Assessing Variation and Diversity of Ethnomedical Knowledge: A Case Study from Malekula Island, Vanuatu
Ethnomedical knowledge is important for health and wellbeing in many rural communities. Bodies of ethnomedical knowledge vary within and between communities, and may be at risk of erosion. However, little work has analyzed knowledge variation in Melanesia. In this study we use structured interview data from 177 participants to analyze richness and diversity of ethnomedical knowledge on Malekula Island in the Republic of Vanuatu. We use an information theoretic approach, a methodology that enables selection between competing hypotheses, and find that ethnomedical knowledge richness is patterned by gender, linguistic preference, and market visitation. We also note that the diversity of ethnomedical knowledge is highest in the oldest, less formally educated participants. These findings may indicate that social and environmental change has impacted the shape and form of ethnomedical knowledge in these communities. In response, we note the importance of vernacular language acquisition for maintenance of ethnomedical knowledge on Malekula. Our approach demonstrates the power of ecological methods, including diversity indices and model selection, for the analysis of ethnobiological data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The medicinal knowledge of local and indigenous people has long piqued the interest of ethnobiologists and anthropologists. Ethnomedical knowledge supports the medicinal needs of some populations, and is a critical aspect of well-being in rural and urban communities around the world (World Health Organization 2008). This knowledge is also of interest for pharmacological exploration (Voeks and Leony 2004) and represents a valuable source of adaptive capacity and resilience to social and ecological changes (Zent and Maffi 2009).
Many commentators have stressed the importance of examining intra- and inter-cultural variation of knowledge (e.g., Pfeiffer and Butz 2005). Such studies enable us to describe the social organization of knowledge as well as the characteristics of knowledgeable individuals (e.g., Boster 1986). Moreover, in an environment where ethnomedical knowledge may be at risk of erosion, studies of knowledge variation may offer clues as to rates and drivers of knowledge loss. In turn, this approach may allow us to develop more effective models for the maintenance of ethnomedical knowledge (Zent and Maffi 2009).
Drivers of variation of ethnobiological knowledge are interactive and complex, and researchers have found that several variables are associated with the structure of bodies of knowledge. Demographic variables often pattern knowledge throughout communities: age tends to be positively associated with ethnobiological knowledge (Voeks and Leony 2004), while gender is a key variable (although the direction of the relationship differs between study sites; Pfeiffer and Butz 2005). Other studies note that market integration impacts on bodies of ethnobiological knowledge, albeit in complex ways (Godoy et al. 1998; Reyes-Garcia et al. 2005). Still others note that other indicators of modernization, such as level of formal education (Quinlan and Quinlan 2007) or lack of proficiency in vernacular languages (Benz et al. 2000), tend to associate negatively with ethnobiological knowledge. Where studies have attempted to infer change in ethnobiological knowledge, several have indicated erosion over time (e.g., Voeks and Leony 2004), though some note persistence (Zarger and Stepp 2004) or net increases (Guest 2002) within some domains of knowledge (or “subject areas”; Berkes 2008) under changing socioeconomic conditions.
In sum, findings thus far have been equivocal, and drivers of variation of ethnomedical knowledge remain unclear (Zent and Maffi 2009). Moreover, there remain a number of opportunities for methodological and analytical advances. For one, untangling the complex relationships between predictor variables in models of ethnobiological knowledge would benefit from careful selection and testing of hypotheses (e.g., Burnham and Anderson 2004). Further, use of a diverse range of analytical tools from parallel fields such as ecology can add depth to data interpretation (Begossi 1996). Finally, a persistent geographic skew in research of this kind means that studies from Oceania, one of the most bioculturally diverse regions on earth, are lacking (Reyes-Garcia et al. 2006).
In this paper we examine variation and diversity of ethnomedical knowledge from Malekula Island in Vanuatu. This work is the first to study variation of ethnobotanical knowledge in Vanuatu, and adds to the few studies of its kind in the region (e.g., Case et al. 2005). We address the opportunities noted above by employing an information theoretic approach (Burnham and Anderson 2004) to parse competing hypotheses, and utilizing methods from ecology to assess diversity, as well as richness, of ethnomedical knowledge (Begossi 1996). This provides a broad foundation for understanding processes of variation and change in ethnomedical knowledge.
Methods
Study Context and Sample Selection
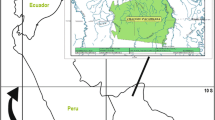
This research was conducted on Malekula Island in the Republic of Vanuatu (Fig. 1). A large (206,756 ha) and rugged island, Malekula has a population of around 27,000 people, who continue to speak around 20 vernacular languages (Lynch and Crowley 2001). Like the rest of Vanuatu, livelihoods on Malekula are based on subsistence horticulture and nearshore reef harvesting (Walter and Lebot 2007). Roughly 75,000 ha of Malekula are forested, with plantations of coconut (Cocos nucifera L.) and cacao (Theobroma cacao L.) dominating the coastal plains. Most people live in coastal areas, surrounded by low hills dominated by secondary forest/garden mosaics.
Geography and a lack of infrastructure mean that, for many communities, access to biomedical care is infrequent and difficult. The only hospital on Malekula is located at the commercial center of Lakatoro/Norsup. For most inhabitants, however, it is only used for the most serious illnesses, as transport expenses can be prohibitive. Health services within more isolated communities tend to lack fundamental medicines such as antibiotics, and visits by doctors are rare.
Accordingly, ethnomedical knowledge remains an important source of medical care for most people (Bradacs et al. 2011). Such remedies are known locally as kastom medicine. Kastom in this sense does not necessarily imply traditional or ancient knowledge; rather, it refers to medicine that was generated from within as opposed to by outsiders. Most Malekulans can self-administer basic remedies for a range of illnesses, but will consult a specialist healer for complex, serious, or unusual illnesses.
We selected four study sites on Malekula (Fig. 1c), in consultation with the Vanuatu Cultural Centre (VCC). Site selection was stratified along a gradient of ease of access to market, because market influence may pattern ethnomedical knowledge within and between communities (Godoy et al. 1998). The focus communities were broadly similar in other respects: all were originally based inland (i.e., in the interior of the island), and moved to the coast after the establishment of the mission stations between 1900 and 1972; all four are relatively small (ranging between 91 and 180 inhabitants); and all four have a VCC fieldworker (community members who participate in a national network for the revitalization of traditional practice and vernacular language). Each community speaks a different vernacular language.
Participant selection was stratified by age (cohorts of 18–30, 31–60, and 60+) and gender, as these variables have been implicated in patterning ethnobiological knowledge (Reyes-García et al. 2006). Within these cohorts, participant selection was opportunistic and based on participant availability.
Data Collection
Primary data were collected using a structured interview with 177 informants. All interviews were conducted in Bislama, a neo-Melanesian creole and national language. Data were collected using freelisting, where participants are asked to list items within a cultural domain (Quinlan and Quinlan 2007). This gives a rapid and simple appraisal of a participant’s knowledge within that domain, based on the assumption that more knowledgeable individuals will list more than less knowledgeable individuals.
We utilized a two-stage freelisting technique (Quinlan and Quinlan 2007). First, a purposively selected sample of 18 expert informants (10 men and 8 women) were asked to freelist illnesses that were treated using kastom medicine in their community. This process resulted in 72 illnesses, which were reduced to the ten most salient using Smith’s S (Smith 1993). The remaining 62 illnesses were discarded (Table 1).
Each of the selected illnesses was vetted by expert informants to confirm that it was appropriate for inclusion in the survey instrument. In particular, we confirmed that we would expect that the illnesses would be known by a wide cross-section of the community rather than being restricted to specialist healers. All participants (n = 177) were then asked to list all the kastom medicines they knew to treat each of the ten illnesses.
Each plant cited through this procedure was identified by its name in the local vernacular, or in Bislama if this was not known. Voucher specimens were collected and identified with the assistance of botanists at the Vanuatu Department of Forests. All specimens are deposited at the National Herbarium of Vanuatu in Port Vila.
Dependent Variables
We generated three measures of ethnomedical knowledge from freelists: richness, abundance, and diversity of citations. Citation abundance refers to the number of kastom medicines listed by each participant, while citation richness refers to the number of folk species recorded. Citation diversity is calculated using the Shannon-Weiner index, an analytical tool developed in ecology (Magurran 2004). This index takes account of both citation richness and evenness (relative abundance) to produce a single measure of citation diversity, denoted by H'. This approach allows us to qualify count data by examining both individual and collective knowledge assemblages.
Predictor Variables
We measured eight variables that have been implicated in variation of ethnobiological knowledge, broadly grouped into demographic and modernization categories (Table 2). Our broad hypothesis was that knowledge will be structured by both demographic and modernization variables: the former because ethnobiological knowledge is held collectively and patterned throughout communities, and the latter because modernization may be weakening transmission of knowledge over time (Voeks and Leony 2004). We also predicted differences between communities, based on the gradient of ease of access to market (Zent and Maffi 2009).
Analysis
First, we compared models that seek to explain variation in individual citation richness, abundance, and diversity using multiple regression under an information theoretic approach (Burnham and Anderson 2004). Information theoretic approaches use explicit criteria (Akaike Information Criterion; AIC) to select the best model, or models, from a set of competing hypotheses. AIC values are derived from the quality of fit of each model as well as the principle of parsimony, and are corrected here to AICc for small sample size (Burnham and Anderson 2004). In this way, this approach avoids information loss associated with the inclusion of surplus predictor variables and considers uncertainty in the choice of explanatory models, in contrast with classical model selection.
We set eleven hypotheses, or combinations of predictor variables, based on the literature noted above in Table 2. We then individually tested each hypothesis by running multiple regression analyses, and ranked models based on their AICc (Table 3). Models are ranked according to the difference in AICc (ΔAICc), and the “best” model subset is generally held to include all models with a ΔAICc < 2 (Burnham and Anderson 2004).
Before multiple regression modeling we completed standard diagnostic tests to ensure the assumptions of regression analysis were met, and that a linear approach was most appropriate. Individual-level citation richness, abundance, and diversity were extremely highly correlated (rs = 0.980 to 0.990), meaning that the use of more than one index would be redundant. As such, we tested only citation richness, which was log transformed to meet assumptions of normality.
Second, we examined citation diversity (H’) at a group level. This is important: because the index is calculated from the total number of citations, calculations at a group level can reveal significant trends where individual-level variation is not evident. We triangulated these data with rarefaction curves, which are used to estimate the richness of ethnomedical knowledge from the pooled total richness of the sample (Gotelli and Colwell 2011).
Statistical analysis was calculated using SPSS 17.0 for Windows (SPSS Inc. 2008). Diversity indices were calculated from raw data using the Shannon Index calculator from Chang Bioscience (2011). Rarefaction curves were calculated using EstimateS 8.2 (Colwell 2009).
Ethical Approach
We adhered to a strict ethical protocol, in response to a number of concerns surrounding the collection of ethnomedical data (e.g., Agrawal 2002). Our protocol was based on the Code of Ethics of the International Society for Ethnobiology (ISE 2006), and was approved by the Human Ethics Committee at Victoria University and the Vanuatu National Cultural Council. The protocol included a number of clauses to ensure a fair, equitable, and accurate research program. Notably, by request of the VCC, all ethnomedical data including species names and uses are confidential and will not be published in this or other reports.
Results
Descriptive Statistics
The freelists inventoried 262 folk species of plants from 73 families and 180 genera, 237 of which were identified to genus level and 216 to species level. The remaining 25 citations could not be identified due to poor specimen condition or because they were unable to be located.
Richness and diversity (H’) of species cited was, in general, highest in the most salient illnesses (e.g., citations for headache treatments, H’ = 4.27; for conjunctivitis, H’ = 2.72). Most individuals cited six plants across the 10 freelists, and the mean number of plants cited per illness was 1.1. Participants commonly knew many treatments for some illnesses (i.e., for treating cuts: mean = 2.05, median = 2) and very few for others (e.g., arthritis: mean = 0 .49, median = 0). Moreover, some plants were commonly used to treat a wide range of ailments: for example, one species of shrub (Euphorbiaceae) was cited by at least one participant in all ten freelists (and 48 times overall).
Model Ranking and Regression
We conducted model ranking as described above. Only one model (Model Eight) had a ΔAICc < 2, and included predictor variables of age, gender, educational attainment, linguistic preference, and market visitation (descriptive statistics in Table 4, regression analysis in Table 5). Gender, linguistic preference, and annual market visitation displayed significant associations with citation richness. When converted from the log transformation, males cited 1.97 more species of medicinal plant than women; participants who preferentially used vernacular languages over Bislama cited 1.11 more medicinal species; and for every additional visit to market, participants cited 1.32 fewer medicinal plants. Both age and educational attainment showed a positive, but insignificant, relationship with citation richness.
Group Level Diversity Indices
We conducted further analysis of citation diversity (H’) in groups for each of the variables tested in the regression analysis (Fig. 2). We created categories within the continuous variables based on sample size: age was split into three cohorts (18–30, 31–60, and 60+); market visitation into four (0 market visits; 1–2 market visits; 3–4 market visits; and 5+ market visits per year); and educational attainment into three (0–5 years of schooling, up to year 6; and over year 6). Differences between categories were tested via Kruskal-Wallis and Mann-Whitney U tests.
Some findings were consistent with the individual level analyses (Appendix 1 [Electronic Supplementary Material]) Men cited a more diverse body of species than women (respectively, H’ = 4.7 and H’ = 4.27; significant at p < .001); participants who preferred to speak vernacular languages displayed a more diverse set of knowledge than those who speak Bislama (respectively, H’ = 4.67 and H’ = 3.8; significant at p < .001); and those with fewer market visits cited a more diverse sample than those with more (e.g., 0 market visits H’ = 4.46, whereas 5+ market visits H’ = 4.2; significant at p < .001). Other differences were significant at the group-level, although similar comparisons had not been in the individual-level analyses: the oldest age cohort cited a significantly more diverse suite of medicinal plants than the youngest (respectively, H’ = 4.62 and H’ = 4.3, significant at p < .001), and those with less education a more diverse body of citations than those with the most (respectively, H’ = 4.68 and H’ = 4.1, significant at p < .001). Citation diversity was highest at Dixon Reef (H’ = 4.36) and significantly lower at Tisvel and Unmet (both H’ = 3.98; difference significant at p < .001).
Finally, we plotted rarefaction curves of the major groupings above (not shown). These plots confirmed significant differences in expected citation richness between the categories above.
Discussion
These results highlight the depth of ethnomedical knowledge within the four focus communities. The 216 specimens identified to species level represent a substantial proportion of the c.1,200 plant species in Vanuatu (Cabalion et al. 1991), and compares favorably with the medicinal plant diversity in other tropical areas (Begossi et al. 2002). Moreover, the lack of variation of individual-level citation richness with age indicates the importance of ethnomedical knowledge across the multiple generations in the sample.
How might we explain the variation of ethnomedical knowledge on Malekula? In the case of gender, these data agree with findings from South America, the Pacific, and Africa in suggesting that men cite a richer body of ethnomedical knowledge than women do (Case et al. 2005). This finding could be interpreted in a number of ways. It is likely, in part, to reflect cultural division of labor in the focus communities, in which men often play a prominent role in dispensing kastom medicine. However, this is unlikely to be the whole story: women are central to the household economy throughout Vanuatu (Regenvanu 2005), and the experts consulted in interview piloting noted that all community members utilize herbal medicine to treat these illnesses. One possibility is that women’s ethnomedical knowledge has eroded in recent years at a faster rate than men’s, although further targeted research is needed to confirm this. Another is that this finding is influenced by the particular categories of knowledge sampled. For example, women have been noted to maintain extensive bodies of ethnomedical knowledge pertaining to the human reproductive cycle (Bourdy and Walter 1992), which was not one of the categories surveyed in our study. While our study design should have ensured we focused on medicines that were relevant to both men and women, further targeted research into variation of women’s ethnomedical knowledge in Vanuatu would be a useful addition.
Linguistic preference and market visitation were also important variables, and may point to the role of social change in patterning richness and diversity of ethnomedical knowledge on Malekula. Vernacular languages, having evolved in close contact with the local environment, can contain rich folk taxonomies that are inherently acquired as a child becomes proficient in that language (Nettle and Romaine 2000). However, linguistic change and attrition are ongoing on Malekula (as elsewhere in Vanuatu; e.g., Vari-Bogiri 2005), driven by social processes such as formal schooling and exogamous marriage (Regenvanu 2005). In these areas, Bislama often becomes prevalent (e.g., Crowley 2006). Bislama has a relatively small vocabulary, and in particular lacks the array of specific terms for the local environment (e.g., names of plant species). This may impede acquisition of ethnomedical knowledge.
The significance of market visitation, too, may be indicative that livelihood changes on Malekula are impacting on patterns of ethnomedical knowledge. Market integration has been found to influence ethnobiological knowledge in a variety of ways (Godoy et al. 1998). In the context of the focus communities, propensity to visit the market town may reflect the likelihood of an individual of accessing hospital care. Research in other areas has found that direct competition from biomedicine has resulted in lower rates of ethnomedical knowledge transmission, or disappearance of specialist healers within the community (Begossi et al. 2002). This hypothesis is indirectly supported by significant differences between communities, where individuals in the southernmost communities reported more diverse sets of ethnomedical knowledge. Given the relatively low sample size within each community, further targeted research would be needed to confirm this finding; however, it offers an interesting corroboration to some of the differences between individuals.
Importantly, although individual knowledge in informants was not structured by some variables (notably age and education), these variables did influence group-level knowledge diversity. Although there was little variation in the number of citations of the oldest, least educated participants, the content of their ethnomedical knowledge was significantly more diverse (Fig. 3). Given recent social and geographic shifts within the focus communities (most notably, the shift to the coast from the hill country inland), it is plausible that elder community members hold idiosyncratic knowledge of the interior environment of Malekula. This knowledge may be no longer salient to younger generations, and may be at risk due to changing systems of cultural transmission (McCarter and Gavin 2011). Of course, this finding may reflect the normal accumulation of knowledge with age (Godoy et al. 2009). However, given that we would expect that an individual would acquire the bulk of their theoretical ethnobotanical knowledge before age 60, the oldest age cohort in this study (e.g., Hunn 2002), differences between the plants cited by age cohorts are important. Indeed, they point to the likely loss of this knowledge over coming decades.
Heuristic diagram of H' of medicinal citations for four individuals. Grey circles represent interviewees. a. represents youngest age cohort (18–30); b. represents oldest (61+). Note each interviewee cites a similarly diverse body of medicines (i.e., the diameter of the circle is the same); however, as a group b. has a more diverse suite of ethnomedical knowledge.
Our analysis emphasizes two methodological points. First, the use of an information theoretic approach allowed the selection of the most appropriate model for the data, and systematically selected between competing hypotheses (Burnham and Anderson 2004). This represents an important refinement of standard regression modeling. Second, the use of diversity indices revealed patterns that were not obvious from the richness and abundance data that are commonly extracted from freelists. Both methods have been extensively utilized within ecology (e.g., Odeli et al. 2008; Spellerberg and Fedor 2003), and have potential for further use in anthropology and ethnobiology.
Conclusion
In this paper we assessed drivers of variation and evidence of erosion in ethnomedical knowledge on Malekula. To do so, we synthesized methods from ethnobiology and ecology and derived data with important implications. Theoretically, the data add to knowledge of ethnomedical knowledge variation, and contribute the first study of this kind from Vanuatu. Methodologically, this paper shows the value of using a range of analytical approaches. In particular, the use of diversity indices allowed us to observe age-cohort related trends and infer erosion of knowledge.
These results have policy implications for those interested in the revitalization of ethnomedical knowledge in Vanuatu. For one, the ability to speak vernacular languages appears to be important for ethnomedical literacy. This supports calls for an increased role for vernacular languages in education in Vanuatu (e.g., Early 1999). Moreover, the more diverse ethnomedical knowledge of older participants adds weight to calls for elders to be more engaged in education, and for the revitalization of pathways for cultural transmission (McCarter et al. 2014; McCarter and Gavin 2014). Finally, ethnomedical knowledge remains important on Malekula, and represents a source of independence and resilience. Given that pressures toward cultural homogenization are likely to increase in coming years, an ongoing interest in the area from policy makers and researchers will be necessary.
Literature Cited
Agrawal, A. 2002. Indigenous knowledge and the politics of classification. International Social Science Journal 54(173):287–298.
Begossi, A. 1996. Use of ecological methods in ethnobiology: Diversity indices. Economic Botany 50(3):280–289.
———, N. Hanazaki, and J. Tamashiro. 2002. Medicinal plants in the Atlantic forest (Brazil): Knowledge, use, and conservation. Human Ecology 30(3):281–299.
Benz, B. F., J. Cevallos, F. Santana, J. Rosales, and S. Graf. 2000. Losing knowledge about plant use in the Sierra De Manantlan Biosphere Reserve, Mexico. Economic Botany 54(2):1874.
Berkes, F. 2008. Sacred ecology: Traditional ecological knowledge and resource management, 2nd edition. Taylor and Francis, London.
Boster, J. 1986. Exchange of varieties and information between Aguaruna manioc cultivators. American Anthropologist 88:429–436.
Bourdy, G. and A. Walter. 1992. Maternity and medicinal plants in Vanuatu. I: The cycle of reproduction. Journal of Ethnopharmacology 37:176–196.
Bradacs, G., J. Heilmann, and C. Weckerle. 2011. Medicinal plant use in Vanuatu: A comparative ethnobotanical study of three islands. Journal of Ethnopharmocology 137(1):434–448.
Burnham, K. and D. Anderson. 2004. Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods and Research 33:261–304.
Cabalion, P., C. Sam, and M. Hoff. 1991. Contribution a l’etude de la flore de Vanuatu. Index synonymique de la flore, liste de specimens d’herbier par especes, listes des especes par ile [Document Interne]. OSRTOM, Port Vila, Vanuatu.
Case, R. J., G. E. Pauli, and D. Soejarto. 2005. Factors in maintaining indigenous knowledge among ethnic communities of Manus Island. Economic Botany 59(4):356–365.
Chang Bioscience. 2011. Shannon-Wiener Diversity Index / Shannon Entropy Calculator. http://www.changbioscience.com/genetics/shannon.html (27 August 2015).
Colwell, R. 2009. EstimateS: Statistical estimation of species richness and shared species samples. Version 8.2 [software].
Crowley, T. 2006. Naman: A vanishing language of Malakula (Vanuatu). Pacific Institute of Linguistics, Australian National University, Canberra.
Early, R. 1999. Double trouble, and three is a crowd: Languages in education and official languages in Vanuatu. Journal of Multilingual and Multicultural Development 20(1):37–41.
Godoy, R., N. Brokaw, D. Wilkie, D. Colon, A. Palermo, S. Lye, S. Sei, D. Colón, and S. Wei. 1998. Of trade and cognition: Markets and the loss of folk knowledge among the Tawahka Indians of the Honduran rainforest. Journal of Anthropological Research 54(2):219–234.
———, V. Reyes-Garcia, J. Broesch, I. Fitzpatrick, P. Giovanninni, M. Rodriguez, T. Huanca, et al. 2009. Long-term (secular) change of ethnobotanical knowledge of useful plants: Separating cohort and age effects. Journal of Anthropological Research 65(c):51–67.
Gotelli, N. J. and R. K. Colwell. 2011. Estimating species richness. Pages 39–54 in A. E. Magurran and B. J. McGill, eds., Biological diversity: Frontiers in measurement and assessment. Oxford University Press, Oxford.
Guest, G. 2002. Market integration and the eistribution of ecological knowledge within an Ecuadorian fishing community. Journal of Ecological Anthropology 6(1):38.
Hunn, E. S. 2002. Evidence for the precocious acquisition of plant knowledge by Zapotec children. Pages 604–613 in J. R. Stepp, F. S. Wyndham, and R. K. Zarger, eds., Ethnobiology and biocultural diversity. International Society for Ethnobiology, Athens, Georgia.
Inc, S. P. S. S. 2008. SPSS for Windows 17.0. SPSS Inc., Chicago.
ISE (International Society of Ethnobiology). 2006. ISE code of ethics (with 2008 additions). http://ethnobiology.net/code-of-ethics/code-in-english/ (27 August 2015).
Lynch, J. and T. Crowley. 2001. Languages of Vanuatu. Pacific Linguists, Canberra.
Magurran, A. E. 2004. Measuring biological diversity. Malden, Massachusetts, and Oxford, U.K: Blackwell Science Ltd.
McCarter, J. and M. C. Gavin. 2011. Perceptions of the value of traditional ecological knowledge to formal school curricula: Opportunities and challenges from Malekula Island, Vanuatu. Journal of Ethnobiology and Ethnomedicine 38(7).
——— and ———. 2014. In situ maintenance of traditional ecological knowledge on Malekula Island, Vanuatu. Society and Natural Resources 27(11):1115–1129.
———, ———, S. Baereleo, and M. Love. 2014. The challenges of maintaining indigenous environmental knowledge. Ecology and Society 19(3).
Nettle, D. and S. Romaine. 2000. Vanishing voices: The extinction of the world’s languages. Oxford University Press, Oxford.
Odeli, E. A., F. M. Pusateri, and G. C. White. 2008. Estimation of occupied and unoccupied black-tailed prairie dog colony acreage in Colorado. The Journal of Wildlife Management 2(6):1311–1317.
Pfeiffer, J. M. and R. J. Butz. 2005. Assessing cultural and ecological variation in ethnobiological research: The importance of gender. Journal of Ethnobiology 25(2):240–278.
Quinlan, M. and R. Quinlan. 2007. Modernization and medicinal plant knowledge in a Caribbean horticultural village. Medicinal Anthropology Quarterly 21(2):169–192.
Regenvanu, R. 2005. The changing face of “custom” in Vanuatu. People and Culture in Oceania 20 (Special edition).
Reyes-Garcia, V., V. Vadez, E. Byron, L. Apaza, W. R. Leonard, L. E. Perez, and D. Wilkie. 2005. Market economy and the loss of folk knowledge of plant uses: Estimates from the Tsimane’ of the Bolivian Amazon. Current Anthropology 46(4):651–656.
———, ———, S. Tanner, T. Huanca, W. R. Leonard, and T. McDade. 2006. Measuring what people know about the environment: A review of quantitative studies. Tsimane Amazonian Panel Study Working Paper #21. http://people.brandeis.edu/~rgodoy/workingpapers/TAPS-WP-21-TEKMETHODS-Jan-2006.pdf.\
Smith, J. 1993. Using ANTHROPAC 3.5 and a spreadsheet to compute a freelist salience index. Cultural Anthropology Methods Letter 5:1–3.
Spellerberg, I. F. and P. J. Fedor. 2003. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the “Shannon-Wiener” Index. Global Ecology and Biogeography 177–179.
Vari-Bogiri, H. 2005. A sociolinguistic survey of Araki: A dying language in Vanuatu. Journal of Multilingual Multicultural Development 26(1):52–66.
Voeks, R. A. and A. Leony. 2004. Forgetting the forest: Assessing medicinal plant erosion in eastern Brazil. Economic Botany 58(Supplement):S294–S306.
Walter, A. and V. Lebot. 2007. Gardens of Oceania. ACIAR, Canberra.
World Health Organization. 2008. Fact sheet on traditional medicine. http://www.who.int/mediacentre/factsheets/fs134/en/ (27 August 2015).
Zarger, R. K. and J. R. Stepp. 2004. Persistence of botanical knowledge among Tzeltal Maya children. Current Anthropology 45(3):413–418.
Zent, S. and E. Zent. 2004. Ethnobotanical convergence, divergence and change among the Hopi of the Venezuelan Guyana. Pages 37–78 in J. Carlson and L. Maffi, eds., Ethnobotany and the conservation of biocultural diversity. New York Botanical Garden Press, New York.
——— and L. Maffi. 2009. Final Report on Indicator No. 2: Methodology for developing a vitality index of traditional environmental knowledge (VITEK) for the project “Global Indicators of the Status and Trends of Linguistic Diversity and Traditional Knowledge.” Terralingua. http://terralingua.org/projects/vitek/VITEK_Report.pdf (3 March 2009).
Acknowledgments
The authors gratefully acknowledge the generous assistance of collaborators in Vanuatu, in particular VCC fieldworkers in the focus communities. Both authors were based at Victoria University of Wellington, New Zealand, while this work was completed. JM was supported by the Ryoichi Sasakawa Young Leaders Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
McCarter, J., Gavin, M.C. Assessing Variation and Diversity of Ethnomedical Knowledge: A Case Study from Malekula Island, Vanuatu. Econ Bot 69, 251–261 (2015). https://doi.org/10.1007/s12231-015-9319-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12231-015-9319-6