophyll a determination: Improvements in methodology. Oikos 30: 438–447.
Hüner, N.P.A., G. Öquist, and F. Sarhan. 1998. Energy balance and acclimation to light and cold. Trends in Plant Science 3: 224–230.
IPCC. 2013. Climate Change 2013. The Physical Science Basis. New York, USA: Cambridge University Press.
Kulk, G., W.H. van de Poll, R.J.W. Visser, and A.G.J. Buma. 2011. Distinct differences in photoacclimation potential between prokaryotic and eukaryotic oceanic phytoplankton. Journal of Experimental Marine Biology and Ecology 398: 63–72.
Lavaud, J., R.F. Strzepek, and P.G. Kroth. 2007. Photoprotection capacity differs among diatoms: Possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnology and Oceanography 52: 1188–1194.
Liang, Y., J. Beardall, and P. Heraud. 2006. Effects of nitrogen source and UV radiation on the growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Journal of Photochemistry and Photobiology, B: Biology 82: 161–172.
Litchman, E., and P.J. Neale. 2005. UV effects on photosynthesis, growth and acclimation of an estuarine diatom and cryptomonad. Marine Ecology Progress Series 300: 53–62.
Longhi, M.L., G. Ferreyra, I. Schloss, and S. Roy. 2006. Variable phytoplankton response to enhanced UV-B and nitrate addition in mesocosm experiments at three latitudes (Canada, Brazil and Argentina). Marine Ecology Progress Series 313: 57–72.
Marcoval, M.A., V.E. Villafañe, and E.W. Helbling. 2007. Interactive effects of ultraviolet radiation and nutrient addition on growth and photosynthesis performance of four species of marine phytoplankton. Journal of Photochemistry and Photobiology, B: Biology 89: 78–87.
Martins, C.C., A.C. Cabral, S.C.T. Barbosa-Cintra, A.L.L. Dauner, and F.M. Souza. 2014. An integrated evaluation of molecular marker indices and linear alkylbenzenes (LABs) to measure sewage input in a subtropical estuary (Babitonga Bay, Brazil). Environmental Pollution 188: 71–80.
Maxwell, D.P., S. Falk, and N.P.A. Huner. 1995. Photosystem II excitation pressure and development of resistance to photoinhibition. Plant Physiology 107: 687–694.
Ogbebo, F.E., and C. Ochs. 2008. Bacterioplankton and phytoplankton production rates compared at different levels of solar ultraviolet radiation and limiting nutrient ratios. Journal of Plankton Research 30: 1271–1284.
Paerl, H.W., and T.G. Otten. 2013. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial Ecology 65: 995–1010.
Pájaro, M., G.J. Macchi, and P. Martos. 2005. Reproductive pattern of the Patagonian stock of Argentine hake (Merluccius hubbsi. Fisheries Research 72: 97–108.
Parizzi, R.A. 2013. Variação sazonal do fitoplâncton e parâmetros ambientais no Canal do Rio Palmital, Baía da Babitonga, Sul do Brasil. Ciência e Natura 35: 41–53.
Parizzi, R.A. 2014. Produtividade primária e dinâmica do fitoplâncton em um estuário subtropical: Uma abordagem em diferentes escalas temporais, Universidade Federal do Paraná Pontal do Paraná
Parkhill, J.-P., G. Maillet, and J.J. Cullen. 2001. Fluorescence-based maximal quantum yield for PSII as a diagnostic of nutrient stress. Journal of Phycology 37: 517–529.
Pereira Camacho, F., and J.M. Souza-Conceição. 2007. Distribuição espaço-temporal da clorofila a e das variáveis ambientais em praias estuarinas da Ilha de São Francisco do Sul (Baía da Babitonga, sul do Brasil). Boletim Técnico-científico do CEPENE 15: 9–16.
Piccolo, M.C., and G.M.E. Perillo. 1999. Estuaries of Argentina: A review. In Estuaries of South America: Their geomorphology and dynamics, ed. G.M.E. Perillo, M.C. Piccolo, and M. Pino Quivira, 101–132. Berlin: Springer.
Porra, R.J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynthesis Research 73: 149–156.
Rabalais, N.N., R.E. Turner, R.J. Díaz, and D. Justic. 2009. Global change and eutrophication of coastal waters. ICES Journal of Marine Science 66: 1528–1537.
Ruban, A.V., J. Lavaud, B. Rousseau, G. Guglielmi, P. Horton, and A.-L. Etienne. 2004. The super-excess energy dissipation in diatom algae: Comparative analysis with higher plants. Photosynthesis Research 82: 165–175.
Ruggaber, A., R. Dlugi, and T. Nakajima. 1994. Modelling of radiation quantities and photolysis frequencies in the troposphere. Journal of Atmospheric Chemistry 18: 171–210.
Schreiber, U., W. Bilger, and C. Neubauer. 1994. Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of photosynthesis, ed. E.-D. Schulze, and M.M. Caldwell, 49–70. Berlin: Springer.
Skewgar, E., P.D. Boersma, G. Harris, and G. Caille. 2007. Sustainability: Anchovy fishery threat to Patagonian Ecosystem. Science 315: 45.
Sobrino, C., M.L. Ward, and P.J. Neale. 2008. Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: Effects on growth, photosynthesis, and spectral sensitivity of photoinhibition. Limnology and Oceanography 53: 494–505.
Staehr, P.A., P. Henriksen, and S. Markager. 2002. Photoacclimation of four marine phytoplankton species to irradiance and nutrient availability. Marine Ecology Progress Series 238: 47–59.
Strickland, J.D.H., and T.R. Parsons. 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada Bull. 167: 1–310.
Thomas, M.K., C.T. Kremer, C.A. Klausmeier, and E. Litchman. 2012. A global pattern of thermal adaptation in marine phytoplankton. Science 338: 1085–1088.
Tittensor, D.P., C. Mora, W. Jetz, H.K. Lotze, D. Ricard, E. Vanden Berghe, and B. Worm. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466: 1098–1101.
van de Poll, W., A.G.J. Buma, R.J. Visser, P.J. Janknegt, V.E. Villafañe, and E.W. Helbling. 2010. Xanthophyll cycle activity and photosynthesis of Dunaliella tertiolecta (Chlorophyceae) and Thalassiosira weissflogii (Bacillariophyceae) during fluctuating solar radiation. Phycologia 49: 249–259.
Villafañe, V.E., and F.M.H. Reid. 1995. Métodos de microscopía para la cuantificación del fitoplancton. In Manual de Métodos Ficológicos, ed. K. Alveal, M.E. Ferrario, E.C. Oliveira, and E. Sar, 169–185. Concepción, Chile: Universidad de Concepción.
Villafañe, V.E., E.S. Barbieri, and E.W. Helbling. 2004. Annual patterns of ultraviolet radiation effects on temperate marine phytoplankton off Patagonia, Argentina. Journal of Plankton Research 26: 167–174.
Villafañe, V.E., A.T. Banaszak, S.D. Guendulain-García, S.M. Strauch, S.R. Halac, and E.W. Helbling. 2013. Influence of seasonal variables associated with climate change on photochemical diurnal cycles of marine phytoplankton from Patagonia (Argentina). Limnology and Oceanography 58: 203–214.
Villafañe, V.E., G.S. Erzinger, S.M. Strauch, and E.W. Helbling. 2014. Photochemical activity of PSII of tropical phytoplankton communities of Southern Brazil exposed to solar radiation and nutrient addition. Journal of Experimental Marine Biology and Ecology 459: 199–207.
Villafañe, V.E., M.S. Valiñas, M.J. Cabrerizo, and E.W. Helbling. 2015. Physio-ecological responses of Patagonian coastal marine phytoplankton in a scenario of global change: Role of acidification, nutrients and solar UVR. Marine Chemistry 177: 411–420.
Weis, E., and A. Berry. 1987. Quantum efficiency of photosystem II in relation to the energy dependent quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 894: 198–208.
Zar, J.H. 1999. Biostatistical analysis. Englewood Cliffs, NJ: Prentice Hall.
Acknowledgments
We thank the help of C. Machado, C. Hack Gumz Correia, and R. Parizzi for their help with analysis of samples and setup of experiments in BB. We also thank the comments and suggestions of three anonymous reviewers that helped us to improve this manuscript. This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas—CONICET (PIP No. 112-201001-00228), Agencia Nacional de Promoción Científica y Tecnológica—ANPCyT (PICT 2012-0271 and PICT 2013-0208), Fundaçao de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), National Counsel of Technological and Scientific Development (CNPQ), Universidade da Região de Joinville, and Fundación Playa Unión. We also thank Cooperativa Eléctrica y de Servicios de Rawson for providing building’ infrastructure in CH. MCJ was supported by the Ministerio de Educación, Cultura y Deporte of Spain through a “Formación de Profesorado Universitario” Ph.D. fellowship (FPU12/01243) and a short-term fellowship (EST13/0666) at EFPU. This is Contribution No. 164 of Estación de Fotobiología Playa Unión.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hans W. Paerl
Rights and permissions
About this article
Cite this article
Villafañe, V.E., Cabrerizo, M.J., Erzinger, G.S. et al. Photosynthesis and Growth of Temperate and Sub-Tropical Estuarine Phytoplankton in a Scenario of Nutrient Enrichment under Solar Ultraviolet Radiation Exposure. Estuaries and Coasts 40, 842–855 (2017). https://doi.org/10.1007/s12237-016-0176-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0176-z