Abstract
Breeding systems play an essential role in plant sexual reproduction and influence speciation and extinction processes. However, our understanding of the breeding systems for particular neotropical angiosperm families is inadequate. The Pineapple family (Bromeliaceae) is one of the few indigenous and highly diverse plant lineages native to the American Continent and is a resource for the ornamental plant industry. Bromeliads have a remarkable history of adaptive radiation, yet the role of breeding systems in their evolution and ecology is still unknown. This review aims to establish the current state of knowledge on breeding systems in Bromeliaceae by identifying general patterns, data limitations, and information gaps. We compiled data on self-compatibility (SC), autonomous self-fertilization (selfing), and apomixis based on a thorough review of the scientific literature from 1990 to 2020. The final database included 177 entries, which represented 26 genera and 152 species (4.1% of the family). Two-thirds of the studies were conducted on species from highly diverse genera: Aechmea, Pitcairnia, Tillandsia, and Vriesea. Bromeliaceae exhibit a wide variety of breeding systems (SC and selfing). Subfamilies Pitcairnioideae (sensu stricto) and Tillandsioideae had higher values of SC and selfing, although some of the most investigated genera in each subfamily exhibited contradictory patterns and data for subfamilies considered ancestral were absent. Complete apomixis was rare, but it was more prevalent in Pitcairnioideae. The evolution of autofertility is likely the combined result of floral herkogamy as well as the species’ self-compatibility. Our present understanding of the evolutionary advantages of selfing in Bromeliaceae is limited and deserves further investigation.
Resumen
Los sistemas reproductivos juegan un papel esencial en la reproducción sexual de las plantas e influyen en los procesos de especiación y extinción. Sin embargo, nuestra comprensión de los sistemas reproductivos de familias de angiospermas neotropicales particulares es inadecuada. La familia de la piña (Bromeliaceae) es uno de los pocos linajes de plantas autóctonas y muy diversas del continente americano y es un recurso para la industria de las plantas ornamentales. Las bromelias tienen una historia notable de radiación adaptativa, pero aún se desconoce el papel de los sistemas de reproducción en su evolución y ecología. Esta revisión tiene como objetivo establecer el estado actual del conocimiento sobre los sistemas de reproducción en Bromeliaceae mediante la identificación de patrones generales, limitaciones de datos y vacíos de información. Recopilamos datos sobre autocompatibilidad (AC), auto-fertilización espontánea y apomixis a partir de una revisión exhaustiva de la literatura científica de 1990 a 2020. La base de datos final incluyó 177 registros, que representan 26 géneros y 152 especies (4.1% de la familia). Dos tercios de los estudios se realizaron en especies de géneros muy diversos: Aechmea, Pitcairnia, Tillandsia y Vriesea. Las bromeliáceas exhiben una amplia variedad de sistemas de reproducción (AC y auto-fertilización). Las subfamilias Pitcairnioideae (sensu stricto) y Tillandsioideae tuvieron valores más altos de SC y auto-fertilización, aunque algunos de los géneros más investigados en cada subfamilia exhibieron patrones contradictorios y no hubo datos para las subfamilias consideradas ancestrales. La apomixis completa fue rara, pero fue más frecuente en Pitcairnioideae. La evolución de la autofertilidad es probablemente el resultado combinado de hercogamia y autocompatibilidad de las especies. Nuestra comprensión actual de las ventajas evolutivas de la auto-fertilización en Bromeliaceae es limitada y merece una mayor investigación.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breeding systems combine morphological and physiological traits of flowers that determine the likelihood that two gametes will unite (Neal & Anderson, 2005) and play a crucial role in the ecology of angiosperms because they have an impact on seed production, demographic stability, gene diversity, and population genetic structure (Goldberg & Igić, 2012). The evolution of breeding systems in plants and its adaptive value are topics of interest mainly due to their micro- and macro-evolutionary consequences (Goodwillie et al., 2005; Charlesworth, 2006; Ferrer & Good, 2012; Karron et al., 2012; Raduski et al., 2012). For many neotropical angiosperm groups, thorough information about their breeding systems is still insufficient.
The Bromeliaceae family (pineapple family or simply bromeliads) is the most diverse group of flowering plants that is mostly endemic to the tropical and subtropical regions of the New World (only one species is present in West Africa). This monocotyledonous lineage consists of approximately 3,600 species in 78 genera (Gouda & Butcher, 2016 cont. updated) and displays extraordinary adaptive radiation associated with morphological, physiological, and ecological traits responsible for their diversification and geographic expansion (Givnish et al., 2014). The role of breeding systems in the evolution and ecology of Bromeliaceae is still unknown, and there is no comprehensive literature review on the diversity of breeding systems in this group. In their earlier publications, Benzing (2000) and Matallana et al. (2010) provided preliminary, albeit incomplete, information regarding the reproductive systems of bromeliads.
Self-incompatibility (SI) is an important element of breeding systems and is defined as the partial or total inability of a bisexual plant to produce zygotes after self-pollination (Becerra & Lloyd, 1992). The SI system activates a series of molecular mechanisms to prevent self-pollen germination and pollen tube development on the flower pistil (de Nettancourt, 2001). SI is regarded as the ancestral condition of flowering plants (Allen & Hiscock, 2008) and has long been acknowledged as the most important mechanism for promoting outcrossing in hermaphroditic plants (Darwin, 1876). SI lineages exhibit greater species diversity and net diversification rates (Ferrer & Good, 2012). The loss or weakening of SI is expressed in different magnitudes as self-compatibility (SC). SC species reproduce through both self- and cross-pollination in a mixed mating system (Goodwillie et al., 2005).
The transition from SI to SC is considered an irreversible evolutionary phenomenon that has occurred independently in numerous plant lineages (Stebbins, 1974; Igić et al., 2008). Self-compatible plants may eventually acquire the ability to self-fertilize autonomously (i.e., selfing or autogamy) without the assistance of pollinators. Selfing is characterized by floral adaptations that facilitate the transfer of one’s own pollen to the stigma, such as homostyly (absence of herkogamy) when the stigma and anthers are at the same height (Webb & Lloyd, 1986) and adichogamy (absence of dichogamy) when stigma receptivity and pollen presentation occur concurrently (Bertin & Newman, 1993). The timing of selfing relative to the opportunity for out-crossing (e.g., prior, competing, or delayed selfing) has reproductive consequences and may shed light on the evolution and maintenance of selfing (Lloyd, 1992; Lloyd & Schoen, 1992). Traditionally, selfing is viewed as a reproductive assurance strategy that evolved as a response to unfavorable ecological conditions for pollination (Darwin, 1876; Muller, 1883; Kalisz & Vogler, 2003; Eckert et al., 2006).
Bromeliads originated in the Guiana Shield in northern South America approximately 100 million years ago and spread to other tropical and subtropical regions of the North and South American continents (Givnish et al., 2011). They are valued in the floriculture sector of international ornamental industry (Negrelle et al., 2012) and are part of the ethnobotany of a number of local cultures (Bennett, 2000). The desire to develop new cultivars has partially motivated the study of their breeding systems (Vervaeke et al., 2001; Souza et al., 2017). Historically, three subfamilies have been recognized: Bromelioideae, Pitcairnioideae, and Tillandsioideae (Smith & Downs, 1974, 1977, 1979) and, after recent molecular studies, five additional subfamilies were separated from within the former Pitcairnioideae: Brocchiniodeae, Hechtioideae, Lindmanioideae, Navioideae, and Puyoideae (Givnish et al., 2007, 2011).
Bromeliaceae inhabit diverse ecosystems, ranging from arid environments and seasonally dry habitats to rainy and cloudy forests; from sea level to high mountains and Paramo regions at an elevation of 5,000 m (Smith & Downs, 1974, 1977, 1979). In the majority of genera, epiphytic growth predominates, followed by terrestrial and rocky habitats (Benzing, 2000). Pollination systems of bromeliads are specialized for specific functional groups of pollen vectors, which include hummingbirds, bats, and insects, primarily bees (Benzing, 2000; Kessler & Kromer, 2000; Aguilar-Rodríguez et al., 2019a; Kessler et al., 2020). Matallana and colleagues (2010) proposed that self-compatibility was the prevalent reproductive condition in the family and, with reference to the evolution of selfing, argued that their data did not support the traditional hypothesis of reproductive assurance.
As a first objective of this review, we compiled and summarized the available information on the breeding systems of Bromeliaceae from the last three decades (1990‒2020) in order to determine the current state of knowledge on this topic. We aimed to describe the magnitude and distribution of breeding system components at various taxonomic levels, as well as to identify broad patterns. As a second objective, we investigated the extent of selfing and its relationship to the species’ self-compatibility condition, herkogamy, and dichogamy, as well as the mechanisms of autonomous self-fertilization. This information was used to examine the evidence in support of the principal hypotheses that would explain the maintenance of selfing in Bromeliaceae.
For this purpose, we defined the breeding systems components as follows: (i) the degree of SC, (ii) the autonomous self-pollination capacity or selfing, and (iii) the presence of agamospermy. Agamospermy or apomixis is the asexual production of seeds (Richards, 2003; Bicknell & Koltunow, 2004) and, if undetected, it might be confused with autogamy and interfere with the interpretation of the breeding system (Raduski et al., 2012). We also highlighted potential data limitations and suggested topics for future research.
Literature Search and Selection Criteria
We systematically searched for papers published during the period from January 1990 to May 2020 using the following internet search engines: Scientific Electronic Library Online (SciELO; https://www.scielo.org/es/), Google Scholar (https://scholar.google.com/), and the Web of Science (ISI; https://login.webofknowledge.com/). The following keywords and their combinations were used: “self-incompatibility”, “reproductive system”, “breeding system”, and “reproductive biology”, accompanied by the word “Bromeliaceae”. Articles published in indexed scientific journals and university theses in English, Spanish, and Portuguese were included. References cited in articles were checked for additional studies.
The selection criteria for inclusion of a study required the reporting of results from any of the following treatments: (i) manual self-pollination, (ii) manual cross-pollination, (iii) autonomous self-pollination, and (iv) agamospermy (Kearns & Inouye, 1993). The information from procedures (i) and (ii) is required to estimate the intensity of the self-compatibility system, an indirect measure of the individual selfing or outcrossing potential (Raduski et al., 2012); procedure (iii) measures the capacity for self-fertilization in the absence of pollinators; and procedure (iv) the ability to produce seeds without pollination. Furthermore, we collected information on sample size (plants and flowers), the floral biology of the species regarding the presence and type of dichogamy and herkogamy, and the timing of self-pollination when reported.
Dataset of Breeding Systems Studies
The initial internet search resulted in 179 references, subsequent review led to the addition of 35 references cited in the bibliography of the reviewed articles. After screening, we eliminated not relevant or duplicated studies, resulting in a final selection of 60 references (52 articles published in peer-reviewed journals, 8 master and doctoral theses) (Supplementary Material: Figure S1). When more than one study for a given species was found (22 cases), the information was considered as separate entries because it came from independent investigations. The final database comprises 177 records representing 152 bromeliad species with bisexual flowers (Supplementary Material: Table S1).
Estimation of Breeding System Components
We extracted the results of controlled pollination treatments for each species and calculated the Self-Compatibility Index (SC-index = Pa / Px) proposed by Becerra & Lloyd (1992) which relates the reproductive success of self-pollinated (Pa) and cross-pollinated (Px) flowers. The SC-index estimates the plant’s ability to produce fruits with its pollen and varies from 0 (totally incompatible) to 1 (totally compatible). Although the self-incompatibility index (SI-index) is frequently reported in the literature, both indexes are related as follows: SI-index = 1– SC-index.
We calculated the autofertility index, AF-index = Pe / Px (Lloyd & Schoen, 1992), using the results of the autonomous self-pollination treatments, which describes the relationship between the reproductive success of flowers excluded from pollinator visitation (Pe) and manually cross-pollinated flowers (Px). This index estimates the ability to produce fruits by autonomous means of self-pollination, and it ranges from 0 (totally autogamous or independent of pollinators) to 1 (non-autogamous or dependent on pollinators).
Results from the apomixis treatment were used to calculate the Agamospermy Index, AG-index = Pag / Px (Riveros et al., 1996) that describes the relationship between the reproductive success of emasculated and excluded flowers from visitation by pollinators (Pag) and manually cross-pollinated flowers (Px). This index varies from 0 (not agamospermous) to 1 (completely agamospermous).
In the abovementioned treatments, the reproductive output represents the proportion of fruits produced after flower manipulation. We opted to calculate the reproductive indexes using the proportion of fruits instead of the number of seeds per fruit because seed formation could be affected by inbreeding depression (Charlesworth & Charlesworth, 1987), a phenomenon unrelated to the mechanisms of rejection and recognition of the own pollen in the stigma and style (de Nettancourt, 2001).
Data Limitations
The majority of compiled studies did not include all necessary manipulations and experimental tests to describe the species’ breeding system using the parameters listed here. The joint data from controlled pollination (manual selfing and crossing), tests of self-fertilization ability and apomixis were only included for 38% of the database records. Manual selfing and cross-pollination are performed together the majority of the time (91% of records). 87% of the records contained information on the capacity for autonomous self-pollination, whereas only 40% of the studied species showed apomixis data (71 species).
Any estimate of the parameters used to describe the breeding system of a species should ideally account for variation within the population. Consequently, the biological representativeness of these estimators is contingent on the number of individuals from a given population that are examined. The indexes used in this review are widely employed in the literature on plant reproduction, but they do not account for sampling. In this regard, the compiled studies revealed a wide range of sample sizes for both manipulated and examined flowers and plants. In manual self- and cross-pollination treatments, for example, sample sizes ranged between 3 and 455 flowers and 1 and 50 plants per species. The trend observed was handling a relatively large number of flowers from a few plants, generally 10 or fewer individuals (Supplemental Material: Figure S2). Notably, the number of plants used for controlled pollination was not reported in the respective methodology for 38 of the studied species. All studies were included in the final database, despite differences in the number of manipulated flowers and individuals, because this review provides a comprehensive overview of the available literature.
Temporal, Geographic, and Taxonomic Scope of the Information
One-half (51%) of the cited studies were conducted during the most recent decade, 2011–2020 (Fig. 1A). In general, studies comprised the investigation of a single species (60%), and the work of Matallana et al. (2010) stands out for its contribution to the study of forty Brazilian species. The majority of studies (ca. 50%) were conducted with plants in field conditions and, to a lesser extent (26%), with plants transferred to greenhouses or common gardens, whereas this aspect was unclear in the rest of studies. In his authoritative book on Bromeliaceae, David H. Benzing (2000) noted that research on breeding systems had been largely neglected and the few prior accounts were mostly anecdotal (e.g., McWilliams, 1974); however, there appears to have been a surge in interest in recent decades.
Geographically, the majority of studies and species investigated have been conducted in northeastern Brazil (Atlantic Forest region), southern Mexico, and southern Central America (Fig. 1B). These regions are centers of bromeliad diversity and endemism (Givnish et al., 2014; Zizka et al., 2019). Other regions of the continent deemed significant for their bromeliad diversity have received less attention and represent knowledge gaps on the topic. These regions include the Andes Mountain range, where the Puyoideae subfamily diversified, and the Guiana Shield, where the clades considered ancestral within Bromeliaceae (Brocchinoideae, Lindmanioideae, and Navioideae) apparently evolved (Givnish et al., 2014; Zizka et al., 2019) (Fig. 1B). Similarly, little attention has been paid to the reproductive biology of the bromeliad flora of the Caribbean islands.
The compiled data set accounts for 4.1% (152 species) of the family’s diversity (Table 1). Bromeliaceae, along with Fabaceae and Solanaceae, is among the few angiosperm families for which the study of their reproductive systems has received the most attention (see Raduski et al., 2012), although that percentage may seem low. The majority of research on Bromeliaceae has focused on species from the three traditional subfamilies and, concurrently, the lineages with the greatest species diversity: Bromelioideae, Pitcairnioideae (sensu stricto), and Tillandsioideae. In each subfamily, a preference exists for the most diverse genera. The genera Tillandsia and Vriesea account for 80% of the research in the Tillandsioideae. In the Bromelioideae, the majority of research has been conducted on Aechmea (35%), Billbergia (10%), and Quesnelia (10%), whereas in the Pitcairnioideae, the focus has been on Pitcairnia (45%) and Dyckia (15%).
Bromeliads typically produce bisexual flowers, with only a few species (2.5%) exhibiting dicliny (mainly dioecy). These groups are restricted to the monotypic subfamily Hechtioideae, half of the species in the genus Catopsis in the subfamily Tillandsioideae and two isolated cases in the Bromelioideae (Androlepis skinneri and Aechmea mariae-reginae) (Benzing, 2000). In our search, we found two studies of the breeding system of dioecious species: Hechtia schottii (Hechtioideae) (Ramírez-Morillo et al., 2008) and A. mariae-reginae (Cascante-Marín et al., 2020).
Distribution and Variation in Self-Compatibility
The general distribution of SC-index values in Bromeliaceae exhibits a bimodal pattern (Fig. 2A), similar to that observed in other angiosperm groups (Raduski et al., 2012). The first peak corresponds to highly SI species (SC-index ≤ 0.10), while the second peak represents highly SC species (SC-index = 0.90–1.0). For 28 species, SC-values significantly exceed 1, but their causes or implications are not addressed in the respective studies. Such values occur when the probability of producing fruits by self-pollination is greater than that of outcrossing; however, the situation described does not appear to have any biological basis (Lloyd & Schoen, 1992). This phenomenon is typically interpreted as the result of experimental artifacts or biases; however, unexpectedly high SC-index values have also been interpreted as evidence of outbreeding depression or early speciation (Ramírez & Nassar, 2017).
(A) Histogram showing the distribution of SC-index values in the Bromeliaceae family. (B) Box-plots of the distribution of SC-index values in the most diverse subfamilies of Bromeliaceae. Different letters indicate significant differences after a Kruskal-Wallis Test (Chi-squared value = 20.576, df = 2, p-value < 0.001). The SC-index indicates the probability of producing fruits by manual self-pollination in relation to manual cross-pollination. Data published in the period 1990–2020
The distribution of SC-values varied significantly between subfamilies, and there was also substantial variation among species within each group (Fig. 2B). In Tillandsioideae and Pitcairnioideae (sensu stricto), the majority of SC values were close to or greater than 1, indicating a tendency toward high SC, whereas in Bromelioideae, SC values were closer to zero, indicating lower SC. In Tillandsia (Tillandsioideae), the SC-index values followed a bimodal pattern, whereas in the closely related genus Vriesea, the pattern was unimodal and centered on high SC values. Pitcairnia and Dyckia (Pitcairnioideae) had unimodal but opposite patterns, while Aechmea and Billbergia (Bromelioideae) had unimodal patterns that were skewed toward low SC values (Fig. 3).
SC is a quantitative feature that covers a gradient from full SI to entire SC among individuals and between populations (Good-Avila et al., 2008); nonetheless, it is typically described as a binary variable in the literature. Species are therefore classified as either SI or SC based on arbitrary cut-off values of the SC-index (see Bawa, 1974; Ramirez & Brito, 1990; Wolowski et al., 2013; Souza et al., 2017). Based on the SC-index ≥ 0.30 criterion, Matallana et al. (2010) concluded that the majority of Bromeliaceae species (75%) were self-compatible. Following the same cutoff threshold, our estimation of SC species decreased to 65%. Remember that these estimates include both partial and complete SC species, and they may also reflect some bias due to the fact that the majority of studies focused on four genera that account for nearly two-thirds of the available data: Aechmea (N = 12), Pitcairnia (N = 24), Tillandsia (N = 25), and Vriesea (N = 37). Matallana et al. (2010) noted that SC may appear to be a common reproductive feature among Bromeliaceae but, thus far, it is primarily confined to the Pitcairnioideae and Tillandsioideae. Moreover, within subfamilies, different patterns may exist within genera. This tremendous variety at several taxonomic levels prevents generalizations about the whole family.
Benzing (2000) observed anecdotally considerable variation in selfed seed production between Costa Rican and Mexican populations of Tillandsia caput-medusae (Tillansioideae). This type of variation in SC has been documented in a number of plant taxa (e.g., Ortiz et al., 2006; Theiss et al., 2010; Roda & Hopkins, 2019), but no study has yet attempted to document the variance in self-compatibility systems between populations in Bromeliaceae. In general, the research accumulated on the reproductive systems of bromeliads has concentrated on evaluating particular populations. Nonetheless, we discovered 22 bromeliad species for which data on SC from two or three populations are available. Although derived from different investigations, these data imply that SC in Bromeliaceae may vary between populations.
Aechmea distichanta (Bromelioideae) with high compatibility (SC-index = 0.90) in a mesic Araucaria forest from Brazil (Scrok & Varassin, 2011) and nearly full SI (SC-index = 0.06) in a population from the dry Chaco forest in northern Argentina (Bianchi et al., 2000) are examples of inter-population variation in SC in bromeliads. Two Brazilian populations of Vriesea carinata (Tillandsioideae) revealed differing self-compatibility expressions: self-incompatible in a lowland wet forest (SC-index = 0.0) and extremely self-compatible in a montane humid forest (SC-index = 0.95) (Araujo et al., 1994; Wolowski et al., 2013; respectively). Tillandsia geminiflora (Tillandsioideae) from two Brazilian populations exhibited a similar contrasting expression of SC (SC-index = 1.62; Matallana et al., 2010; SC-index = 0.0; Wolowski et al., 2013).
Variation in SC between populations presumably reflects the effect of local selection forces on the mating system and genetic diversity of the species (Busch, 2005). Benzing (2000) argued that marginal populations of some Tillandsia species may represent founder events of individuals with the capacity for autonomous self-fertilization. The research by Paggi et al. (2022) illustrates this idea, they estimated the mating system of Vriesea gigantea in populations at the center and periphery of the species’ range and discovered greater selfing rates in marginal populations, which they ascribed to insufficient pollination conditions and historical events. The majority of the aforementioned examples of among-population variation in SC in bromeliads may be the result of colonizing events by SC individuals associated with limiting pollination conditions, but additional comparative studies are required to fully comprehend this source of variation in the breeding systems of Bromeliaceae.
Distribution and Mechanisms of Autonomous Selfing
Nearly half of the species (47%) examined for spontaneous selfing in our database had high autofertility values (AF-values ≥ 0.30; N = 64), and the variation range for this reproductive feature was large (Fig. 4A). The degree of autofertility was moderately correlated with the SC strength of the species (Pearson test: r = 0.49, d.f.= 86, p-value < 0.001; Fig. 4B), and this pattern held true for each of the three major subfamilies: Bromelioideae, Pitcairnioideae (sensu stricto), and Tillandsioideae (p-values < 0.01). The considerable diversity in selfing capability in the presence of SC (Fig. 4B) may be attributable to the expression of floral characteristics that affect pollen deposition on the same flower, such as herkogamy and dichogamy (Lloyd & Schoen, 1992).
(A) Histogram showing the distribution of Autofertility values and (B) scatterplot between autofertility and self-compatibility (SC-index) in the three main subfamilies of Bromeliaceae. The shaded area along the regression line indicates the interval of confidence (Pearson correlation test: t = 5.2389, df = 86, r = 0.49, P-value < 0.001). Data from 87 species published in the period 1990–2020
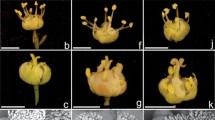
In our database, dichogamy was documented in 31% of the examined species, with protandry (Table 2) being the most prevalent dichogamy type, in which the anthers develop first. Although, protandry might promote selfing, as well as the lack of dichogamy or adichogamy observed in a number of the examined bromeliads, their relation with the species’ autofertily was unclear. Information on the relative position of anthers and stigma was provided for nearly half (44%) of the studied cases (Table 2). There are herkogamous and homostylous (lack of herkogamy) species in the three major subfamilies (Fig. 5); however, when herkogamy was present, it was recorded qualitatively and defined as approach (stigma above the anthers) or reverse (stigma below the anthers) type or present but undescribed.
Some examples of floral herkogamy in Bromeliaceae. Homostylous flowers in (A) Guzmania polycephala, Tillandsioideae; (B) Pitcairnia atrorubens, Pitcairnioideae; (C) Tillandsia fasciculata, Tillandsioideae. Herkogamous flowers in (D) Fosterella micrantha, Pitcairnioideae; (E) Tillandsia ionantha, Tillandsioideae; (F) Werauhia ampla, Tillandsioideae. Photos from the first author, except (D) courtesy of J. F. Morales and (E) modified from Wikipedia Commons (Edu, São Paulo, Brazil; https://commons.wikimedia.org/w/index.php?curid=5594251)
In a number of other flowering plant taxa, the degree of herkogamy has been associated with the potential for autofertility (Opedal, 2018). We studied this connection by defining bromeliad species as herkogamous (approach, reverse, or merely present) or homostylous, and found that species with homostylous flowers were associated with high autofertility (AF-index ≥ 0.30) (Fig. 6). Bromeliads with herkogamous flowers were also less capable of self-fertilization (Chi-squared value = 21.6, df = 3, p-value < 0.001). This functional link between herkogamy and autofertility has the ability to affect the plant mating system, that is, the percentage of self- and cross-pollinated seeds and the genetic diversity of the population (Opedal, 2018).
Given the significant frequency of homostylous species with high autofertiliy (AF-index ≥ 0.30) in Bromeliaceae, one would predict lower rates of outcrossing and genetic variation in these species relative to herkogamous species. To assess this idea, there are little data on the herkogamy and mating systems of bromeliads. Evidence in this regard comes from Costa Rican populations of Guzmania monostachia and Tillandsia fasciculata, two tillandsioid bromeliads pollinated by hummingbirds, with homostylous flowers and high autofertility (> 90% fruit set in pollinator-exclusion treatments) that resulted in very low values of outcrossing rates (tm) estimated with microsatellites and indistinguishable from predominant selfing (Cascante-Marín et al., 2006). Using isozymes markers, Soltis et al. (1987) studied the population genetics of two tillandsioid species with contrasting floral traits. For the herkogamous Tillandsia ionantha, they found high genetic variation and a low fixation index, indicative of an outcrossing species, whereas for the homostylous T. recurvata, they found low genetic variation and a high fixation index, indicative of a highly selfing species. More evidence is required to substantiate the generality of this link; if present, the variance in herkogamy in Bromeliaceae might serve as a proxy for their mating systems and genetic diversity (Opedal, 2018).
An extreme form of selfing is cleistogamy, which occurs when flowers do not open and self-fertilization occurs (Lord, 1981; Culley & Klooster, 2007). This phenomenon appears to be uncommon in Bromeliaceae but has been documented mostly in species of Tillandsia (subgenus Diaphoranthema) that produce flowers with reproductive organs inserted within closed corollas and covered by the calyx (Gilmartin & Brown, 1985; Bianchi & Vesprini, 2014). All plants from the studied populations of T. capillaris, T. recurvata, and T. tricholepis from northwestern Argentina were cleistogamous, the reduced size of their flowers did not allow any manipulation; nonetheless, these species had a high level of autonomous self-pollination (Bianchi & Vesprini, 2014). The population of T. capillaris investigated by Gilmartin & Brown (1985) in La Paz, Bolivia, had both cleistogamous and chasmogamous flowers, indicating that its expression is unstable.
The Adaptive Value of Selfing in Bromeliaceae
The evolutionary significance of selfing and mixed mating systems in Bromeliaceae is an understudied issue. In the general literature on plant reproduction, selfing is regarded as: (1) a reproductive assurance mechanism when pollination conditions are inadequate (Jain, 1976; Goodwillie et al., 2005); and (2) a reproductive isolation mechanism to maintain the genetic integrity of the species and prevent fitness costs associated with hybrid progeny (Levin, 1971; Jain, 1976). In an ecological context of sympatry, Wendt et al. (2002) and Matallana et al. (2010) proposed that selfing in bromeliads is a reproductive isolation strategy that decreases the transfer of heterospecific pollen and the undesirable results of hybridization. If selfing functions as a preventative strategy to safeguard against fitness losses owing to hybridization with congeners, it should occur before the heterospecific pollen transfer opportunities, as in “prior selfing”, as recently postulated by Brys et al. (2016) in their “preemptive selfing hypothesis.“ Nonetheless, Wendt et al. (2002) found that self-pollination occurred during the time of flower withering (“delayed selfing”) in their investigated Pitcairnias and the study of Matallana et al. (2010) did not provide information on the timing of selfing.
Selfing is one of several reproductive isolation strategies in flowering plants, and it should be evaluated in conjunction with other reproductive barriers occurring before or after pollination (Coyne & Orr, 1989; Ramsey et al., 2003; Lowry et al., 2008). These barriers include phenology and floral morphology (prezygotic barriers) and interspecific incompatibility or incongruity (poszygotic barriers) (Levin, 1971; Grant, 1994; Widmer et al., 2009; Sobel & Chen, 2014). No study to date has attempted to evaluate these reproductive barriers in bromeliads. The degree and relative impact of these barriers to total reproductive isolation, including selfing, may be estimated and compared using standardized indices that take into account the reduction in gene flow experienced by taxa (sensu Sobel & Chen, 2014; Lowry et al., 2008).
On the other hand, a number of studies have suggested that selfing functions as a reproductive assurance mechanism in bromeliads (Bush & Guilbeau, 2009; Scrok & Varassin; 2011; Ríos & Cascante-Marín, 2017; Gomes et al., 2020), but without formal evaluation. Goodwillie and Weber (2018) have shown that delayed selfing promotes reproductive assurance more effectively than “prior” or “competing” selfing, because it ocurrs at the end of the flower´s life, when the opportunity for cross-pollination has happened. Most bromeliads exhibit pollination systems that promote outcrossing, although plants with such specialized pollination systems frequently exhibit mechanisms of delayed selfing (Fenster & Martén-Rodríguez, 2007). In spite of a large number of Bromeliaceae species capable of self-fertilization, recording the precise time of selfing was uncommon (only in ten species). Those reported as exhibiting delayed selfing included two pitcairnioid species, W. flammea and P. corcovadensis (Wendt et al., 2002). In the tillandsioid group, delayed selfing was reported for W. gladioliflora (Cascante-Marín et al., 2005), Werauhia noctiflorens and W. nutans (Aguilar-Rodríguez et al. 2019b), Tillandsia schiedeana (Orozco-Ibarrola et al., 2015), and Vriesea gigantea (Paggi et al., 2015), but the true role of this selfing mechanism as a reproductive safeguard was untested. Its confirmation would require estimating the contribution of selfing to reproductive success by comparing intact and emasculated flowers, both under natural pollination conditions, and assessing the viability of selfed progeny to identify any potential fitness cost due to inbreeding depression (Schoen & Lloyd, 1992; Eckert et al., 2006).
Self-Incompatibility Type
Brewbaker & Gorrez (1967) performed controlled crosses and determined the segregation pattern of the incompatibility system in two sibling families of Ananas comosus (Bromelioideae). These authors proposed the existence of a homomorphic gametophytic self-incompatibility system (GSI), controlled by a single multiallelic S-locus. GSI involves interbreeding plants with the same floral morphology (homomorphic), in which the SI phenotype of the pollen is determined by its haploid genotype (gametophytic) (Newbigin et al., 1993). Subsequent studies that have observed pollen tube growth in controlled self-pollinations in Aechmea (Bromelioideae) and Tillandsia (Tillandsioideae) species (Vervaeke et al., 2001; Bianchi & Vesprini, 2014; Souza et al., 2017) supported GSI due to pollen tubes ceasing to develop in the style. Most of the incompatibility systems in monocots that have been genetically characterized exhibit gametophytic control (Allen & Hiscock, 2008), and although evidence in Bromeliaceae points to GSI, the physiological and molecular mechanism that regulates the reactions of incompatibility has not been described yet.
The highest frequency of species considered SI (SC-index ≤ 0.10) have been identified in the Bromelioideae subfamily and, thus far, this condition is present in half of the genera studied in this group: Aechmea, Ananas, Araeococus, Billbergia, Bromelia, Neoregelia, and Quesnelia. In Pitcairnioideae (sensu stricto), species of genus Dyckia are reported as SI: D. tuberosa (Vosgueritchian & Buzato, 2006) and D. distachya (Wiesbauer, 2008). In Tillandsioideae most SI species have been documented in genus Tillandsia, subgenera Tillandsia and Anoplophytum (sensu Smith & Downs, 1977). A single SI species is known from Vriesea (V. carinata: Araujo et al., 1994; Souza et al., 2017).
Apomixis
The agamospermy index varied from low (0.06) to high values (1.06). Apomixis in Bromeliaceae appears to have evolved in the main subfamilies and has been detected in the most studied genera (except Billbergia, Quesnelia, and Werauhia) and, in addition to Hohenbergia, Lymania, and Wittmackia (Bromelioideae), Encholirium (Pitcairnioideae sensu stricto) and Brocchinia (Brocchinioideae). The incidence of agamospermy was greater in Pitcairnioideae subfamily (sensu stricto) (80% of the investigated species). Notable is that several species documented as highly apomictic also exhibited high SC values: Aechmea bracteata (Pitcairnioideae, AG-index = 0.94) (Pool-Chalé et al., 2018), Encholirium horridum (Pitcairnioideae, AG-index = 1.00) (Hmeljevski et al., 2017), Lymania smithii (Bromelioideae; AG-index = 1.16) (Siqueira Filho, 2003), and Tillandsia heterophylla (Tillandsioideae, AG-index = 1.06) (Aguilar-Rodríguez et al., 2015). However, apomixis was not taken into account while calculating the respective SC-indices.
In the putatively apomictic Bromeliaceae, the particular processes of apomixis, i.e. aplospory, diplospory, and adventitious embryony (sensu Bicknell and Koltunow, 2004), have not yet been documented. In species where apomixis is substantial, confirmation would be appropriate using a convenient method such as calculating the DNA content in embryo and seed endosperm using flow cytometry techniques (Matzk et al., 2000). Low values of the AG-index may be attributable to experimental artifacts during emasculation caused by unintentional contamination of the stigma when it is not removed or when anther dehiscence occurs before anthesis and pollen is deposited on the stigma.
Conclusions and Recommendations
Compared to numerous other angiosperm families, Bromeliaceae reproductive systems have been relatively well studied, although the number of documented species still represents just a small portion of the family’s overall diversity (152 out of ca. 3650 species). There is a previous suggestion that SC is widespread in Bromeliaceae (Matallana et al., 2010). However, this reproductive trait is only representative of the subfamilies Pitcairnioideae and Tillandsioideae, and the degree of SC varies widely among species in these groups. At this taxonomic level, there are certain biases in the number of represented genera, as well as opposing patterns in SC distribution. When data from the remaining five subfamilies becomes available, it will be possible to make a stronger generalization about the breeding systems of the entire family. The addition of early diverging lineages (i.e., Brocchinioideae, Lindmanioideae, and Navioideae) and poorly represented taxa in the best-studied subfamilies would enrich the overall picture.
The high selfing ability of several examined bromeliads, mostly tillandsioid and pitcairnioid species, is most likely the combined result of an SC system and reduced herkogamy or homostyly, floral traits linked with low outcrossing rates. Correlative analyses of mating systems and herkogamy are required to confirm this functional relationship in Bromeliaceae, in addition to determining the predicted consequences for population genetics. Insufficient empirical evidence exists to explain the evolutionary benefit of the high frequency of selfing in Bromeliaceae. We propose that future reproductive biology research considers evaluating the main hypotheses in this regard.
The inconsistencies detected in the sampling designs of the compiled studies may weaken the biological representativeness of any parameter describing a species’ breeding system. We strongly recommend taking the potential intra-population variation into consideration when determining the sample size. Even when the available indices to define breeding systems do not account for individual variation, the addition of a representative sample size from the population under study will result in a more accurate biological assessment. When applicable, gathering extensive information on the floral biology (dichogamy, herkogamy, and temporal mode of self-fertilization) of the researched species would increase our understanding of the diversity and function of selfing mechanisms in Bromeliaceae.
Data Availability
The primary data on breeding systems parameters collected and analyzed during this study are available as supplementary information files.
References
Aguilar-Rodríguez, P. A., T. Krömer, J. G. García-Franco & M. C. MacSwiney G. 2015. From dusk till dawn: Nocturnal and diurnal pollination in the epiphyte Tillandsia heterophylla (Bromeliaceae). Plant Biology 18: 37–45. https://doi.org/10.1111/plb.12319
Aguilar-Rodríguez, P. A., M. Tschapka, J. G. García-Franco, T. Krömer & M. C. MacSwiney. 2019b. Bromeliads going batty: pollinator partitioning among sympatric chiropterophilous Bromeliaceae. AoB PLANTS 11: 1–19. https://doi.org/10.1093/aobpla/plz014
Aguilar-Rodríguez, P. A., T. Krömer, M. Tschapka, J. G. García-Franco, J. Escobedo-Sarti & M. C. MacSwiney G. 2019a. Bat pollination in Bromeliaceae. Plant Ecology and Diversity 12: 1–19. https://doi.org/10.1080/17550874.2019.1566409
Allen, A. & S. Hiscock. 2008. Evolution and phylogeny of self-incompatibility systems in angiosperms. Pp. 73–102. In: V. Frankling-Tong (ed.), Self-incompatibility in plants. Springer-Verlag, Berlin.
Araujo, A. C., E. A. Fischer & M. Sazima. 1994. Floração sequencial e polinização de três espécies de Vriesea (Bromeliaceae) na região de Juréia, sudeste do Brasil. Revista Brasileira de Botânica 17: 113–118.
Bawa, K. S. 1974. Breeding systems of tree species of a lowland tropical community. Evolution 28: 85–92. https://doi.org/10.1111/j.1558-5646.1974.tb00729.x
Becerra, J. X. & D. G. Lloyd. 1992. Competition-dependent abscission of self-pollinated flowers of Phormium tenax (Agavaceae): a second action of self-incompatibility at the whole flower level? Evolution 46: 458–469. https://doi.org/10.1111/j.1558-5646.1992.tb02051.x
Bennett, B. 2000. Ethnobotany of Bromeliaceae. Pp. 587–608. In: D. H. Benzing (ed.), Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge.
Benzing, D. H. 2000. Bromeliaceae: Profile of an adaptive radiation. Cambridge University Press, Cambridge.
Bertin, R. I. & C. M. Newman. 1993. Dichogamy in angiosperms. The Botanical Review 59: 112–152. https://doi.org/10.1007/BF02856676
Bianchi, M. B. & J. L. Vesprini. 2014. Contrasting breeding systems in six species of Tillandsia L. (Bromeliaceae) from woody areas of Santa Fe Province: Argentina. Plant Biosystems 148: 956–964. https://doi.org/10.1080/11263504.2013.806965
Bianchi, M. V., P. E. Gibbs, D. E. Prado & J. L. Vesprini. 2000. Studies on the breeding systems of understorey species of a Chaco woodland in NE Argentina. Flora 195: 339–348.
Bicknell, R. A. & A. M. Koltunow. 2004. Understanding Apomixis: recent advances and remaining conundrums. The Plant Cell 16: 228–246. https://doi.org/10.1105/tpc.017921.Apomixis
Brewbaker, J. L. & D. D. Gorrez. 1967. Genetics of self-incompatibility in the monocot genera Ananas (Pineapple) and Gasteria. American Journal of Botany 54: 611–616. https://doi.org/10.1002/j.1537-2197.1967.tb10684.x
Brys, R., J. van Cauwenberghe & H. Jacquemyn. 2016. The importance of autonomous selfing in preventing hybridization in three closely related plant species. Journal of Ecology 104: 601–610. https://doi.org/10.1111/1365-2745.12524
Busch, J. W. 2005. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae). American Journal of Botany 92: 1503–1512. https://doi.org/10.3732/ajb.92.9.1503
Bush, S. P. & J. E. Guilbeau. 2009. Early autonomous selfing in the hummingbird-pollinated epiphyte Pitcairnia brittoniana (Bromeliaceae). Journal of the Torrey Botanical Society 136: 313–321. https://doi.org/10.3159/08-RA-119.1
Cascante-Marín, A., C. Trejos, R. Madrigal & E. J. Fuchs. 2020. Genetic diversity and reproductive biology of the dioecious and epiphytic bromeliad Aechmea mariae-reginae (Bromeliaceae) in Costa Rica: implications for its conservation. Botanical Journal of the Linnean Society 192: 773–786. https://doi.org/10.1093/botlinnean/boz083
Cascante-Marín, A., J. G. B.Oostermeijer, J. H. D. Wolf & J. C. M. den Nijs. 2005. Reproductive biology of the epiphytic bromeliad Werauhia gladioliflora in a premontane tropical forest. Plant Biology 7: 203–209. https://doi.org/10.1055/s-2005-837584
Cascante-Marín, A., M. de Jong, E. D. Borg, J. G. Oostermeijer, J. H. D. Wolf & J. C. M. den Nijs. 2006. Reproductive strategies and colonizing ability of two sympatric epiphytic bromeliads in a tropical premontane area. International Journal of Plant Sciences 167:1187–1195. https://doi.org/10.1086/507871
Charlesworth, D. 2006. Evolution of plant breeding systems. Current Biology 16: 726–735. https://doi.org/10.1016/j.cub.2006.07.068
Charlesworth, D. & B. Charlesworth. 1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. https://doi.org/10.1146/annurev.es.18.110187.001321
Coyne, J. A. & H. A. Orr. 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381. doi:https://doi.org/10.2307/2409213
Culley, T. M. & M. R. Klooster. 2007. The cleistogamous breeding system: a review of its frequency, evolution, and ecology in Angiosperms. The Botanical Review 73: 1–30. http://www.bioone.org/doi/abs/https://doi.org/10.1663/0006-8101(2007)73[1:TCBSAR]2.0.CO;2
Darwin, C. 1876. The effects of cross and self fertilisation in the Vegetable Kingdom. Murray, London.
de Nettancourt, D. 2001. Incompatibility and incongruity in wild and cultivated plants (2nd. Edition). Springer-Verlag, Berlin.
Eckert, C. G., K. E. Samis & S. Dart. 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. Pp. 183–203. In: L. D. Harder & S. C. H. Barrett (eds.), Ecology and Evolution of Flowers. Oxford University Press.
Fenster, C. B. & S. Martén-Rodríguez. 2007. Reproductive assurance and the evolution of pollination specialization. International Journal of Plant Sciences 168: 215–228. https://doi.org/10.1086/509647
Ferrer, M. M. & S. V. Good. 2012. Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates. Annals of Botany 110: 535–553. https://doi.org/10.1093/aob/mcs124
Gilmartin, A. J., & G. K. Brown. 1985. Cleistogamy in Tillandsia capillaris (Bromeliaceae). Biotropica, 17: 256–259. https://doi.org/10.2307/2388227
Givnish, T. J., M. H. J. Barfuss, B. van Ee, R. Riina, K. Schulte, R. Horres, P. A. Gonsiska, R. S. Jabaily, D. M. Crayn, J. A. C. Smith, K. Winter, G. K. Brown, T. M. Evans, B. K. Holst, H. Luther, W. Till, G. Zizka, P. E. Berry & K. J. Sytsma. 2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: Insights from an eight-locus plastid phylogeny. American Journal of Botany 98: 872–895. https://doi.org/10.3732/ajb.1000059
Givnish, T. J., M. H. J. Barfuss, B. van Ee, R. Riina, K. Schulte, R. Horres, P. A. Gonsiska, R. S. Jabaily, D. M. Crayn, J. A. C. Smith, K. Winter, G. K. Brown, T. M. Evans, B. K. Holst, H. Luther, W. Till, G. Zizka, P. E. Berry & K. J. Sytsma. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. https://doi.org/10.1016/j.ympev.2013.10.010
Givnish, T. J., Millam, K. C., Berry, P. E., & K. J. Sytsma. 2007. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae Inferred from ndhF sequence data. Aliso, 23: 3–26
Goldberg, E. E. & B. Igić. 2012. Tempo and mode in plant breeding system evolution. Evolution 66: 3701–3709. https://doi.org/10.1111/j.1558-5646.2012.01730.x
Gomes, A. C., B. H. S. Ferreira, C. S. Souza, L. M. M. Arakaki, C. Aoki, G. M. Paggi, & M. R. Sigrist. 2020. Adaptive response of extreme epiphyte Tillandsia species (Bromeliaceae) is demonstrated by different sexual reproduction strategies in the Brazilian Chaco. Botanical Journal of the Linnean Society 192: 840–854. https://doi.org/10.1093/botlinnean/boz104
Good-Avila, S. V., J. I. Mena-Alí & A.G. Stephenson. 2008. Genetic and environmental causes and evolutionary consequences of variations in self-fertility in self incompatible species. Pp. 33–51. In: V. Frankling-Tong (ed.), Self-incompatibility in plants. Springer-Verlag, Berlin.
Goodwillie, C. & J. J. Weber. 2018. The best of both worlds? A review of delayed selfing in flowering plants. American Journal of Botany 105: 641–655. https://doi.org/10.1002/ajb2.1045
Goodwillie, C., S. Kalisz & C. G. Eckert. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36: 47–79. https://doi.org/10.1146/annurev.ecolsys.36.091704.175539
Gouda, E. J. & D. Butcher, D. (2016, October). A list of accepted Bromeliaceae names (cont. updated). University Botanic Gardens, Utrecht. Retrieved November 16, 2020, from http://bromeliad.nl/bromNames/
Grant, V. 1994. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences USA 91: 3–10. https://doi.org/10.1073/pnas.91.1.3
Hmeljevski, K. V., M. Wolowski, R. C. Forzza & L. Freitas. 2017. High outcrossing rates and short-distance pollination in a species restricted to granitic inselbergs. Australian Journal of Botany 65: 315–326. https://doi.org/10.1071/BT16232
Igić, B., R. Lande & J. R. Kohn. 2008. Loss of self-incompatibility and its evolutionary consequences. International Journal of Plant Sciences 169: 93–104. https://doi.org/10.1086/523362
Jain, S. K. 1976. The evolution of inbreeding in plants. Annual Review of Ecology and Systematics 7: 469–495. https://doi.org/10.1146/annurev.es.07.110176.002345
Kalisz, S. & D. W. Vogler. 2003. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84: 2928–2942. https://doi.org/10.1890/02-0519
Karron, J. D., C. T. Ivey, R. J. Mitchell, M. R. Whitehead, R. Peakall, R. & A. L. Case. 2012. New perspectives on the evolution of plant mating systems. Annals of Botany 109: 493–503. https://doi.org/10.1093/aob/mcr319
Kearns, C. A. & D. W. Inouye. 1993. Techniques for pollination biologists. University Press, Colorado.
Kessler, M. & T. Kromer. 2000. Patterns and ecological correlates of pollination modes among bromeliad communities of Andean forests in Bolivia. Plant Biology 2: 659–669. https://doi.org/10.1055/s-2000-16642
Kessler, M., S. Abrahamczyk & T. Kromer. 2020. The role of hummingbirds in the evolution and diversification of Bromeliaceae: unsupported claims and untested hypotheses. Botanical Journal of the Linnean Society 192: 592–608. https://doi.org/10.1093/botlinnean/boz100
Levin, D. A. 1971. The origin of reproductive isolating mechanisms in flowering plants. Taxon 20: 91–113. https://doi.org/10.2307/1218538
Lloyd, D. G. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370–380. https://doi.org/10.1086/297041
Lloyd, D. G. & D. J. Schoen. 1992. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences 153: 358–369. https://doi.org/10.1086/297040
Lord, E. 1981. Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. The Botanical Review 47: 421–449. https://doi.org/10.1007/BF02860538
Lowry, D. B., J. L. Modliszewski, K. M. Wright, C. A. Wu & J. H. Willis. 2008. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philosophical Transactions of the Royal Society B 363: 3009–3021. https://doi.org/10.1098/rstb.2008.0064
Matallana, G., M. A. S. Godinho, F. A. G. Guilherme, M. Belisario, T. S. Coser & T. Wendt. 2010. Breeding systems of Bromeliaceae species: evolution of selfing in the context of sympatric occurrence. Plant Systematics and Evolution 289: 57–65. https://doi.org/10.1007/s00606-010-0332-z
Matzk, F., A. Meister & I. Schubert. 2000. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal 21: 97–108.
McWilliams, E. L. 1974. Evolutionary ecology. Pp. 40–58. In: L. B. Smith & R. J. Downs. Pitcairnioideae (Bromeliaceae). Flora Neotropica 14: 661–1401.
Muller, H. 1883. The fertilisation of flowers. Macmillan, London.
Neal, P. R. & G. J. Anderson. 2005. Are “mating systems” “breeding systems” of inconsistent and confusing terminology in plant reproductive biology? Or is it the other way around? Plant Systematics and Evolution 250: 173–185. https://doi.org/10.1007/s00606-004-0229-9
Negrelle, R., D. Mitchell & A. Anacleto. 2012. Bromeliad ornamental species: conservation issues and challenges related to commercialization. Acta Scientiarum - Biological Sciences 34: 91–100. https://doi.org/10.4025/ACTASCIBIOLSCI.V34I1.7314
Newbigin, E., M. A. Anderson & A. E. Clarke. 1993. Gametophytic self-incompatibility systems. The Plant Cell 5: 1315–1324, https://doi.org/10.1105/tpc.5.10.1315
Opedal, Ø. H. 2018. Herkogamy, a principal functional trait of plant reproductive biology. International Journal of Plant Sciences 179: 677–687.
Orozco-Ibarrola, O. A., P. S. Flores-Hernández, E. Victoriano-Romero, A. M. Corona-López & A. Flores-Palacios. 2015. Are breeding system and florivory associated with the abundance of Tillandsia species (Bromeliaceae)? Botanical Journal of the Linnean Society 177: 50–65. https://doi.org/10.1111/boj.12225
Ortiz, M. A., S. Talavera, J. L. García-Castaño, K. Tremetsberger, T. Stuessy, F. Balao & R. Casimiro-Soriguer. 2006. Self-incompatibility and floral parameters in Hypochaeris sect. Hypochaeris (Asteraceae). American Journal of Botany 93: 234–244.
Paggi, G. M., C. Palma-Silva, M. H. Bodanese-Zanettini, C. Lexer & F. Bered. 2015. Limited pollen flow and high selfing rates toward geographic range limit in an Atlantic forest bromeliad. Flora 211: 1–10. https://doi.org/10.1016/j.flora.2015.01.001
Paggi, G. M, C. Palma-Silva, C. M. Zanella, M. Goetze, M. V. Büttow, C. Lexer & F. Bered. 2022. Spatiotemporal variation on fertility, mating system, and gene flow in Vriesea gigantea (Bromeliaceae), an Atlantic Forest species. Frontiers in Forest Global Change 5: 893548. doi: https://doi.org/10.3389/ffgc.2022.893548
Pool-Chalé, M., I. Ramírez-Morillo, G. Carnevali Fernández-Concha & C. T. Hornung-Leoni. 2018. Reproductive biology of Aechmea bracteata (Sw.) Griseb. (Bromelioideae: Bromeliaceae). Plant Biology 20: 113–120. https://doi.org/10.1111/plb.12645
Raduski, A. R., E. B. Haney & B. Igić. 2012. The expression of self-incompatibility in angiosperms is bimodal. Evolution 66: 1275–1283. https://doi.org/10.1111/j.1558-5646.2011.01505.x
Ramirez, N. & Y. Brito. 1990. Reproductive biology of a tropical palm swamp community in the Venezuelan Llanos. American Journal of Botany 77: 1260–1271. https://doi.org/10.2307/2444587
Ramírez, N. & J. M. Nassar. 2017. Breeding systems in Angiosperms: novel inferences from a new analytical approach. Plant Systematics and Evolution 303: 19–137. https://doi.org/10.1007/s00606-016-1357-8
Ramírez-Morillo, I. M., F. Chi May, G. Carnevali Fernández-Concha & F. May Pat. 2008. Reproductive biology of Hechtia schottii, a dioecious Bromeliaceae, in Mexico. Revista de Biología Tropical 56: 279–289. https://doi.org/10.15517/rbt.v56i1.5524
Ramsey, J., H. Bradshaw & D. Schemske. 2003. Components of reproductive isolation between the monkey flowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57: 1520–1534. https://doi.org/10.1554/01-352
Richards, A. J. 2003. Apomixis in flowering plants: an overview. Philosophical Transactions of the Royal Society B 29: 1085–1093. https://doi.org/10.1098/rstb.2003.1294
Ríos, L. D. & A. Cascante-Marín. 2017. High selfing capability and low pollinator visitation in the hummingbird-pollinated epiphyte Pitcairnia heterophylla (Bromeliaceae) at a Costa Rican mountain forest. Revista de Biología Tropical 65: 735–743. https://doi.org/10.15517/rbt.v65i2.25948
Riveros M., A. M. Humaña & M. K. Arroyo. 1996. Sistemas de reproducción en especies del bosque Valdiviano (40 Latitud Sur). Phyton (Buenos Aires) 58: 167–176.
Roda, F., & R. Hopkins. 2019. Correlated evolution of self and interspecific incompatibility across the range of a Texas wildflower. New Phytologist 221: 553–564. https://doi.org/10.1111/nph.15340
Schoen, D. J. & D. G. Lloyd. 1992. Self- and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. International Journal of Plant Sciences 153: 381–393. https://doi.org/10.1086/297042
Scrok, G. J. & I. G. Varassin. 2011. Reproductive biology and pollination of Aechmea distichantha Lem. (Bromeliaceae). Acta Botanica Brasilica 25: 571–576. https://doi.org/10.1590/s0102-33062011000300009
Siqueira Filho, J. A. 2003. Fenologia da floração, ecologia da polinização e conservação de Bromeliaceae na Floresta Atlântica Nordestina. Doctoral thesis. Universidade Federal de Pernambuco, Brazil.
Smith, L. B. & R. J. Downs. 1974. Pitcairnioideae. Flora Neotropica 14: 663–1401.
Smith, L. B. & R. J. Downs. 1977. Tillandsioideae. Flora Neotropica 14: 1–609.
Smith, L. B. & R. J. Downs. 1979. Bromelioideae. Flora Neotropica 14: 1493–2142.
Sobel, J. M. & G. F. Chen. 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68: 1511–1522. https://doi.org/10.1111/evo.12362
Soltis, D. E., A. J. Gilmartin, L. Rieseberg & S. Gardner. 1987. Genetic variation in the epiphytes Tillandsia ionantha and T. recurvata (Bromeliaceae). American Journal of Botany 74: 531–537. https://doi.org/10.1002/j.1537-2197.1987.tb08673.x
Souza, E. H., L. M. Versieux, F. V. D. Souza, M. L. Rossi, M. A. P. Costa & A. P. Martinelli. 2017. Interspecific and intergeneric hybridization in Bromeliaceae and their relationships to breeding systems. Scientia Horticulturae 223: 53–61. https://doi.org/10.1016/j.scienta.2017.04.027
Stebbins G. L. 1974. Flowering Plants: evolution above the species level. Belknap, Cambridge.
Theiss, K. E., K. E. Holsinger & M. E. K. Evans. 2010. Breeding system variation in 10 evening primroses (Oenothera sections Anogra and Kleinia; Onagraceae). American Journal of Botany 97: 1031–1039. https://doi.org/10.3732/ajb.0900260
Vervaeke, I., E. Parton, L. Maene, R. Deroose & M. P. De Proft. 2001. Prefertilization barriers between different Bromeliaceae. Euphytica 118: 91–97. https://doi.org/10.1023/A:1004016709231
Vosgueritchian, S. B. & S. Buzato. 2006. Reprodução sexuada de Dyckia tuberosa (Vell.) Beer (Bromeliaceae, Pitcairnioideae) e interação planta-animal. Revista Brasileira de Botanica 29: 433–442. https://doi.org/10.1590/S0100-84042006000300010
Webb, C. J. & D. G. Lloyd. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. herkogamy. New Zealand Journal of Botany 24: 163–178. https://doi.org/10.1080/0028825X.1986.10409726
Wendt, T., M. B. F. Canela, D. E. Klein & R. I. Rios. 2002. Selfing facilitates reproductive isolation among three sympatric species of Pitcairnia (Bromeliaceae). Plant Systematics and Evolution 232: 201–212. https://doi.org/10.1007/s006060200043
Widmer, A., C. Lexer & S. Cozzolino. 2009. Evolution of reproductive isolation in plants. Heredity 102: 31–38. https://doi.org/10.1038/hdy.2008.69
Wiesbauer, M. B. 2008. Biologia reprodutiva e diversidade genética de Dyckia distachya Hassler (Bromeliaceae) como subsídio para conservação e reintrodução de populações extintas na natureza. Master thesis. Universidade Federal de Santa Catarina, Brazil.
Wolowski, M., C. F. Saad, T. L. Ashman & L. Freitas. 2013. Predominance of self-compatibility in hummingbird-pollinated plants in the Neotropics. Naturwissenschaften 100: 69–79. https://doi.org/10.1007/s00114-012-0995-0
Zizka, A., J. Azevedo, E. Leme, B. Neves, A. F. da Costa, D. Caceres & G. Zizka. 2019. Biogeography and conservation status of the pineapple family (Bromeliaceae). Diversity and Distributions 26: 183–195. https://doi.org/10.1111/ddi.13004
Acknowledgements
This research article is part of the master’s thesis of the junior author. To all researchers who have contributed to the knowledge of Bromeliaceae breeding systems. To Ivón Ramírez-Morillo for her comments on an early version of this manuscript. Two anonymous reviewers contributed to improving the presented ideas with their valuable comments and recomendations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statements and Declarations
The first author received support from the Vicerrectoría de Investigación at the Universidad de Costa Rica (Project C0-060).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cascante-Marín, A., Núñez-Hidalgo, S. A Review of Breeding Systems in the Pineapple Family (Bromeliaceae, Poales). Bot. Rev. 89, 308–329 (2023). https://doi.org/10.1007/s12229-023-09290-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-023-09290-0