Abstract
Coastal wetlands, well recognized for their ecosystem services, have faced many threats throughout the USA and elsewhere. While managers require good information on the net impact of these combined stressors on wetlands, little such information exists. We conducted a 4-month mesocosm study to analyze the multiple stressor effects of precipitation changes, sea level rise, and eutrophication on the salt marsh plant Spartina alterniflora. Pots containing plants in an organic soil matrix were positioned in tanks and received Narragansett Bay (RI, USA) water. The study simulated three precipitation levels (ambient daily rain, biweekly storm, and drought), three levels of tidal inundations (high (15 cm below mean high water (MHW)), mean (MHW), and low (15 cm above MHW)), and two nutrient enrichment levels (unenriched and nutrient-enriched bay water). Our results demonstrate that storm and drought stressors led to significantly less above- and belowground biomass than those in ambient rain conditions. Plants that were flooded at high inundation had less belowground biomass, fine roots, and shoots. Nutrients had no detectable effect on aboveground biomass, but the enriched pots had higher stem counts and more fine roots than unenriched pots, in addition to greater CO2 emission rates; however, the unenriched pots had significantly more coarse roots and rhizomes, which help to build peat in organogenic marshes. These results suggest that multiple stressors of altered precipitation, sea level rise, and nutrient enrichment would lead to reduced marsh sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas in the Northeastern USA have long been associated with high population densities, residential land development, and high nutrient loadings (Deegan et al. 2012). In addition to increasing cultural eutrophication, salt marshes in this area are also subject to increasing rates of sea level rise (SLR) and changing precipitation patterns (Sallenger et al. 2012; Boon 2012; IPCC 2014; Kirwan and Megonigal 2013). The Northeastern USA has been a particular hot spot for SLR, where over the past four decades increases in SLR rates were 3–4 times higher in the Northeastern USA than the worldwide average, with sea level rate differences on the Atlantic coast from Cape Hatteras north to Boston averaging 0.38 ± 0.01 cm year−1 compared to the global average of 0.098 ± 0.04 cm year−1 (Yin et al. 2009; Church and White 2011; Sallenger et al. 2012; Boon 2012). This accelerated rate of SLR is expected to limit the ability of coastal salt marshes to build peat and maintain elevation (Cahoon et al. 2009), especially in the Northeastern USA where sediment supply to coastal wetlands is naturally low or diminished due to human activities (Weston 2014). In addition, the Northeastern USA is predicted to experience more frequent storms, heavier and more intense rainfall, and episodic droughts in the future (IPCC 2014). The interactions and compounding effects of multiple stressors such as these are difficult to predict and can result in unexpected marsh system responses (Cahoon and Guntenspergen 2010; Fagherazzi et al. 2012; Kirwan and Megonigal 2013).
Recent studies have examined the effects of multiple stressors on coastal salt marshes. In coastal California, the ability of salt marsh systems to serve as robust coastal nutrient filters was severely reduced when SLR caused loss of marsh plants through water logging, while nitrogen retention in plants increased when exposed to higher nutrient loads (Nelson and Zavaleta 2012). In order to persist and counteract inundation effects such as these, marshes exposed to increasing rates of SLR will need to increase rates of soil elevation. This will be dependent upon several processes, but most importantly to the organogenic soils of the Northeastern USA, mineral sediment deposition, and belowground organic matter accumulation (Kirwan and Mudd 2012; Kirwan and Megonigal 2013). In a separate study, increases in atmospheric CO2 stimulated both belowground root productivity and elevation gain in tidal brackish marshes (Langley et al. 2009). Surprisingly, the CO2 effect was enhanced under increasing salinity and flooding conditions, suggesting that increases in CO2 also increase the capacity of coastal wetlands to tolerate SLR; nitrogen addition, however, tended to negate elevation gains caused by increased CO2 levels alone (Langley et al. 2009). Another study examining the interactive effects of the rate of SLR and warming on marsh plant productivity using model simulations found that plant responses also differed depending upon the elevation of the marsh relative to sea level (Kirwan and Mudd 2012). However, no study to our knowledge has examined the combined effects of accelerated sea level rise, nutrient enrichment, and changing precipitation patterns on salt marshes. Because these three stressors are present-day management concerns, we used a factorial design in a greenhouse experiment to examine effects and interactions. In concurrent studies, we reported the marsh system level responses to these three stressors (Watson et al. 2014; Oczkowski et al. 2016), and here, we present the plant community-level effects.
A better understanding of the effects of multiple stressors and their compounding effects on coastal systems is needed to improve predictive models and restoration approaches that assist coastal resource managers in selecting appropriate climate adaptation practices (Crain et al. 2008; Beier et al. 2012; Fagherazzi et al. 2012; Stein et al. 2013; Wigand et al. 2016). To study multiple stressor effects and responses, we carried out a mesocosm greenhouse experiment which examined changing precipitation patterns and SLR, in both unaltered and nutrient-enriched seawater in tanks using the Northeastern USA low salt marsh dominant species, S. alterniflora. We hypothesized that nutrient enrichment would lead to an increase in aboveground production coupled with a decrease in belowground production and that multiple stressors would not ameliorate the effects of one another, but would overall lead to deteriorating marsh conditions in the treatments exposed to stressors. Further, we predicted that longer periods of drought would lead to a decrease in plant production and the rate of peat buildup and an increase in CO2 efflux from the soil, while prolonged tidal inundation would negatively affect above- and belowground biomass, as well as S. alterniflora stem height and count.
Methods
Experimental Design
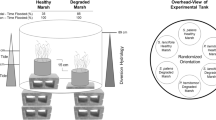
We examined the main and compounding effects of three stressors (precipitation patterns, SLR, and nutrient loading) with mesocosms using a 3 × 3 × 2 factorial design, to test the main effects and interactions of stressors on aboveground growth and biomass, belowground growth of roots and rhizomes, and soil CO2 emission rates. Exposure mesocosms were plant pots fabricated from PVC piping and maintained for four months (July to November 2011) in a greenhouse at the U.S. EPA Atlantic Ecology Division in Narragansett, RI. The tanks that held the pots were constructed to simulate different inundation treatments by using the “marsh organ” design of Morris et al. (2002) (Fig. 1). Seventy-two pots (Fig. 1a), each containing one nursery-grown S. alterniflora 16-cm3 plug, were placed in six tanks (700 L, 1.2 m diameter, 0.6 m height). Each pot was filled with a homogenous mixture of peat from southern New England salt marshes and mature sieved compost soil to mimic organic rich soils in the Northeastern USA (Allen 1990).
Experimental setup. a A schematic of the six tanks with circles representing different treatments. Tanks in the left column were filled with seawater as our control (unenriched), but the tanks in the right column were also filled a daily fertilizer aliquot (enriched). The top row represents the two control ambient precipitation tanks (small raindrops), the middle row shows the drought tanks, and the bottom row shows the storm tanks (storm clouds and large raindrops). The lengths of the pots (n = 4) in each circle represent the three different marsh habitats, where medium-sized pots represent our control middle marsh (mean inundation level), the longer pots represent a high marsh (low inundation), and the shorter pots represent a low marsh (high inundation). b A schematic that shows the height of the water level during the tides. The two waterlines represent the inundation levels reached during each high and low tide relative to the pot heights
The tanks received raw seawater pumped in from Narragansett Bay and were filled and drained twice daily on a semidiurnal tidal regime. Tanks were filled from low tide height (0.2 m) to high tide height (0.42 m) over the course of 4 h, remained at high tide over a 2-h slack period, and then drained back down to low tide followed by another 2-h slack period. The mesocosms were set up as a 3 × 3 × 2 factorial design (n = 4) to receive three levels of precipitation (ambient daily rain, biweekly storm, and drought), three inundation levels (high, mean, and low), and two levels of nutrient enrichment (unenriched bay water and nutrient-enriched bay water, Fig. 1a).
Inundation Treatments
Pots had an open top and a bottom fitted with a fine mesh nylon screen (125 μm) to allow vertical water exchange upwards. Each pot was 10.2 cm in diameter and one of three different heights (30, 45, and 60 cm) to represent three different elevations and inundation regimes on a marsh platform; the S. alterniflora in 30 cm pots represented a low elevation creek bank (−15 cm mean high water (MHW)), 45 cm pots represented the middle marsh platform at MHW, and 60 cm pots represented a high marsh elevation (+15 cm MHW, Fig. 1b). Since high tide reached 0.42 m, pots at the high inundation were completely inundated with seawater during high tide, while the tide reached near the pot surface at the mean inundation level. At low tide, no pots were inundated with seawater. Based on previous studies examining the optimal elevation for S. alterniflora growth relative to MHW (e.g., Morris et al. 2002; Fagherazzi et al. 2012; Watson et al. 2014), we considered the MHW (mean inundation) at middle marsh elevation our control treatment, representing the most optimal elevation for S. alterniflora in our study.
Precipitation Treatments
Precipitation was added to the tanks as artificial rainwater with a nitrate concentration of 65 μM from potassium nitrate, as determined by averaging the summer nitrogen concentrations in rainwater measured from local monitoring stations (2007–2009 at CT 15, MA 01, and MA 08; http://nadp.sws.uiuc.edu). The two ambient rain tanks received 3 mm of rainwater daily, the two storm tanks received 10 cm rainwater once every fortnight, and the two drought tanks received no freshwater for the duration of the experiment. Ambient rainwater volumes were based on actual rainfall measurements collected locally at T.F. Green State Airport, Rhode Island (ID: GHCND:USW00014765; http://www.ncdc.noaa.gov/). Total rainfall values for the summer months (i.e., growing season) were divided by the number of days in summer to determine average daily rain to apply to the ambient rain tanks (3 mm). Storm treatment rain volumes (10 cm) were based on summer rainfall totals and were designed to represent an intense storm event. Water was sprinkled over the top of the tanks using a watering can and evenly dispersed among the pots and overlying tank seawater. We considered ambient rainfall to be our control, representing a condition not associated with future climate change projections.
Nutrient Treatments
Nutrients were added to one of each precipitation treatment tank, while the other corresponding three tanks received no nutrient additions (Fig. 1a). The unenriched tanks were considered our control treatment, as it resembled a scenario with no additional anthropogenic nitrogen and phosphorus inputs. Dissolved nutrients (1.155 g ammonium nitrate and 0.128 g phosphorus pentoxide) were applied in 100 mL aliquots once daily into the water column during the tanks’ incoming tide. The nitrogen addition concentrations (340 mg N m−2 day−1) were based on sewage effluent loadings reported for the highly enriched Jamaica Bay estuary, New York (Benotti et al. 2007), and phosphorus loading to 47 mg P m−2 day−1 to achieve a molar N/P ratio of 16:1.
Overlying tank water samples were collected thrice weekly and pH was measured using an Accumet AP115 meter. The remaining sample volumes were then stored in a −10 °C freezer until ready for nutrient analysis. Nitrate (NO3 −), nitrite (NO2 −), ammonia (as NH4 +), and orthophosphate (PO4 3−) were quantified on an Astoria2 Autoanalyzer (Astoria-Pacific, OR). Nitrate and nitrite concentrations were determined using cadmium reduction (U.S. EPA method 353.2), and ammonia and orthophosphate were also analyzed using EPA methods (U.S. EPA methods 350.1 and 365.1, respectively) with blanks after every 10 samples and standard checks every 15 samples for all analyses.
In a concurrent study, we observed and reported blooms of phytoplankton, microphytobenthos, and macroalgae in the enriched tanks, which competed with S. alterniflora for dissolved nutrients (Oczkowski et al. 2016). Measured nutrient concentrations were higher in the enriched tanks than the unenriched tanks (63.55 ± 2.11 and 5.78 ± 0.54 μM DIN, respectively). Salinity and pH of overlying water were also monitored throughout the experiment. The pH ranged from 7.86 to 8.00 across all tanks and salinity ranged from 31 to 33 ‰ (Table S1) throughout the experiment, matching the marine water pumped in from Narragansett Bay; these conditions were similar in all tanks throughout the experiment.
Vegetation and Soil Parameters
Stem Counts, Heights, and Aboveground Biomass
Once monthly, heights and numbers of all stems were measured. At the conclusion of the experiment, all aboveground growth was clipped and harvested, rinsed clean, dried in a 60 °C oven to remove all moisture, and then weighed. Aboveground biomass was only determined for the same pots that were later analyzed for belowground biomass, only n = 3 for each treatment combination.
Soil CO2 Efflux
After aboveground plants were clipped at the surface, the exposed stems were plugged with silicone sealant to prevent gas exchange. Soil CO2 emissions were measured at this time for each pot using a Li-COR 8100 CO2 flux system and dome (Wigand et al. 2009) during daytime low tide. Each pot was measured once for CO2 efflux.
Belowground Structure and Biomass
Computer-aided tomography (CT) was used to image the belowground coarse roots and rhizomes in the pots (Davey et al. 2011). Three of the four S. alterniflora pot replicates were prepared for CT scanning by inserting calibration rods of air, water, and glass into the pots; these provided reference standards to estimate the specific particle densities of the coarse roots and rhizomes. The pots were scanned at RI Medical Imaging (East Greenwich, RI) on a GE Medical Systems Light Speed 16 Helios Version 1 CT scanner. The cores were spirally scanned in a horizontal position from the top to the bottom of the core with the resolution set at a slice thickness of 0.625 mm. Images were analyzed using the software OsriX (Pixmeo; Geneva, Switzerland) and ImageJ (National Institutes of Health) to quantify belowground coarse root and rhizome abundance, counts, and volume. Coarse roots were defined as having diameters of greater than or equal to 1 mm but less than 2 mm, and rhizomes as having diameters greater than or equal to 2 mm (Davey et al. 2011). Fine roots, defined as having diameters less than 1 mm, were not able to be detected through CT imaging due to resolution limitations.
After CT imaging, pots were kept upright in the freezer until ready for soil characterization. Belowground parameters were only measured in the active root zone, to a depth of 20 cm (Valiela et al. 1976). Pots were defrosted, extruded, and sectioned into 10 cm lengths to 20 cm depth and returned to the freezer until roots and rhizomes were ready to be rinsed to remove the soil, dried at 60 °C, and weighed. Three samples for each treatment (representing each possible combination of precipitation × elevation × nutrient enrichment) were measured for belowground characteristics, with 54 samples analyzed in total. Fine root dry mass was estimated as the difference between the hand-sieved belowground biomass and the estimated dry mass of the coarse roots and rhizomes measured with CT imaging. The dry mass of the coarse roots and rhizomes was estimated from the CT wet volume using a conversion factor of 2.2 determined from the wet density/dry density equation reported in Davey et al. (2011). All fine root biomass totals that were estimated to be negative values after the equation calculation were treated as zeros.
Statistics
Three-way ANOVA models with precipitation (ambient, storm, drought), inundation (high, mean, low), and nutrients (unenriched, enriched) as main effects were used to analyze above- and belowground responses. CO2 efflux values were natural log transformed and estimated fine root biomass data were natural log plus 1 transformed (ln (x + 1)) prior to ANOVA analyses. When statistically significant interactions were present, differences between means of treatment combinations from the two variables were compared using Bonferroni’s correction. Bonferroni was used because not all factor combination comparisons were of interest (e.g., if precipitation interacted significantly with nutrients, ambient/unenriched vs. storm/unenriched would be of interest, but not ambient/unenriched vs. storm/enriched), and therefore, Bonferroni’s correction resulted in the more statistically powerful tests. When no interaction was present, differences between means were tested with Tukey’s pairwise comparison method, which is more powerful than Bonferroni when all comparisons are of interest. All ANOVAs were tested at the 95 % confidence level. Analyses were performed using SAS 9.3 statistical software (SAS Institute 2011).
Results
Aboveground Biomass, Stem Counts, and Heights
The precipitation × inundation and inundation × nutrient enrichment interaction terms were both significant (p = 0.0001 and p = 0.04, respectively) for aboveground biomass at the end of the growing season (Table S2). The precipitation × inundation interaction was driven by the low inundation ambient rain treatment, which had greater aboveground biomass than comparable drought, storm, and ambient inundation treatments (Table S3). Unenriched ambient treatment at low inundation averaged 3594 g m−2 compared to the averaged 2134 g m−2 in the nutrient-enriched ambient tank (Fig. 2a). The significant result of the inundation × nutrient interaction was driven by unenriched treatments, where unenriched mean and low inundations (1717 ± 168 and 1964 ± 454 g m−2, respectively) were greater than unenriched high inundations (1106 ± 74 g m−2) (Table S3). However, nutrient additions had no effect on aboveground biomass as a main effect. Generally, inundation and precipitation main effects both had a negative effect on aboveground biomass (Fig. 2a). Plants receiving storm and drought precipitation treatments (1337 ± 111 and 1218 ± 70 g m−2, respectively) showed decreased aboveground biomass compared to ambient precipitation treatments (2006 ± 228 g m−2). Pots at the low inundation treatment showed greater biomass (1730 ± 258 g m−2) and those at the high inundation had a lower biomass (1261 ± 81 g m−2), relative to mean inundation (1569 ± 103 g m−2) (Fig. 2a). Overall, the aboveground biomass in pots at the low inundation was greater than the biomass at the high inundation.
Above- and belowground biomass for Spartina alterniflora. Standard errors (n = 6) are displayed with each average. a Average aboveground biomass (g m−2); values were averaged across treatment combinations. Significant differences were observed in precipitation × inundation and inundation × enrichment interactions, and precipitation and inundation main effects. b Average hand-sieved belowground biomass (g m−2) to a depth of 20 cm with values averaged across all treatments combinations. Significant differences were observed in: precipitation × inundation two-way interaction, and precipitation and inundation main effects
The final sampling average stem count three-way interaction was significant (p = 0.01) as well as the interactions for precipitation × inundation at both peak and final samplings (p = 0.01 and p = 0.03, respectively) (Table S2). At peak (September 2011), the differences were again observed in the ambient treatment at mean inundation, which had greater stem counts than the ambient treatment at high inundation (15 ± 2 and 8 ± 1, respectively), while enrichment was not significant (Table S3). At the end of the experiment (November 2011), the results were more ambiguous due to the three-way interaction. While enriched drought and storm treatments showed some instances of having greater stem counts than in corresponding unenriched treatments, this was not observed between the ambient enriched and unenriched treatments, and both the ambient and storm treatments showed instances of having greater counts than the drought treatments (Fig. 3; Table S4). Stem counts measured during peak biomass showed no significant differences between plants in unenriched and enriched tanks (Table S2). However, measurements at the end of the growing season showed trends that average stem count was greater in the enriched tanks (16 ± 1 in enriched and 13 ± 1 in unenriched; Fig. 3), while the average height per pot was greater in the unenriched tanks (40 ± 2.2 cm in unenriched and 32 ± 1.6 cm in enriched; Fig. 4, Table S3).
Average monthly stem counts with associated standard errors for Spartina alterniflora throughout the duration of the experiment. Statistical analyses were run at peak (Sept) and final (Nov) times and significant differences were observed in final precipitation × inundation × enrichment three-way interaction, peak and final precipitation × inundation, and peak inundation and final precipitation, inundation, and enrichment main effects
Average monthly stem heights (cm) with associated standard errors for Spartina alterniflora throughout the duration of the experiment. Statistical analyses were run at peak (Sept) and final (Nov) times and significant differences were observed in peak precipitation × inundation and precipitation × enrichment and final precipitation × enrichment, and peak precipitation and inundation and final precipitation, inundation, and enrichment main effects
The precipitation × nutrient enrichment interaction was significant for average stem heights at both peak and final samplings (p = 0.01 and p = 0.02, respectively); the only other significant interaction was at peak for precipitation × inundation (p = 0.05) (Table S2). These results were driven by the ambient rain tanks, particularly in the unenriched tank (36 ± 2 peak and 49 ± 4 cm final), which had significantly taller stems than the enriched ambient tank (29 ± 3 peak and 33 ± 3 cm final), and the unenriched drought (24 ± 2 peak and 38 ± 3 cm final) and storm (24 ± 2 peak and 32 ± 2 cm final) tanks at both peak and final samplings (Fig. 4; Table S3). At peak, these significant interactions are also evident in the low inundation ambient treatment which had the tallest stems, and similarly at the end of the experiment when plants at the low inundation across all precipitation and enrichment treatments were significantly taller than those at other inundations (p = 0.003) (Tables S2 and S3).
Belowground Structure and Biomass
The hand-sieved total belowground biomass (0–20 cm depth) precipitation × inundation interaction term was significant (p = 0.0004), which was again driven by the ambient low inundation (2026 ± 308 g m−2), which had greater belowground biomass than both storm and drought low inundations (1081 ± 176 and 658 ± 77 g m−2, respectively) and ambient mean and high inundations (1174 ± 152 and 597 ± 118 g m−2, respectively) (Tables S3 and S5). The biomass of low inundation exposures (1255 ± 187 g m−2) was greater than the mean inundation (960 ± 117 g m−2), which was greater than high inundation biomass (606 ± 96 g m−2; p < 0.0001; Fig. 2b).
Although the hand-sieved total belowground biomass showed no significant difference between unenriched and enriched treatments (p > 0.05), the CT-scanned average abundances of coarse roots and rhizomes did display a trend of greater abundances in the unenriched than the enriched pots (4193 and 3290 n m−2, respectively; p = 0.0009) (Fig. 5, S1, S2; Table S5). However, the three-way precipitation × inundation × enrichment interaction term was significant (p = 0.04). The results of the Bonferroni correction show that the enrichment differences were found within the low inundation treatments, where the unenriched ambient (5656 ± 283 n m−2) was greater than the enriched ambient (3670 ± 324 n m−2), and the unenriched storm (4899 ± 443 n m−2) was greater than the enriched storm (2948 ± 355 n m−2) (Figs. 5 and S2; Table S4). Additionally, the precipitation differences were driven by both the ambient and storm unenriched low inundation treatment plants having greater coarse root and rhizome abundances than the drought unenriched low inundation plants (Fig. S2). The inundation differences were due to differences in the unenriched drought treatments, where the high inundation plants (5070 ± 589 n m−2) had greater abundances than the mean inundation and low inundation plants (2884 ± 352 and 2840 ± 211 n m−2, respectively). In contrast, the enriched pots had a greater estimated fine root mass than the unenriched pots (565 ± 89 and 387 ± 100 g m−2, respectively; p = 0.002) (Tables 1, S3, and S5). The greater coarse root and rhizome abundance in the unenriched pots and the greater fine root biomass in the enriched pots support the finding of overall belowground biomass not being significantly different between the two treatments. The greater mass of estimated fine roots in the enriched pots might be the result of shorter, yet more abundant stem counts observed in the enriched aboveground plants.
CT scan images showing belowground root structure from six pots representing a pot at the mean inundation from all six tanks. The three graphs in the middle show the averaged root and rhizome abundances (n m−2) from the mean inundation. Bars show the unenriched and enriched averages with standard errors for 0–20 cm depth. Significant differences were observed in precipitation × inundation × enrichment three-way interaction and precipitation × inundation two-way interaction, and precipitation, inundation, and enrichment main effects
Both hand-sieved belowground biomass and CT-derived coarse roots and rhizome abundance were greatest in the low inundation, and the mean inundation was greater than the high inundation, indicating that pots not subjected to sea level rise conditions had greater belowground biomass than pots flooded in seawater (Figs. 2b, 5, S1, and S2).
Soil CO2 Efflux
At the conclusion of the experiment, precipitation × inundation (p = 0.001), inundation × enrichment (p = 0.006), and the three-way interaction precipitation × inundation × enrichment (p = 0.004) were all significant, suggesting that all three stressors can contribute to significantly altered CO2 efflux rates (Table S5). The precipitation differences were driven by the unenriched tanks, where high inundation ambient treatments (0.23 ± 0.08 μmol m−2 s−1) had lower rates than high inundation storm and drought treatments (2.22 ± 0.34 and 0.84 ± 0.27 μmol m−2 s−1, respectively), but mean inundation ambient treatments (2.52 ± 0.27 μmol m−2 s−1) had greater rates than mean inundation storm and drought (0.74 ± 0.19 and 0.79 ± 0.31 μmol m−2 s−1, respectively) (Table S4). The enrichment differences were driven by the high inundation ambient treatments where the enriched pots had greater rates than unenriched pots (2.1 ± 0.48 and 0.23 ± 0.08 μmol m−2 s−1). The inundation treatments showed less clear patterns in the three-way interaction, but overall, the low inundation had greater rates than the mean and high inundation treatments (Fig. 6). This was further shown in the main effects, where low inundation pots showed trends of greater soil CO2 efflux rates than the mean and high inundation pots (5.79 ± 0.6, 1.65 ± 0.24, 1.65 ± 0.25 μmol m−2 s−1) (Fig. 6, Table S5). The enriched tanks also had greater rates than the unenriched tanks (3.25 ± 0.4 and 2.81 ± .3 μmol m−2 s−1, respectively).
Carbon dioxide emission rates (μmol m−2 s−1) and associated standard error measured at the end of the experiment for each treatment (n = 4). Significant differences were observed in precipitation × inundation × enrichment three-way interaction, precipitation × inundation and inundation × enrichment two-way interactions, and precipitation, inundation, and enrichment main effects
Discussion
S. alterniflora at high inundation compared to mean inundation was associated with less belowground biomass, fine roots, and aboveground stem count, and this was particularly true when the plants were exposed to ambient precipitation (Figs. 2 and 3; Tables S2 and S5). Furthermore, nutrient enrichment generally resulted in a decrease of root and rhizome abundance in the mesocosms. Loss in belowground root structure can contribute to long-term marsh degradation as described by earlier studies (Turner et al. 2009; Turner 2011; Deegan et al. 2012). In addition to the negative effects on marsh structure due to nutrients, in a concurrent study with the presented one, Watson et al. (2014) reported that increased flooding at the high inundation caused higher sulfide levels. High sulfide levels can impact marsh sustainability by inhibiting root production (King et al. 1982; Koch et al. 1990). Larger diameter coarse roots and rhizomes coupled with decreased belowground biomass have in the past been associated with heavily impacted, degrading organogenic marshes, receiving high nutrient loads (Wigand et al. 2014). Furthermore, the results of the present study suggest that the compounding effects of the additional stress of either drought or storm will further reduce S. alterniflora biomass already stressed by nutrient inputs, with the greatest impact on the low marsh plants and less impact on plants exposed to less tidal inundation. The results from our analyses suggest that when inundation has a negative impact on belowground structures, the addition of an additional stressor such as drought or storm further exacerbates changes in belowground structure, as has been observed in other similar multiple stressor experiments associated with climate change (Charles and Dukes 2009; Fox et al. 2012; Porter et al. 2013). Combined, the responses of the S. alterniflora to the treatments of changing precipitation and increased inundation suggest that accelerated SLR in the Northeastern USA will have a negative effect on belowground production and subsequent accumulation of organic matter (Fig. 2b; Table S5). Since SLR rate is expected to increase, and over 85 % of southern New England S. alterniflora salt marshes are currently estimated to be at an elevation below their optimal level for growth (Watson et al. 2014), S. alterniflora growth may be even further limited in the future unless there is an opportunity for upland migration. With less marsh habitat at an elevation appropriate for optimal plant growth, the remaining marsh habitat at higher elevations may become increasingly rare, especially if there are barriers to upland migration, further limiting options available to coastal resource managers to carry out actions to adapt to accelerated sea level rise.
S. alterniflora showed greatest aboveground biomass growth at the low inundation treatment (+15 cm above MHW, i.e., high elevation), which can mostly be attributed to the high biomass in the unenriched ambient tanks (Fig. 2a, Table S3). The plants exposed to mean inundation had higher stem abundance than the low inundation at both peak and final measurements, indicating that the low inundation plants grew taller throughout the experiment, rather than producing new small stems (Table S2). The importance of the observed high growth rates in mesocosms at the mid and high elevations exposed to less tidal inundation was predicted by observations of under natural conditions where plants have optimal elevations for growth and have elevation capital and would thus able to migrate upland when there are no barriers to migration (Cahoon and Guntenspergen 2010). Also, S. alterniflora is able to establish residence throughout a large salinity gradient, further increasing its elevation capital and likelihood of maintaining an optimal elevation (Sutter et al. 2015). Our results demonstrate the success of S. alterniflora in areas of lower tidal inundations.
According to the IPCC report (2014), the Northeastern USA is predicted to receive less frequent precipitation but heavier pulses of precipitation during storm events (more than 5 cm precipitation in 24 h), as modeled in our storm tanks. Ambient precipitation conditions in our study overall trended toward having greater belowground biomass, aboveground biomass, and average plant heights than in the storm treatment (Figs. 2a, b and 4; Table S3), differing from a recent experiment where precipitation led to no or negative changes in plant production (Charles and Dukes 2009). While plants seemed to thrive under the ambient precipitation conditions, growth was clearly stunted under storm and drought conditions. The response of the plants to the storm treatment more closely resembled that observed in the drought tanks, instead of the response in the ambient precipitation tanks. Even though the storm tanks received nearly double the volume of rain water with its associated nutrients than the ambient precipitation tanks each month, it appears that the timing of delivery of precipitation mattered much more than the volume. This was evidenced in a companion paper which found greater 15N tracer retention in the leaves and roots of S. alterniflora during peak growth in the ambient treatment pots in comparison to the storm treatment pots (Oczkowski et al. 2016). A valuable function of salt marshes is their ability to retain nitrogen during the summer growing season and reduce nutrient loads into coastal waters. After the growing season and during and after senescence, marshes release some nitrogen into the estuary, but at these times, the estuary’s vulnerability to the negative impacts of excess nutrient loading has lessened (e.g., Valiela et al. 2000). The results of this paper along with our companion paper demonstrate that not only will changes in precipitation patterns reduce the growth of above- and belowground S. alterniflora production, but it will reduce the capability of the salt marsh to retain nitrogen (Oczkowski et al. 2016).
In the nutrient-enriched treatment, an initial increase of fine roots during the growing season could improve soil strength, but increased decomposition rates (Watson et al. 2014) and increased soil CO2 efflux rates under enriched conditions suggest that labile fine roots could quickly decompose over an annual cycle. Furthermore, the nutrient-enriched treatments had fewer coarse roots and rhizomes (Table S4) which are more refractory than fine roots and important for organic matter accumulation (Wigand et al. 2014). The nutrient-enriched tanks also had higher average stem counts than the unenriched tanks (Fig. 3), particularly due to the higher counts in the enriched storm and drought treatments (Table S4), while the average height per pot was greater in the unenriched tanks, notably in the ambient unenriched tank (Table S3), indicating that unenriched plants grew taller and the enriched plants grew many small stems. This observed effect of more stems in fertilized pots has been previously observed in field mesocosms (Wong et al. 2015). These small stems could be associated with the higher average fine root biomass we observed in the enriched tanks. However, fine roots may not lead to accumulation of peat, because fine roots decompose more rapidly than coarse roots or rhizomes (Morris et al. 2013; Wigand et al. 2014).
Long-term field manipulative nitrogen enrichment experiments have demonstrated decreases in belowground production and soil strength (Turner et al. 2009; Deegan et al. 2012). The results observed due to increased nitrogen loads in these long-term experiments complement our results of increased decomposition rates coupled with decreased coarse root and rhizome production (Watson et al. 2014). Although our experimental design did not directly capture responses that might occur over longer time periods, we propose that the changes in belowground root and rhizome structure demonstrated in our study support the hypothesis that the combined stressors of altered precipitation, accelerated SLR, and nutrient enrichment will have negative long-term effects on the buildup of peat in organogenic marsh systems receiving low sediment inputs (as is often the case in the Northeastern USA, Weston 2014). Furthermore, decreased productivity of S. alterniflora in organogenic marshes reduces system sustainability and the provision of ecosystem services such as flood abatement, water quality maintenance, and nursery and feeding habitat for valued fish, crustaceans, and birds.
References
Allen, J.R.L. 1990. The formation of coastal peat marshes under an upward tendency of relative sea-level. Journal of the Geological Society of London 147: 743–745.
Beier, C., C. Beierkuhnlein, T. Wohlgemuth, J. Peñuelas, B. Emmett, C. Körner, H. de Boeck, J. Hesselbjerg Christensen, S. Leuzinger, I.A. Janssens, and K. Hansen. 2012. Precipitation manipulation experiments—challenges and recommendations for the future. Ecology Letters 15: 899–911. doi:10.1111/j.1461-0248.2012.01793.x.
Benotti, M. J., M. Abbene, and S.A. Terracciano. 2007. Nitrogen loading in Jamaica Bay, Long Island, New York: predevelopment to 2005. U.S. Geological Survey Scientific Investigations Report 2007–5051. http://pubs.usgs.gov/sir/2007/5051/.
Boon, J.D. 2012. Evidence of sea level acceleration at U.S. and Canadian tide stations, Atlantic coast, North America. Journal of Coastal Research 28(6): 1437–1445. doi:10.2112/JCOASTRES-D-12-00102.1.
Cahoon, D.R., and G.R. Guntenspergen. 2010. Climate change, sea-level rise, and coastal wetlands. National Wetlands Newsletter 32: 8–12.
Cahoon, D.R., D. J. Reed, A.S. Kolker, M.M. Brinson, J.C. Stevenson, S. Riggs, R. Christian, E. Reyes, C. Voss, and D. Kunz. 2009. Coastal wetland sustainability, Chapter 4. In: Coastal sensitivity to sea-level rise: a focus on the Mid-Atlantic Region. Report by the US Climate Change Science Program and the Subcommittee on Global change Research. Synthesis and Assessment Product 4.1, pages 57–72.
Charles, H., and J.S. Dukes. 2009. Effects of warming and altered precipitation on plant and nutrient dynamics of a New England salt marsh. Ecological Applications 19: 1758–1773.
Church, J.A., and N.J. White. 2011. Sea-level rise from the late 19th to the early 21st century. Surveys in Geophysics 32: 585–602.
Crain, C.M., K. Kroeker, and B.S. Halpern. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11: 1304–1315. doi:10.1111/j.1461-0248.2008.01253.x.
Davey, E., C. Wigand, R. Johnson, K. Sundberg, J. Morris, and C. Roman. 2011. Use of computed tomography imaging for quantifying coarse roots, rhizomes, peat, and particle densities in marsh soils. Ecological Applications 21: 2156–2171.
Deegan, L.A., D.S. Johnson, R.S. Warren, B. Peterson, J.W. Fleeger, S. Fagherazzi, and W. Wollheim. 2012. Coastal eutrophication as a driver of marsh loss. Nature 490: 388–392.
Fagherazzi, S., M.L. Kirwan, S.M. Mudd, G.R. Guntenspergen, S. Temmerman, A. D’Alpaos, J. van de Koppel, J. Rybczyk, E. Reyes, C. Craft, and J. Clough. 2012. Numerical models of salt marsh evolution: ecological, geomorphic, and climatic factors. Reviews of Geophysics 50, RG1002. doi:10.1029/2011RG000359.
Fox, L., I. Valiela, and E.L. Kinney. 2012. Vegetation cover and elevation in long-term experimental nutrient-enrichment plots in Great Sippewissett salt marsh, Cape Cod, Massachusetts: implications for eutrophication and sea level rise. Estuaries and Coasts 35: 445–458.
IPCC. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Summaries, frequently asked questions, and cross-chapter boxes. In A contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change, ed. C.B. Field, V.R. Barros, D.J. Dokken, K.J. Mach, M.D. Mastrandrea, T.E. Bilir, M. Chatterjee, K.L. Ebi, Y.O. Estrada, R.C. Genova, B. Girma, E.S. Kissel, A.N. Levy, S. MacCracken, P.R. Mastrandrea, and L.L. White. Geneva: World Meteorological Organization. 190 pp.
King, G.M., M.J. Klug, R.G. Wiegert, and A.G. Chalmers. 1982. Relation of soil-water movement and sulfide concentration to Spartina alterniflora production in a Georgia salt-marsh. Science 218(4567): 61–63.
Kirwan, M.L., and J.P. Megonigal. 2013. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504: 53–60.
Kirwan, M.L., and S.M. Mudd. 2012. Response of salt-marsh carbon accumulation to climate change. Nature 489: 550–553.
Koch, M.S., I.A. Mendelssohn, and K.L. McKee. 1990. Mechanism for the hydrogen-sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography 35: 399–408.
Langley, J.A., K.L. McKee, D.R. Cahoon, J.A. Cherry, and J.P. Megonigal. 2009. Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proceedings of the National Academy of Sciences 106: 6182–6186.
Morris, J.T., P.V. Sundareshwar, C.T. Nietch, B. Kjerfve, and D.R. Cahoon. 2002. Responses of coastal wetlands to rising sea-level. Ecology 83: 2869–2877.
Morris, J.T., G.P. Shaffer, and J.A. Nyman. 2013. Brinson review: perspectives on the influence of nutrients on the sustainability of coastal wetlands. Wetlands 33: 975–988.
Nelson, J.L., and E.S. Zavaleta. 2012. Salt marsh as a coastal filter for the oceans: changes in function with experimental increases in nitrogen loading and sea-level rise. PLoS ONE 7(8), e38558. doi:10.1371/journal.pone.0038558.
Oczkowski, A., C. Wigand, A. Hanson, E. Markham, and R. Johnson. 2016. Nitrogen retention in salt marsh systems across elevation and precipitation regimes: a multiple stressor experiment. Estuaries and Coasts 39: 68–81.
Porter, E.M., W.D. Bowman, C.M. Clark, J.E. Compton, L.H. Pardo, and J.L. Soong. 2013. Interactive effects of anthropogenic nitrogen enrichment and climate change on terrestrial and aquatic biodiversity. Biogeochemistry 114: 93–120.
Sallenger Jr., A.H., K.S. Doran, and P.A. Howd. 2012. Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nature Climate Change. doi:10.1038/nclimate1597.
SAS Institute. 2011. SAS software, version 9.3. Cary: SAS Institute.
Stein, B.A., A. Staudt, M.S. Cross, N.S. Dubois, C. Enquist, R. Griffis, L.J. Hansen, J.J. Hellmann, J.L. Lawler, E.J. Nelson, and A. Pairis. 2013. Preparing for and managing change: climate adaptation for biodiversity and ecosystems. Frontiers in Ecology and the Environment 11: 502–510.
Sutter, L.A., R.M. Chambers, and J.E. Perry III. 2015. Seawater intrusion mediates species transition in low salinity, tidal marsh vegetation. Aquatic Botany 122: 32–39.
Turner, R.E. 2011. Beneath the saltmarsh canopy: loss of soil strength with increasing nutrient loads. Estuaries and Coasts 34: 1084–1093.
Turner, R.E., B.L. Howes, J.M. Teal, C.S. Milan, E.M. Swenson, and D. Goehringer-Toner. 2009. Salt marshes and eutrophication: an unsustainable outcome. Limnology and Oceanography 54: 1634–1642.
Valiela, I., J.M. Teal, and N.Y. Persson. 1976. Production and dynamics of experimentally enriched salt marsh vegetation: belowground biomass. Limnology and Oceanography 21: 245–252.
Valiela, I., M.L. Cole, J. McClelland, J. Hauxwell, J. Cebrian, and S.B. Joye. 2000. Role of salt marshes as part of coastal landscapes. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 23–38. Dordrecht: Kluwer Academic.
Watson, E.B., A.J. Oczkowski, C. Wigand, A.R. Hanson, E.W. Davey, S.C. Crosby, R.L. Johnson, and H.M. Andrews. 2014. Nutrient enrichment and precipitation do not enhance resiliency of salt marshes to sea level rise in the northeastern US. Climatic Change 125: 501–509.
Weston, N.B. 2014. Declining sediments and rising seas: an unfortunate convergence for tidal wetlands. Estuaries and Coasts 37: 1–23.
Wigand, C., P. Brennan, M. Stolt, M. Holt, and S. Ryba. 2009. Soil respiration rates in coastal marshes subject to increasing watershed nitrogen loads in southern New England, USA. Wetlands 29: 952–963.
Wigand, C., C.T. Roman, E. Davey, M. Stolt, R. Johnson, A. Hanson, E.B. Watson, S.B. Moran, D.R. Cahoon, J.C. Lynch, and P. Rafferty. 2014. Below the disappearing marshes of an urban estuary: historic nitrogen trends and soil structure. Ecological Applications. doi:10.1890/13-0594.1.
Wigand, C., T. Ardito, C. Chafee, W. Ferguson, S. Paton, K. Raposa, C. Vandemoer, and E. Watson. 2016. Development of a climate-change adaptation strategy for management of coastal marsh systems in southern New England, USA. Estuaries and Coasts. doi:10.1007/s12237-015-0003-y.
Wong, J.X.W., C. Van Colen, and L. Airoldi. 2015. Nutrient levels modify saltmarsh responses to increased inundation in different soil types. Marine Environmental Research 104: 37–46.
Yin, J., M.E. Schlesinger, and R.J. Stouffer. 2009. Model projections of rapid sea-level rise on the northeast coast of the United States. Nature Geoscience 2: 262–266.
Acknowledgments
We thank Beth Watson, Kristen Jones, Joe Bishop, Gabrielle Sousa, and Kirk Silver for field and laboratory assistance; Kevin Kelly, John Sardelli, Bob Dow, Adam Kopacsi, and Russ Ahlgren for maintenance and construction of tanks and mesocosms; Kenneth Miller for statistical analysis; and Patricia DeCastro for graphical arts support. Sandi Robinson, Rick McKinney, and Giancarlo Cicchetti provided helpful input on an earlier version of the manuscript. This is ORD tracking number ORD-010361 of the U.S. EPA’s Office of Research and Development, National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division. Although the information in this document has been funded by the U.S. Environmental Protection Agency, it does not necessarily reflect the views of the agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carles Ibanez Marti
Rights and permissions
About this article
Cite this article
Hanson, A., Johnson, R., Wigand, C. et al. Responses of Spartina alterniflora to Multiple Stressors: Changing Precipitation Patterns, Accelerated Sea Level Rise, and Nutrient Enrichment. Estuaries and Coasts 39, 1376–1385 (2016). https://doi.org/10.1007/s12237-016-0090-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0090-4