Abstract
The ability of seeds to regenerate from soil seed banks has long been recognized as a key survival strategy for plants establishing new niches in highly variable climates of alpine environments. However, the fundamental aspects of evolutionary/selective forces for seed bank development in alpine ecosystems are largely unknown. Here, we developed a model that describes dormancy, a high temperature requirement and a specific light/darkness regime at the time of seed shedding can preclude autumn germination, thus contributing to seed persistence until the next growing season. The benefits of these factors synchronising germination with the growing season are reviewed. Additionally, the importance of climatic variations of maternal environment affecting some of these factors is also discussed. It is suggested that the environmental conditions during the growing season partly control the seed persistence and seeds that fail to germinate are carried over to the next season. Species that have small (<3 mg) and round-shaped seeds tend to persist more easily in soil for over 5 years, than do the large or flat seeds. However, some large-seeded species also have the potential to establish short-term persistence bank. A literature survey reveals 88 % of the alpine seeds have a mass <3 mg. Seed size has only a weak relationship with mean germination timing (MGT) indicating that reduced persistence in large-seeded species cannot be counteracted by quicker germination, but combined effects of other factors stimulating germination remain an open area to be studied. It is proposed that long distance dispersal (LDD) is limited in most-but not all-species, primarily due to the absence of specialized dispersal structures. However, among numerous dispersal modes, most species tend to be dispersed by wind. Thus, spermatophytes of alpine environments have a greater tendency to establish seed banks and spread the risk of germination to many years, rather than being dispersed to other micro-climates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ecological significance of seeds in establishing plant communities has been extensively documented for an appreciable number of alpine species (Bliss, 1958; Baskin & Baskin, 1998; Molau & Larsson, 2000; Welling & Laine, 2000; Welling et al., 2004; Bu et al., 2009; Ma et al., 2011). Unlike other ecosystems, alpine seed germination is principally determined by the short and erratic growing season (Bell & Bliss, 1980). Consequently, seeds do not often have a favorable germination environment and would be expected to enter the soil seed bank to postpone the germination to a later year when more favorable conditions occur (reviewed by Arroyo et al., 1999; Cavieres & Arroyo, 2001; Welling et al., 2004). However, there is some evidence to show that the unfavorable conditions preventing seed germination may sometimes persist for two consecutive years (Körner, 2003). Therefore, it is not surprising to discover that evolutionary and ecological processes have resulted in most, if not all, species adapted to alpine regions having seeds with a longevity of at least 2 years.
The ability of alpine seeds to form long-term seed banks is considered as an important ecological trait for population dynamics and species establishment (McGraw & Vavrek, 1989; Körner, 2003). One of the important means by which seeds persist in the soil is by preventing germination immediately after dehiscence. Early efforts to understand alpine soil seed bank dynamics have mostly focused on the seed germination timing per se (see Baskin & Baskin, 1998), thus the mechanisms controlling germination have not been treated adequately. Even less attention has been paid to recognize the factors influencing seed persistence in alpine environments. This dearth of knowledge has become an acute problem, especially given that the effects of global warming conspicuously affect the alpine ecosystem as a whole rather than impacting upon specific species (Walther et al., 2002; Gottfried et al., 2012). Such predictions explicitly require a profound understanding of seed adaptation and it is imperative to model the imminent alpine flora community changes that may result from climate shifts.
The important goal of this review is to explore some of the mechanisms evolved in alpine species that determine how seeds enter and leave the soil. Our model depicts dormancy, a requirement for relatively high temperatures and specific light/darkness regimes are the key factors affecting seed persistence in alpine environment. Moreover, when considering seed persistence in an ecological context, it is imperative to place special emphasis on the comparative biology of alpine plants including the phenology of seed size, germination traits and dispersal affecting the seed turnover and contributing to persistence. We do not mean to imply that only these factors are the pertinent adaptive traits for seed persistence in alpine species; however, our view is that many unknowns impede our understanding and this oversight can be only resolved by paying more attention to these areas. We aim to identify these critical gaps and suggest possible future research directions, rather than summarizing the overwhelming information available on soil seed banks per se, a topic covered in myriad literature (e.g., McGraw & Vavrek, 1989; Körner, 2003; Baskin & Baskin, 1998). To this end, the focus of discussion is limited only to seed bank establishment and persistence. Thus, we do not address topics such as seed density in relation to seed numbers, and species competition during emergence etc. Nevertheless, understanding these subjects is vital, yet out knowledge is rudimentary, but somewhat detailed information can be found elsewhere (see Chambers et al., 1991; Chambers & MacMahon, 1994; Forbis, 2003; Körner, 2003; Bu et al., 2007a, 2008, 2009).
Definitions and Terms
At the outset, it is necessary to explain our terms. Here we consider a seed to be the product of sexual reproduction dispersed at natural dispersal time with all its dispersal structures, e.g., wings, plumes etc. We do not use specific terms such as achenes, and refer to all these propagules as seeds. We also adopt the seed bank classification scheme of Bakker et al. (1996) and Thompson et al. (1997) on a broader level. Hence, seeds that persist in soil for less than 1 year are termed as ‘transient’. Whereas, seeds that persist for more than 1 year but less than 5 years are characteristically considered as ‘short-term persistent’ and any species that continue to persist in soil for more than 5 years are ‘long-term persistent’. Although, in most of the discussion, the term ‘transient’ and ‘persistent’ are loosely used to differentiate the seed longevity of less than or more than 1 year respectively, whenever possible, clear distinctions of seed bank types are made. We define dormancy as a state in which seed germination does not take place even when the favorable environmental factors (temperature, moisture, light/dark etc.) that stimulated germination in a non-dormant or dormancy broken seed are provided. Five types of dormancy have been recognized in seeds (Baskin & Baskin, 1998, 2004) namely physiological (PD), morphological (MD), morpho-physiological (MPD), physical (PY) and combinational (PD + PY), we attempt to specify dormancy types when information is available. For the purpose of this review, we define ‘long-distance dispersal’ (LDD) as seed movement which is over 100 m (sensu Cain et al., 2000).
Dormancy
Dormancy is one of the remarkable traits evolved in seeds that synchronize germination with the most favorable season for seedling survival (Vleeshouwers et al., 1995; Baskin & Baskin, 1998; Thompson, 2000; Bewley et al., 2013). Since Amen (1966) reviewed the extent and role of seed dormancy in alpine species, there has been an abrupt increase in the number of studies documenting alpine dormancy from species to community level (Baskin & Baskin, 1998; Liu et al., 2011). This increase in literature has refuted two of the early notions that (i) dormancy is a rare phenomenon in alpine systems (see Amen, 1966) and (ii) dormancy is highly associated with seed-coat inhibition (Billings & Mooney, 1968). The literature available on alpine seed dormancy is in fact growing and this topic has been comprehensively reviewed periodically (Billings & Mooney, 1968; Kaye, 1997; Baskin & Baskin, 1998). It is beyond the scope here to present such an exhaustive review yet again. Instead, we discuss the role of dormancy (and breaking requirements) in seed persistence.
Cavieres (1999) and Cavieres and Arroyo (2001) postulated that dormant seeds have some advantage in establishing persistent seed banks in temporally and spatially unpredictable alpine environments. This is partly because seeds shed in the autumn are mostly dormant and require a period of cold-stratification satisfied under snow before they germinate in spring (see below) and partly due to the ability of the seeds to re-enter dormancy (in the case of PD) if they did not germinate. This view is in fact supported by (i) Rees’ (1996) assertions that in order to maximize the progeny number, species adapted to an environment that varies from 1 year to another select dormancy as an adaptive trait (also see Rees & Long, 1992) and (ii) as Bewley et al. (2013) recently generalized, the number of species with dormant seeds is likely to increase with poleward movement because the unpredictability in climate increases.
Apart from these simple theoretical considerations, direct evidence delineating whether or not dormancy contributes to seed persistence in alpine regions is scarce. This is ascribable to the paucity of information amassed on a specific species for which both the dormancy and longevity are known. Some progress has been made recently by Schwienbacher et al. (2011) to bridge this gap (but see conclusion section). In an attempt to investigate the seed dormancy types in 28 alpine species of central European Alps, Austria, those authors concluded 26 species were dormant and only 2 species were non-dormant. Out of 26 dormant species, PD (with all sub-classes namely non-deep, intermediate and deep) was present in 20 species. MD and PY were recognized in 4 and 2 species respectively. In addition to assigning the type of dormancy, their study included 9 species for which the seed longevity had previously been assessed by artificial burial method (Schwienbacher et al., 2010). The findings of their study clearly indicate that all seeds survived for at least 2 years in an artificial burial experiment (see ‘Seed size and persistence’, below) and had some type of dormancy - mostly PD or PY (Table 1).
The assertion that dormant seeds persist in soil is in marked contrast with Thompson et al. (2003) who concluded that “dormancy is neither a necessary nor a sufficient condition for the accumulation of a persistent seed bank, and there is no realistic prospect of predicting persistence from dormancy” based on their data from north-west Europe (Thompson et al., 1997). They further supported this conclusion by showing that most of the temperate shrubs have deep dormancy, but these species rarely persist in soil. However, we presume that two different issues have been raised and these can be encapsulated in the questions (1) do seeds of species establishing persistent seed banks have dormancy and (2) do all dormant seeds enter persistent seed banks? Thus, not all dormant seeds persist in alpine soil, but being non-dormant could reduce the ability of seeds to enter persistent seed bank in alpine environments. For example, despite Geum reptans and Oxyria digyna being dormant (Table 1), they were both short-term persistent corroborating the claim that some dormant seeds are also short-lived in soil.
Indeed, it was initially thought that factors such as light requirements, germination inhibitors etc. cause dormancy in alpine seeds (Amen, 1965). Consequently, Billings and Mooney (1968) concluded ‘seed dormancy in most alpine species is under environmental control’. In the central European Alps, however, Schwienbacher et al. (2011) explicitly classified the dormancy type in accordance with Baskin and Baskin (2004), therefore these species do not represent ‘enforced dormancy’. One plausible reason for the disagreement observed between North West Europe and central European Alps datasets may lie in the geographical distribution of the species. In the alpine environment, many species (ca. >70 % as estimated by Baskin & Baskin, 1998 cited in Schwienbacher et al., 2011) produce dormant seeds. Further evidence to safely conclude that most alpine seeds are likely to produce dormant seeds comes from the data set assembled by Jurado and Flores (2005) which suggests plants growing in environments with frost and/or drought contain some form of seed dormancy (PD, MPD and PY) than species in more benign environment.
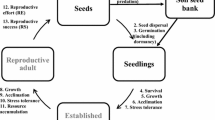
It is generally agreed that seeds dispersed in autumn are held in seed banks until spring because of dormancy breaking requirements (Fig. 1, Table 2). These dormant seeds overwinter under snow cover and alleviate dormancy mostly by cold-wet stratification. They then germinate at the first opportunity immediately after the snow melt in spring to maximize growing periods in favorable conditions (Baskin & Baskin, 1998). Germination of non-dormant seeds in autumn, on the other hand, is exclusively controlled by the local microclimate of a particular year. Interannual variation in autumn temperature should therefore impose selective pressures on alpine communities conferring advantages on species with dormant seeds (Rees & Long, 1992; Allen & Meyer, 1998).
Fate of a seed after dispersal from the mother plant. The horizontal dotted line distinguishes the transient and persistent bank. If the seeds buried in soil (or close to the surface) germinate or die within 5 years, they are transient seed banks. The remainder develop persistent seed banks. Seeds can die or be dispersed to other locations at any stage of this model (see also Chambers & MacMahon, 1994). Similarly, the soil surface may have increased seed rain, due to dispersed seeds from other locations reaching the local micro-site. For the purpose of this model, ‘soil surface’ means the buried depth at which seeds can receive dormancy breaking cues and/or light, germinate and establish seedlings. Seeds buried in the soil undergo seasonal cycling in dormancy status and the depth of the dormancy changes with season
The influence of environmental conditions acting as selective force in the evolution of seed dormancy is gaining recognition. Studies focusing on alpine species, e.g., Chenopodium bonus-henricus (Dorne, 1981), Artemisia tridentata ssp. vaseyana (Meyer & Monsen, 1991), genus Penstemon (Meyer & Kitchen, 1994b; Meyer et al., 1995), Linum perenne (Meyer & Kitchen, 1994a) Phacelia secunda (Cavieres & Arroyo, 2001), and Koenigia islandica (Wagner & Simons, 2008), revealed seeds collected from lower altitudes are non-dormant or conditionally dormant, seeds from mid-altitudes are dormant and require cold-stratification for dormancy alleviation, and seeds from high-altitudes are dormant and require longer cold-wet stratification duration compared to seeds collected from mid-altitudes. Insights gained from these studies suggest dormancy and longer stratification duration must have co-evolved in seeds adapted to higher altitudes. The differential effects of environmental conditions on dormancy may also explain why Oxyria digyna has been classified both as dormant (Stöcklin & Bäumler, 1996; Schwienbacher et al., 2011) and non-dormant (Mooney & Billings, 1961; Mondoni et al., 2012). This species is common to both arctic and alpine communities and therefore is subject to a range of conditions which may or may not induce dormancy.
The opposite situation was observed in some of the recent studies which showed seeds of many alpine plants lack dormancy when they develop and therefore germinate immediately in autumn. Seed germination experiments performed in 37 endemic rare or endangered taxa of Sierra Nevada high mountain (SE Spain), for instance, showed most of the species tested were non-dormant and do not require any pretreatment for germination (Lorite et al., 2007). Giménez-Benavides et al. (2005) reached a similar conclusion when working with 20 species collected from high altitudes of Spain. It is possible that these areas experience Mediterranean climate and seeds develop in a slightly warmer temperature than in true alpine locations, to be less dormant (see also conclusion). One recent study by Sommerville et al. (2013) shows species with non-endospermic seeds did not require any stratification to germinate, however, endospermic seeds needed 8 weeks of cold stratification for germination. Although they concluded that ‘alpine species with endospermic seed and a restricted distribution are most likely to contract in range under climate change and would be appropriate to prioritize for ex-situ conservation’, non-endospermic seeds are also at risk in warming climates as the absence of dormancy/breaking requirement impose the risk of autumn germination and seedling mortality in winter, particularly on exposed sites (see Mondoni et al., 2012).
Dormancy has generally not been specifically addressed in studies of seed persistence in the soil. Nevertheless, the available evidence makes it tempting to conclude that dormancy plays a crucial role in persistence at least for alpine vegetation. However, it is too early to generalize at ecosystem level. This is mainly due to the species-specific breaking requirements evolved in spermatophytes adapted to alpine environment (Table 2). Given most species with PD, these seeds can cycle back to dormancy if the conditions are unfavorable during early spring. Such cycling is also expected to be common in buried seeds (Baskin & Baskin, 1998). The evolution of a dormancy cycle, regardless of the seed position in soil, can be viewed as a specific control in preventing germination outside growing season. For example, if the buried seeds are brought back to the soil surface (Fig. 1), dormancy cycling checks the germination time to synchronize with growing season. Supposing such seasonal change of dormancy had not evolved in alpine species, there would be a chance for buried seeds (when they reach soil surface) to germinate any time of the year, provided other environmental conditions are satisfied.
Temperature Requirement for Germination
Most of the seeds endemic to alpine environments do not germinate immediately after shedding because the temperature required for germination is high (Sayers & Ward, 1966; Billings & Mooney, 1968; Densmore & Zasada, 1983; Nishitani & Masuzawa, 1996; Baskin & Baskin, 1998; Cavieres & Arroyo, 2001; Shimono & Kudo, 2003) and in autumn this temperature is rarely - if ever - met. After overwintering, cold-wet stratification reduces the high temperature requirement and seeds develop a tendency to germinate at a wide range of temperatures (Baskin & Baskin, 1998; Table 1).
It is not uncommon for some alpine species to shed nondormant seed that remain ungerminated during autumn due to the prevailing low temperatures (Billings & Mooney, 1968). Seeds of Oxyria digyna can germinate immediately after dispersal if the temperature is between 10 and 15 °C, but some seeds can even germinate below 10 °C, if the temperatures fluctuate between 13 and 2 °C and have light for 12 h (Mooney & Billings, 1961). Neither cold-wet stratification, nor after-ripening is a strict requirement for germination, rather the germination after seed shedding was impaired by prevailing low temperatures in autumn. In accordance with this view, a warmer autumn temperature is likely to increase the germination of some nondormant seeds, e.g., Geum reptans, Veronica alpina and Oxyria digyna to autumn (Mondoni et al., 2012). Such seeds are poorly represented in alpine communities, as the winter temperatures decimate seedlings (but see below).
Typically, 25 to 35 °C is the most favorable temperature eliciting autumn germination in the proportion of non-dormant/conditionally dormant seeds (Clebsch & Billings, 1976; Kibe & Masuzawa, 1994; Nishitani & Masuzawa, 1996; Körner, 1999; Novak et al., 2011). Autumn germination is facilitated further if the temperature fluctuates between 20 and 30 °C (Sayers & Ward, 1966; McDonough, 1970). However, these relatively high temperatures do not occur in alpine environments during autumn (Billings, 1974; Bell & Bliss, 1980; Reynolds, 1984). Elkington (1971) showed freshly collected seeds of Dryas octopetala germinated to 48 % when sown at 25 °C under controlled germination conditions, but field sown seeds in autumn germinated only in the following spring, suggesting the temperature required for germination was not met. Likewise, Marchand & Roach (1980) also reported seeds of Arenaria groenlandica, Juncus trifidus and Potentilla tridentata germinated to 88, 88 and 52 % when tested in laboratory at 23 to 28 °C, 19 to 23 °C and 21 to 26 °C respectively, however, only 38, 17 and 6 % of the seeds germinated in the field.
The mean autumn, spring and summer temperatures decrease with altitude. In addition, snow cover often comes early but lasts longer at higher elevations (Lütz, 2011). Evidence is mounting that the autumn and spring temperature optima for germination is different for species occurring at different altitudes. In general, seeds from higher altitudes where temperature minima is low have a tendency to germinate at lower temperature after cold stratification, but seeds from lower altitudes experiencing warmer conditions during seed development require higher temperature than high-altitude species (Dorne, 1981; Mariko et al., 1993; Nishitani & Masuzawa, 1996). Thus, seeds moved from a low altitude in a reciprocal transplant experiment germinated in the autumn at higher elevations, but when seeds from higher altitudes were moved to lower altitudes seeds germinated only in the second spring following transplant (Jaganathan et al., unpublished).
A simple germination experiment using Polygonum weyrichii var. alpinum and P. cuspidatum seeds at a range of temperatures including 5, 10, 20, 25 and 35 °C, showed that germination of seeds of both species could only be achieved in the autumn at temperatures of 35 °C both in light and dark conditions (Nishitani & Masuzawa, 1996). Importantly, after cold-wet stratification, seeds of P. cuspidatum from Mt. Fuji (a site experiencing relatively high temperatures) germinated equally well at 10, 20, 25 °C, contrasting with the poor germination of seeds from Shizuoka (relatively moderate temperatures) at 10 °C. Similarly, in the case of Phacelia secunda, freshly collected seeds did not germinate under simulated diurnal fluctuations at 20/10 °C or at 10/5 °C at the time of collection in autumn, but seed germination increased proportionately with cold wet stratification duration (Cavieres & Arroyo, 2000). In particular, seeds from low altitudes required lesser stratification duration and germinated equally well at both the temperatures tested than did seeds from higher elevations (Cavieres & Arroyo, 2000). In another study, Blionis & Vokou (2005) confirmed the ability of higher altitude seeds to germinate at low temperature after overwintering in Campanula populations. Also, Mondoni et al. (2009) showed seeds of Silene elisabethae collected from the coldest site in their investigation germinated better at low temperature after cold-wet stratification.
Vera (1997) studying the effect of altitude and seed size on germination and seedling establishment of Calluna vulgaris, Erica cinerea and E. vagans collected from heathlands in northern Spain found that small sized seeds originating at higher altitudes germinated well at 20 °C. A more recent study by Liu et al. (2013) on seed germination of 445 species in a grassland community on the eastern Tibet Plateau, demonstrated that after-ripened seeds germinate better at alternating temperatures (either at 5/25 °C or 10/20 °C) than at a constant temperature of 15 °C especially if they were small forbs. More studies are needed to offer clearer understanding of autumn and spring germination ecology especially at the community level.
The local microclimate in fellfield environments is harsher than that of snowbeds due to little snow accumulation, and reduced snow cover thickness and duration (Körner, 2003). Snow patches covering soil for a longer time in snowbed fields offer refuge to the seeds from low temperature. For instance, seeds under snow cover are held in stasis and showed significantly lower level of damage and mortality than seeds kept artificially snow free (Williams, 1987). On average, growing season length decreases by 30–50 % from fell-field to snow-bed. Thus, habit-specific temperature requirement for autumn germination is expected but there is no evidence for this trend. For example, seeds of Potentilla matsumurae collected from both fellfield and snowbed failed to germinate at the time of collection at 15/5 °C, but with increasingly warm temperature cycles (20/10 °C, 25/15 °C, 30/20 °C, 35/25 °C) the germination percentage increased (Shimono & Kudo, 2003).
In the same study, Shimono and Kudo (2005) disclosed that seeds of some species e.g., Arnica unalaschkensis, Bupleurum ranunculoides, Vaccinium ovalifolium at the time of dispersal in autumn have a more moderate optimum temperature regime, thus germination at higher temperatures of 35/25 °C is unfavorable. However, in these species cold-wet stratification has widened the temperature at which germination occurs. Overall, it seems logical to conclude that the temperature requirement for germination both in autumn and after overwintering in field is highly species-specific. Despite this, several sound pieces of evidence indicate that it is safe to generalize that the requirement for high temperatures for germination in autumn in alpine species can be beneficial to postpone germination to spring, especially in non-dormant seeds. Furthermore, studies that document germination temperature requirements have revealed both inter and intra-species variation in autumn germinating temperature, but acknowledge that the temperatures required for germination are not likely to be met in autumn.
Light Requirement
Light is an important environmental factor stimulating germination in many seeds (Milberg et al., 2000). The requirement of light for germination has been one of the potential determinants driving species to accumulate a persistent seed bank (Baskin & Baskin, 1998; Milberg et al., 2000; Pons & Fenner, 2000). A strict light requirement in some alpine species not only prevents germination in late autumn, it also cues the timing of germination to spring (Densmore, 1997; Fig 1).
Seasonally changing day length acts as a bottle neck in controlling alpine seed germination. In Alaska, nonstratified seeds of Diapensia lapponica, Chamaedaphne calyculata, Saxifraga tricuspidata and Ledum decumbens showed no or few germinating seeds under short-day conditions (Densmore, 1997). However, cold stratification increased the proportion of germinated seeds of all species, significantly both under short-day and long-day conditions. Similarly, longer-day conditions promoted germination in all PD seeds tested, except Poa alpina (see below; Schwienbacher et al., 2011). Haggas et al. (1987) reported that seeds of Carex paysonis collected in Beartooth Mountain achieved good germination only under complete darkness followed by light conditions at constant temperature (25 °C). Seeds incubated at 25 °C under complete light or darkness resulted in poor germination. In addition to being dormant, seeds of C. pendula required light for germination even after the dormancy is broken (Brändel & Schütz, 2005). Seeds of Silene elisabethae, showed increased germination percentage with increase in cold-wet stratification duration, but germination was tightly controlled by light (Mondoni et al., 2009). Novak et al. (2011) also noted seeds of Peucedanum ostruthium are better germinated at 26 °C only in light. These studies clearly depict light plays a pivotal role in controlling the timing of seed germination form seed banks.
By contrast, for some alpine species light is not required for germination, instead light inhibits it. Examples of dark-requiring species include Poa alpina (Acharya, 1989; Schwienbacher et al., 2011), Trifolium pallescens (Schwienbacher et al., 2011), Anthyllis vulneraria subsp. alpicola (Schwienbacher et al., 2011), Agropyron latiglume (Acharya, 1989), and Koenigia islandica (Heide & Gauslaa, 1999; Wagner & Simons, 2009). Both dark and light requirement in seeds can play a pivotal role in delaying germination, thus contributing to seed persistence (Fenner & Thompson, 2005; Bewley et al., 2013). Therefore, it seems reasonable to suppose that dormancy and light requirement both circumvent autumn germination in alpine species, thus affecting the composition of soil seed banks. In dark-requiring species (e.g., Trifolium pallescens), dormancy can act as the main mechanism in preventing seed germination until the growing season (Table 1). However, in spring, seeds experience light/dark cycles, therefore it is not known if cold-stratification in winter imposes a light requirement in the otherwise dark-requiring seed.
Important results from recent ecological studies suggest that environmental conditions significantly contribute to the development of a light requirement in alpine species. Seeds of Koenigia islandica collected from Iqaluit (Nunavut, Canada), Yukon (Canada), and Jasper (Alberta, Canada) did not have a significant light-requirement for germination, whereas seeds collected from more severe climates, Svalbard (Norway) and Colorado (USA), required light for germination (Wagner & Simons, 2009). Mondoni et al. (2012) showed that seeds of Oxyria digyna germinated at 2 °C higher than the current alpine climate (12/12 h photoperiod) achieved higher germination, but that there is a tendency for the seeds to become autumn germinators because dormancy is lacking. Unfortunately, the role of light in germination control was not considered in their study. However, Billings & Mooney (1968) worked with O. digyna seeds and reported that those collected from an American alpine location had greater cumulative germination in light than in dark, but seeds of arctic origin did not have strict requirement for light. The aforementioned studies suggest the evolution of a requirement for light is more likely location-specific.
Light requirement in seeds has also been reported to be of great significance in controlling the depth of burial at which germination occurs (Pons & Fenner, 2000). Since light can only penetrate a few millimeters from the top of the soil surface, seeds found below this depth do not germinate; and even in dark-requiring species, those seedlings would die before reaching the soil surface, especially if they are small owing to the smaller seedling size (see below). Data on other ecosystems suggest that seeds that are smaller are more likely to require light for germination than larger seeds (Milberg et al., 2000; Jankowska-Blaszczuk & Daws, 2007). In a recent study, Wu et al. (Wu et al., 2013) found a similar pattern in alpine species of Qinghai-Tibetan Plateau. Using twenty species, those authors reported significant negative correlation between strength of light required for germination and seed mass, thus large-seeded species were less likely to require light for germination than those of small-seeded species. If small seeds are buried to greater depths, the strong requirement for light in small-seeded species suggests that disturbance is an inevitable requirement in the germination of seeds from seed banks. Nothing, however, is known of the light requirement of seed in different stages of the seed bank cycle (Fig. 1).
While the studies illustrated above lend some support to the assertion that light plays an important role in controlling seed persistence, this perspective requires detailed experimental investigations. Because our knowledge on the relationship between light requirement and germination is limited merely to genus Carex (Amen & Bonde, 1964; Schütz & Rave, 1999; Schütz, 2002) and Koenigia islandica (Heide & Gauslaa, 1999; Wagner & Simons, 2008, 2009), the understanding of the role of light in controlling the autumn germination of some non-dormant alpine seeds is still rudimentary. Nevertheless, we surmise that the long-day requirement for germination might be common (but not ubiquitous) in alpine species. This is because, in any year with an unfavorable growing season, long-day conditions will not be repeated until the following year, a condition typical to all alpine environments. However, in favorable years, a significant amount of light reaches the soil surface due to the lack of taller plants (Billings, 1974; Densmore, 1997). Hence, in addition to dormancy, light requirements also hold germination in check, thus contributing to seed persistence in soil.
Factors Affecting Seed Persistence
In many alpine species, the evolution of highly specific germination requirements, such as those described above, delay the germination of autumn-dispersed seeds until spring. Thus, the interplay of factors including dormancy, specific temperature requirement to germinate and highly specific light or dark requirements in a particular species offers triple safety measures against the harsh alpine climate; without these controls seeds are more likely to have asynchronous germination thereby posing a serious risk of seedling mortality (Billings & Bliss, 1959). For example, when seeds develop in a relatively warm climate due to interannual variation, the degree of dormancy (sensu Baskin & Baskin, 1998) may be reduced. In such cases, autumn germination may be prevented by a high temperature requirement and/or light requirement instead of the depth of dormancy acting as the primary obstacle to germination. If only one mechanism for preventing germination has been evolved in alpine seeds, then the autumn germination ability is likely to depend on the maternal environmental condition during seed development and opportunistic temperature at autumn. Evolution of such complex germination requirements is not unique to all ecosystems around the world. The obvious example are flood meadows, where many seeds are non-dormant but germination is controlled by light and a narrow temperature requirement, hence dormancy has no role in seed persistence (Hölzel & Otte, 2004 and references therein).
In addition to a complex suite of responses to varying environmental cues, it has been suggested that not all the seeds of a particular species may respond to germination cues stimulated by seasonal temperature cycles in alpine environments, thus carryover of a proportion of seeds to next year is expected to maximize species fitness (Meyer & Kitchen, 1994a; Meyer et al., 1995). Carry over mechanisms are common in unpredictable conditions because the risk of germination is spread to different years. For some alpine species, e.g., Carex frigida, low germination in the growing season tends to favor carry over mechanisms (Schütz, 2002). Although the success of seeds carried over to germinate in the next year depends on various phenotypic and genotypic factors (Meyer & Kitchen, 1994a), how a species selects potential candidates for carry over is not understood completely. Thus, it is not known if the seeds to be carried over were predetermined at the time of shedding or the selection is a result of phenotypic variability or simply that all the non-germinated seeds at the end of growing season are carried over (but see Meyer & Kitchen, 1994a). However, the degree of dormancy, position of seeds in soil and local microclimate are certainly important factors determining which seeds are carried over. Furthermore, for various reasons that are typical to alpine environments, e.g., frequent summer frost (Marcante et al., 2012), only a proportion of seeds germinate even in favorable growing seasons (Cavieres & Arroyo, 2001), leaving the reminder to be part of the persistent seed bank.
Seed Size and Persistence
A commonly accepted prerequisite for seeds to enter persistent seed banks is the small size and round shape (Thompson et al., 1993). Despite their suggestion that this phenomenon is likely to be universal, subsequent studies testing the prevalence of small, round seeds in persistent seed banks have argued both for (Bekker et al., 1998; Thompson et al., 2001; Zhao et al., 2011), and against (Leishman & Westoby, 1998; Moles et al., 2000; Yu et al., 2007) the hypothesis. There have been few works that studied the significance of seed size in establishing persistent seed bank in alpine landscapes. In Argentinean mountain grassland, the available evidence demonstrated that small seeded species have an increased likelihood of entering the persistent seed bank (Funes et al., 1999; Fig. 2). Conversely, Cerabolini et al. (2003) showed a negative correlation between seed size and persistence in species tested on Italian Alps (Fig. 2).
Relationship between seed mass (log scale) in mg and variance of seed dimension adopted from (a) Italian Alps; 114 species (re-drawn only for alpine species from Cerabolini et al., 2003 with permission), b Central Austrian Alps (Schwienbacher et al., 2010) after excluding the standard deviation given in the original data set and c Argentinean mountain grassland; 71 species (re-drawn from Funes et al., 1999 with permission). (∆) Transient, (●) Long-term persistence, (■) short-term persistence, and (♦) persistence (but no information on whether short or long-term), (X) uncertain or could not classified. More detailed information of how to calculate the variance of dimension can be found in Thompson et al. (1993). In order to make some extremely small seeded data point readable in the Argentinean mountain grassland (c) we have rescaled the x-axis of this study to begin from 0.001
Recent years have witnessed a surge of studies determining seed longevity in soil using artificial burial experiments. Schwienbacher et al. (2010) conducted such an experiment in the Central European Alps (ca. 3500 m a.s.l; Austria) for Achillea moschata, Artemisia genipi, Anthyllis vulneraria subsp. Alpicola, Geum reptans, Linaria alpina, Oxyria digyna, Saxifraga aizoides, S. oppositifolia and Trifolium pallescens. Seed germination following excavation at 1, 2 and 5 years revealed, with exception of Geum reptans and S. oppsitifolia (which have extremely tiny seeds), at least some of the seeds buried at a depth of 3 cm survived for a minimum of 2 years (Fig. 1; Table 2). However, their study had two surprising results. Firstly, in contrast to the suggestion that round seeded species have increased tendency to enter the persistent seed bank, flattened L. alpina seeds survived for 5 years forming a long-term persistent bank, whereas other flattened seeds of O. digyna did not survive for 5 years. Secondly, species classified as transient by Cerabolini et al. (2003), were shown to form short-term persistent or long-term persistent seed banks (Table 1). The only other attempt to use an artificial burial approach to test seed viability for >1 year in alpine tundra was a study in the Chilean Alps (Arroyo et al., 2004). From a total of 15 species studied, all but two species namely Senecio magellanicus (with cylindrical shape but smaller size) and Berberis buxifolia (6.33 mg), survived for 2 years. However, they terminated the experiment in 2 years, therefore a distinction between short-term and long-term seed persistence is not feasible.
There are several lines of evidence showing that small seeds easily become buried, thereby escaping post-dispersal predation, remaining in the same environment in which they had developed and becoming part of the persistent seed bank (Peco et al., 2003; Ma et al., 2010b). If this holds true then seeds at depth in the soil are expected to be smaller than the seeds in the surface layer. However, there are only a few experimental studies addressing the vertical movement of seeds in alpine flora. In the Austrian Alps, Klug-Pümpel and Scharfetter-Lehrl (2008) found small seeded species move to greater depths than large seeded species, but Ma et al. (2010b) failed to find such pattern in 122 species of Tibetan Plateau, China. The important question perhaps then is: what depth is safe for seeds? Ma et al. (2010b) showed that seeds buried (naturally) to 15 cm can germinate when excavated and sown in optimal germinating conditions. Seeds artificially buried to 3 cm deep germinated after 5 years (Schwienbacher et al., 2010; Table 1). However, over the same time period but at 8–10 cm depth, Adzhiev et al. (2011) reported no germination in 45 out of 63 species of the Northwest Caucasus.
It is difficult to draw any firm conclusion on the impacts of seed size on persistence in alpine seed banks from these studies mainly because of the overriding effect of other factors influencing results, three of which are discussed here. The first to be considered is the type of soil surface where seeds land. If seeds are deposited on a surface having smaller particles, there is an increased likelihood that small seeded species rather than those with large seeds, and those with adhesive seed coats are trapped amongst the soil particles and buried in the soil (Chambers et al., 1991; Chambers, 1995, 2000). Small soil particle size may enable the large seeded species to move horizontally over longer distances until they encounter soils with large particle sizes. The smaller seeds frequently trapped by soil of smaller particle size often move to a deeper position than the larger seeds trapped in large particle soil. In contrast, both small and large seeded species are buried easily if seeds were deposited on soil surface having larger particle size (see Chambers et al., 1991 for a detailed discussion).
A second confounding factor when trying to generalize on the relationship between seed size and persistence is derived from the understanding that entry to the soil seed bank confers longevity. It is perhaps an over-simplification to say that ease of burial improves the longevity of small seeded species in the alpine system. Indeed, seeds buried to greater depths do not germinate, unless the soil is overturned but, if disturbance is very infrequent, these species may be lost from the aboveground community and eventually from the seed bank also. For example, in the central Chilean Andes, Arroyo et al. (1999) found buried seeds of Chaetanthera pusilla were viable, although the species did not occur in the standing vegetation. Similar results were obtained in the alpine regions of Tibet for a wide range of species: a total of 87 species were identified in the soil seed banks, but only 62 appeared in the standing vegetation (Ma et al., 2010a). There are no data available on how long these seeds can be viable but presumably, this longevity will be species-specific. Nonetheless, dating by accelerator mass spectrometry revealed seeds of Carex bigelowii and Luzula parviflora in Alaskan tundra had at least persisted for two to three centuries (McGraw et al., 1991).
A third problem with linking seed size and persistence is the inconsistency in methods with which seed longevity is determined; this causes subsequent confusion when trying to build causal relationships between size and viability. For example, seeds of some species classified as transient by Cerabolini et al. (2003), survived more than 1 year when tested with artificial burial experiments (Table 2). It has been suggested that the discrepancy in the findings of these studies has resulted from different methods employed in testing seed longevity (Schwienbacher et al., 2010). Cerabolini et al. (2003) used published information and germination experiments conducted with seeds excavated from soil kept in glass houses to classify the seed bank type, whereas Schwienbacher et al. (2010) classified the seed bank type based on data from artificial burial experiment. Each method has its own advantages and disadvantages; the artificial burial approach often overlooks the mechanism of burial, which is arguably a complex process itself (Thompson et al., 1997). Therefore, the conclusion of Schwienbacher et al. (2010) that Linaria alpina retains its viability in the soil for 5 years as estimated by artificial burial experiments, can be called into question because the soil environment may not enable adequate burial and subsequent avoidance of disturbance to maintain the conditions generated in their experiment. Thus, whether shape is a good predictor for seed persistence or not, remains largely equivocal for alpine species. More meaningful answers to this question will only come from novel seed bank studies that combine both natural soil sampling and artificial burial approach incorporating small and large seeds.
While some small seeded species more easily enter persistent seed banks than large seeded species, careful consideration of seed persistence studies point to the possibility that not all small seeded species can potentially establish persistent seed banks (Fig. 2). Similarly, generalizations on large seeded species are inappropriate as some of these can also establish short-term persistent seed banks (Fig. 2). As yet, there is no evidence available to demonstrate whether large seeded species possess a long-term persistent seed bank in alpine landscapes (Fig. 2). However, a short-term persistent seed bank tends to be adequate for maintaining species in alpine conditions, because the favorable weather conditions can occur at least once every few years (Körner, 2003). Furthermore, most of the plants adapted to alpine tundra are perennials and produce seeds in fairly large amount, at least in the favorable years, and although a long-term persistent seed bank is adaptive to the alpine climate, it is not necessary for a species to succeed in colonization.
Seedling Establishment
An additional factor that complicates our understanding of the significance of seed size in persistence is the hypothesis related to seedling establishment. With finite resources allotted to reproductive success, a given species may produce more small seeds or less large seeds, leading to a variation in seed mass often spanning five to six orders of magnitude across species found in a particular ecosystem (Thompson, 1987; Jakobsson & Eriksson, 2000; Leishman et al., 2000; Leishman & Murray, 2001). A growing body of literature is specifically devoted to understand how such variation in seed size influences the fitness of plant communities (Leishman & Murray, 2001). Although small-seeded species benefit by producing more number of seeds than large seeded-species and have an increased likelihood of entering soil banks, the latter group might counteract the advantage by establishing high number of successful seedlings than the small seeded-species.
Because seed germination is an irreversible event, timing of germination is a critical component in the life-cycle of alpine plant species. Seeds, once germinated, have to continue growing or die. Seedling establishment in alpine environments is a slow process and often lasts for several years (Jaganathan, personal observation). Although some of the seeds germinating under snow cover are very slow requiring up to 6 months to achieve 50 % germination (Meyer et al., 1995), spring germinators must attain a suitable size to tolerate subsequent winter conditions (see Leishman et al., 2000), if not, seedling mortality becomes prevalent. If this holds true, then it is reasonable to expect that large-seeded species establish seedlings more quickly and there may be a trade-off between seed size and mean germination time (MGT). Consequently, we anticipate that large-seeded species must germinate faster than small ones to maintain an advantage over the seed bank strategy prevalent in small-seeded species.
We tested this hypothesis in 653 Qinghai-Tibet Plateau species (original data from Bu et al., 2007a; Wu et al., 2013). Species were only included in this analysis if they were recorded as germinated. Hence, species recorded with MGT of 0 (failed germination) were excluded from this analysis. In this way, we were able to select a total of 585 species from 39 families. We performed a linear regression to explain the relationship between seed mass and their respective MGT. In contrast to our expectation, we observed no significant relationship between seed mass and MGT (slope = 1.8, R 2 = 0.01, d.f. = 584, p > 0.05) (Fig. 3).
The relationship between seed mass and MGT (Mean Germination Time) estimated for 565 Qinghai-Tibet Plateau species. Number of days required for germination increased with increase in seed size (slope = 1.8) but the relationship is not significant (R 2 = 0.01, d.f. = 584, p > 0.05). Each point represents one species
If large seed size does not confer an advantage with respect to MGT an alternative benefit may be bestowed through greater seedling size (Vera, 1997; Moles & Westoby, 2004). However, dormancy can regulate germination only in terms of when in the growing season emergence occurs for more successful seedling establishment. Further, there is little evidence that large-seeded species have some advantage in survival over small-seeded species (even within same genus, see Vera, 1997). We conclude that this advantage may not counterbalance the large number of seeds produced by small-seeded species and their ability to persist in soil.
Seed Dispersal
At the ecosystem level, microhabitat and seed availability determine the composition and diversity of species (Bullock et al., 1995; Tackenberg et al., 2003; Ozinga et al., 2004). Seed dispersal has a marked effect on seed availability in any given location and the distribution of the ‘seed rain’ is potentially very different to that of the parent plants. Plants adapted to short-growing seasons in unpredictable environments are believed to disperse seeds over a longer distance, so that seeds experience different weather conditions and germinate in the environments where conditions are favorable (Bakker et al., 1996; Baskin & Baskin, 1998; Tackenberg et al., 2003). Given the unpredictability in climate, it is reasonable to expect alpine species have long distance dispersal (LDD). In spite of a pullulating interest on understanding the seed dispersal in low-altitude ecosystems, our knowledge on seed-dispersion patterns for alpine ecosystem remains conjectural (McGraw & Vavrek, 1989; Muñoz & Arroyo, 2002). In particular, research on alpine seed dispersal has mostly concerned the mode of seed movement, rather than precisely estimating how long seeds could move. One reason for this lacuna is the difficulty in tracking all routes a seed could move from mother plant to the germination site. Even for the studies that attempted to quantify the distance, results were influenced by several other factors such as height from which seeds are dispersed; size and morphology of seeds; morphological adaptations for dispersal; dispersal vectors availability; wind speed at the time of dispersal and wind direction.
Seed movement within alpine landscapes involves numerous dispersal vectors. Most frequently seeds are dispersed by wind (Van der Pijl, 1982; Willson et al., 1990; Table 3). A simulation model in the glacier foreland of the Dischma Valley, Switzerland has shown that more than half of the alpine species were dispersed a long distance by wind, whereas only 25 % of low altitude species tend to have LDD (Tackenberg & Stöcklin, 2008). Because the horizontal movement of seed can be affected by the wind speed, Tackenberg and Stöcklin (2008) also measured the wind speed in alpine and low-altitude, but found only a small difference between these two environments. However, wind speed may not only vary between season but also years (Billings & Bliss, 1959). Furthermore, secondary movement of seeds also depends on the soil characteristics (Chambers et al., 1991; Chambers, 1995, 2000). The small-sized seeds frequently trapped by smaller particle size move vertically, thus do not move to long-distances (see Chambers et al., 1991 for a detailed discussion). Therefore, it is not known if the simulation model has overestimated the role of soil characteristics and wind speed in secondary seed dispersal.
The dogma that seed dispersal agents are rare in alpine environments (McGraw & Vavrek, 1989), has been disproved by active research over the last two decades. Seeds of many alpine plants could successfully survive passage through the intestinal tract of animals, i.e., endozoochory (Bruun et al., 2008; Yu et al., 2012). For some species, this passage promoted germination, whereas for others it suppressed the total percentage of germinated seeds. A good example is the Andean (Chile) shrub Berberis empetrifolia dispersed in the faecal deposition of Liolaemus bellii. After passing through the digestive tract, germination increased significantly when compared to the untreated control (Celedon-Neghme et al., 2008).
In the alpine forest of southwestern China, species of Pinus armandii, P. densata, Abies sp. and Viburnum sp. were predated by rodents (Wang et al., 2012). Two years of a seed caching experiment showed not only that seeds of P. armandii were dispersed to < 10 m but also rodent seed selection for caching was size-specific. In the experiments conducted with two populations of endangered Spanish black pine P. nigra in the Cuenca Mountains of Central Spain, seed predation in a mast year was significantly lower compared to the low seed producing year (Lucas-Borja et al., 2012) suggesting seed predation varies between years. Mountain snowberries (Gaultheria depressa) endemic to New Zealand alpine ecosystems was consumed by scree weta (Deinacrida connectens) and moved long distances (Larsen & Burns, 2012), however such dispersal events are rare.
The ability of ants to disperse seeds was assessed experimentally in Central Chilean Andes for Sisyrinchium arenarium (Iridaceae) (Muñoz & Arroyo, 2002). Insightful measurements made at two different altitudes (2700 m and 2000 m) revealed greater activity of ants and birds at higher altitude compared to lower altitude, however, the overall seed removal by ants was significantly greater than birds at both elevations. In a subsequent study those authors showed that 9 species namely Alstroemeria pallida, Anarthrophyllum cumingii, Chuquiraga oppositifolia, Laretia acaulis, Rhodophiala rhodolirion, Sisyrinchium arenarium, Taraxacum officinale, Azorella monantha and Pozoa coriacea, were dispersed by ants and birds, but only over a short distance (Muñoz & Cavieres, 2006). In the sub-alpine locations of Mt. Norikura, Kita-Alps, Japan, seeds of Dicentra peregrina were observed to be dispersed by Formica gagatoides, but the distance is not known (Komatsu et al., 2014). To the best our knowledge, experimental evidence of ants dispersing seeds in other alpine environment has not been documented elsewhere. However, a growing number of studies are explicitly showing the significance of seed dispersal by birds in other alpine locations. For instance, in New Zealand, some fruit species were dispersed only by a fruit-eating parrot, kea (Nestor notabilis) (Young et al., 2012), but the specific distance of seed movement was not measured.
In New Zealand, 10 Veronica species disperse their seeds by the mechanism of hygrochasy (Garnock‐Jones & Lloyd, 2004; Pufal et al., 2010). Hygrochasy is generally understood as the ability of seed holding structures to dehisce when the moisture content of those structures increases to a certain threshold level. This mechanism of seed dispersal is common in desert species, where water availability and seed dispersal (and germination) must coincide. The evolution of hygrochasy beyond these alpine endemics needs further clarification, although it is assumed that this mode of dispersal does not favor LDD.
Many alpine species were able to float in water for more than 1 year, when empirically tested (Danvind & Nilsson, 1997). Nevertheless, long-distance movement of seeds by floating may be constrained by several factors (Danvind & Nilsson, 1997). Since alpine landscapes are covered by snow for most of the year, there is a greater probability that the seeds dispersed outside the snow-free growing season land on the snow blanket. Although these seeds are dispersed by wind (Matlack, 1989; Chambers & MacMahon, 1994), accumulating snow cover precludes further movement once the seed has come into contact with the snow surface. During snow melting these seeds move, but only over a short distance. For example, the work by Scherff et al. (1994) showed melting snow moved seeds of Ranunculus adoneus only to a distance of 10 cm. Similarly, Glaser (1981) observed extremely scarce movement of seeds when snowbeds melted in Mount McKinley National Park, Alaska. Evidence of this kind clearly demonstrates that the distance seeds move by this mode is inconsequential (Greene & Johnson, 1997).
In recent years, the influence of humans on alpine vegetation has become more pronounced. The construction of roads or walkways, regular visits to specific areas for research and education, and the recent surge of interest in mountain tourism frequently disturb vulnerable alpine communities. In addition, man and man-made machines are more likely to move lowland seeds in to alpine environments. Emerging studies have just started to understand the severity of the consequences for alpine plants, but for some lengthy treatment of this subject consult Pauli et al. (2001). Trampling damage due to walkers entering the Central Tasmanian alpine vegetation affects the shrubs, shrubland and grassland, although fen tend to be more resistant (Whinam & Chilcott, 1999). Non-native seeds belonging to 17 families have been identified in polar regions as a result of accidental transport by visitors (Ware et al., 2012). In Mount Norikura (Japan), construction of road has changed the species composition as a result of alteration in canopy and soil nutrition (Takahashi & Miyajima, 2010).
Except for wind dispersal, our knowledge of seed movement and potential dispersal distances by other modes remains far from satisfactory. Indirect evidence suggests that limited seed availability due to short-distance dispersal impedes the regeneration potential in open alpine landscapes rather than microhabitat availability. For example, restricted dispersal of Vaccinium myrtillus, Rhinanthus minor, Rumex acetosa, Cicerbita alpina, Trollius europaeus and Campanula thyrsoides appears to be the foremost reason for the patchy distribution, because seeds added manually to unoccupied sites resulted in successful germination (Lindgren et al., 2007; Frei et al., 2012).
It has been argued above that LDD is a sporadic event in alpine species. Whilst this is based on limited evidence, we nonetheless expect a larger set of data might yield similar results. As of now, the notion that LDD is rare in alpine species should be viewed as a working hypothesis rather than conclusion.
Dispersal vs. Persistence
Given finite reserves of energy to establish their off-spring successfully, many plants have evolved different ways of ensuring dispersal to different locations or persistence in soil to reduce the so-called ‘colonizing effect’. One way is to invest in seed dispersal structures to attain longer dispersal. Information regarding the seed dispersal structures for alpine flora are incomplete, but a few alpine plants have been reported to invest in seed dispersal structures (Chambers & MacMahon, 1994). Bu et al. (2008) provided substantial data on the seed dispersal structure at community level in eastern Qinghai-Tibet plateau (Fig. 4). This data set suggests that most of the alpine plants present do not invest in any specific dispersal structures (Fig. 4), although a few species do invest in wind dispersal structures. In their work on comparing seed dispersal patterns for various plant communities, Willson et al. (1990) noted many alpine species could have morphological adaptations for wind, thus seed dispersal by wind may be common, amongst other available dispersal vectors.
Dataset of 633 species collated by Bu et al. (2008) showing number of species investing in specific dispersal structure
While the studies by Willson et al. (1990) and Bu et al. (2008) generally agree that most alpine species neither invest in specialized morphological structure nor move long distances, the question as to why this is the case cannot be answered from available evidence. There may be several plausible explanations, although none of them have been rigorously treated in experimental works. Due to plant development in extremely cold-climates, the parent plant may have only limited resources allocated for seed development (Forbis, 2003; Körner, 2003). Larger seeds with specific dispersal structure means augmented investment, a reason that might explain why there were fewer fleshy seeded species observed by Willson et al. (1990). Furthermore, seed development must take place in a short time, if not, the development is carried over to next year – principally because most alpine plants (and plant parts) are held in stasis by the snow cover except the seed producing and growing season.
A decade ago, Körner (2003) tentatively concluded that most of the alpine species may have seed mass of less than 4 mg. Extending his suggestion to a larger data set of more than 1000 species by reworking the data presented by Ma et al.(2010b), Funes et al. (1999), Cerabolini et al. (2003), Körner (2003), Giménez-Benavides et al. (2005), Pluess et al. (2005), Shimono and Kudo (2005), Bu et al. (2007a), Schwienbacher et al. (2010), Ma et al. (Ma et al., 2013) [from the seed size summary data] and Wu et al. (2013), we show that 88 % of the species have seed mass below 3 mg and nearly 93 % have seed mass below 4 mg, reinforcing the selection of small-seeded species in alpine vegetation. Furthermore, in literature where seed mass data are not presented for individual species, those studies have some useful summary of seed mass range. For example, of the 122 species reported in the study by Ma et al. (2010b), only few were above 3 mg, with the seed mass of 4.3618 mg (Vicia multicaulis) as the largest seed.
Baker (1972) first proposed that seed weight decreases with elevation using a data set from California. However, successive studies have either rejected (Mariko et al., 1993; Pluess et al., 2005) or accepted (Bu et al., 2007b; Guo et al., 2010; Dainese & Sitzia, 2013) this proposition, thereby undermining the generality of this pattern. One plausible reason for lack of unanimity in results is because in these studies the climatic conditions in which the seeds have developed were poorly measured or ignored. It has been stated that the short length of summer and low temperatures at high-altitude potentially limits the seed development duration and ‘there may simply be inadequate time available for producing and stocking large, heavy seeds’ (Baker, 1972). To support this view, seed developmental studies of Gentianella germanica comparing histogenesis and maturation drying over 3 years, have indicated that seeds developed in colder years were smaller and poorly developed when compared with seeds from the warmer year (Wagner & Mitterhofer, 1998). Similar conclusions have been presented for numerous species including Potentilla pulcherrima (Stinson, 2004).
Another limitation of the debate on the impact of seed size is due to the differing objectives and methods used to determine if a relationship exists between seed size and elevation. For Baker (1972) the average seed weight of species occurring at a particular altitude compared with the mean seed weight at a different (higher) altitude shows a decreasing trend, although discussions on one (Penstemon) genera and possibly same results on other genera were presented. Pluess et al. (2005) measured the seed size in 29 Swiss alpine congeneric species and found seed size increased with altitude for 55 % of the species tested, but decreased or remained equal for 3 % and 41 % of species respectively. Nevertheless, populations of Scabiosa lucida, Saxifraga oppositifolia, Epilobium fleischeri and Carex flaccafound occurring at different altitudes showed a decrease in seed size with altitude. Mariko et al. (1993) working with Reynoutria japonica seeds collected from various altitudes of Mt. Fuji, concluded that mean seed weight of the upland population was significantly heavier than that of the lowland populations. Interestingly, the proposition that seed size increases with altitude is attributed to the fact that seedlings from larger seeds are more likely to survive extreme conditions than seedlings developed from small seeds. However, if seedling size advantage is a reason for the increased seed size observed with altitudes, this does not explain why large seeded species are rare in high altitudes (Table 4). Although we are inclined to believe that having a larger seed in comparison to low altitude species may be advantageous for alpine species of higher altitudes, we also accept that this ‘trend’ is not universal - even within an individual plant the seeds produced vary in size significantly, presumably due to numerous intrinsic and extrinsic factors, e.g., maternal environment, heteromorphism etc.
The underlying hypothesis describing the evolution of alpine plants has not yet been completely understood. However, it is suggested that some of the spermatophytes colonized tundra vegetation by dispersing seeds with differing phenotypes, some of which could survive extremely complex and uncertain alpine weather (Billings & Mooney, 1968; Marchand & Roach, 1980). The exact empirical evidence verifying this hypothesis cannot be possibly secured, but this view appears true, especially in terms of recent literature contemplating the encroachment of low-altitude species into the alpine vicinity as a consequence of global warming providing optimal survival conditions (e.g., Pauli et al., 1996; Gottfried et al., 2001; Parolo & Rossi, 2008; Walther et al., 2009).
Many theories have been proposed to explain the drivers behind evolution of seed selection and adaptation. Hubbell’s (2001) neutral theory of biodiversity assumes all individuals of all species are functionally equivalent, thus species abundance in a community is driven by the stochastic events such as dispersal, local extinction and speciation. This is in contrast to niche theory, which claims each species has its own traits and these independent species co-exist within a community (Coomes et al., 2002). Leishman and Murray (2001) argued that seeds of early successional species were often smaller with good dispersal capacities. Such seed traits were advantageous in evolution, as a higher number of seed increases the probability to find different climates and germinate on suitable microsite. However when succession proceeds, both small and large-seeded species become more common, possibly due to the ability to persist in soil seed banks (either influenced by environment or maternal affects). More recent studies have therefore rejected the prediction of neutral theory (McGill, 2003; Gilbert et al., 2006), indirectly supporting the overriding role of environment in selecting the phenotype.
It has been shown in Qing-Hai Tibet Plateau that early successional species were small but achieved high abundances, thus, seed size acted as a main determinant of species abundance (Chu et al., 2007). In addition, seed mass was negatively related to species density early in the succession and this relationship became insignificant with time, implying large-seeded species started colonizing in later successional stages. This idea has been reinforced by examining the community assembly changes in Qinhghai-Tibetan Plateau China, where abundant species in an early-successional meadow were shown to be small-seeded species (Zhang et al., 2012). However, the question as to what extent environment acted as a selective pressure and determined the evolutionary origin of alpine seeds along the successional gradient still remains unclear.
These studies, although few in number, offer insights suggesting that, owing to the advantage that species with smaller seeds have in entering seed banks (Funes et al., 1999; Cerabolini et al., 2003; Schwienbacher et al., 2010), most alpine species may benefit from the seed banks rather than dispersal. Furthermore, seeds in alpine soil remain frozen most of the year and therefore secondary dispersal could be limited during these periods. This feature essentially drives the seeds to establish a soil seed bank. Consequently, entry into a seed bank may be critically advantageous not only because little to no investment for separate structures is required but also because the low soil temperature ensuring increased longevity of seeds (McGraw et al., 1991). We therefore suggest that most alpine seeds have an increased tendency to be found in a seed bank in the model developed by Chambers and MacMahon (1994).
This review has thus far dealt with various factors that influence the entry and exit of seeds into alpine seed banks but all of these bar the external environment are strongly affected by the phylogeny of the species themselves. The relatedness of taxa in alpine systems therefore has the potential to explain some of the variation in seed size and morphology, dispersal strategy, dormancy mechanisms and response to germination cues, all of which affect the composition of seed banks. Links between phylogeny and traits affecting seed bank membership have been made in temperate systems (Mazer, 1989; Leishman et al., 1995), tropical communities (Norden et al., 2009) and alpine/subalpine vegetation (Bu et al., 2007a, 2008; Xu et al., 2014; Zhang et al., 2004). More generally, Finch-Savage and Leubner-Metzger (2006) highlight the common evolutionary path for dormancy that means it is present throughout the higher plant families with only a few exceptions, and in all major climate zones. However, associations between phylogenetic groupings and germinability (measured as germination percentage, germination time, or both) whilst being significant, are very variable and models point to a number of sometimes unidentified alternative explanators. For example, Bu et al. (2008) could explain only 12 % of the variation of germination time in a one way ANOVA by using phylogeny, whilst Bu et al. (2007a, 2007b) attributed 65.4 % of germinability variation to genus-level groupings using GLMs that also included habitat, a non-significant explanatory variable. In addition to these ambiguous results, there is a geographic focus to the plateau communities of central Asia and until researchers in other regions emulate their efforts, no generalities can be made.
Conclusions/Future Research Needs
Understanding how alpine seeds establish seed banks is of extensive ecological and practical importance, because changes induced by global warming directly affect seed regeneration potential, thus influencing the floristic changes of alpine environments. Our ecological understanding of seed persistence in alpine ecosystems is still in its infancy, although seed persistence in soil seed banks is clearly an ecologically important trait for most alpine species. It is therefore not surprising that most of our discussion stems from studies addressing seed adaptation in alpine environment, rather than seed bank studies per se. Many areas have received only sporadic attention and data from those studies can be subjected to criticism for their disagreement in results and also due to bias in sampling. For example, a handful of studies (e.g., Schwienbacher et al., 2011) have stored seeds at a condition that would benefit the physiological changes favoring dormancy alleviation and progress the seeds towards germination before being used in original experiments. Needless to say, such shortcomings must to be avoided in order to precisely understand the effects of maternal environments imposed on seed development. In addition, we have identified several areas that require future research with emphasis on the following:
-
(1)
The inherited traits of dormancy, germination temperature and light requirement could be affected to some extent by the maternal environment, which exert a selective pressure in developing physiological mechanisms that are location-specific. The effect of maternal climate affecting some of the adapted traits clearly suggests that these traits are likely to be altered in a future warming climate. Evidence for these patterns is already becoming apparent in some of the alpine locations. However, much remains to be studied.
-
(2)
It is suggested that climatic conditions in the growing season only partly controls seed carry-over. Because of specific germination temperatures required for each species present in a population, some temperatures preclude germination in some species and carry the seeds forward; the same temperature would initiate germination in other species. Since not all dormant and small seeded species enter persistence seed banks, there is a possibility that some other (physiological) factors are also involved in persistence under alpine climate. In addition to finding such factors, if any, contributing persistence, we need more rigorous appraisal of seed characteristics in relation to the environment. These approaches should also take inter alia inter-annual and inter-population variability into account. Thus, more specific studies delineating the point at which seeds bury in the model (Fig. 1) are needed.
-
(3)
Driving forces selecting seeds for seed banks must be evaluated in detail. Seed loss by germination and dispersal should be documented clearly. More especially, knowledge on dispersal is important in spatial demography studies particularly on soil seed banks. Many studies have shown vegetation of alpine regions is patchy possibly due to limited dispersal. LDD rarely occurs in alpine regions. If seeds do migrate to long-distance, habitat-dependent germination requirement are not met often, thereby seeds either germinate asynchronously to the dispersed environment or remain in soil for long time.
-
(4)
While a limited number of recent studies mostly on Qinghai-Tibet plateau, China, have focused on dispersal structure, little –if any- information exists on communities from other locations. This limited data on seed dispersal structure and dispersal distance dataset clearly underpins the need for more community level studies, paying particular attention to altitudinal and climatic influence.
-
(5)
Further phylogenetic investigations are needed to tease out the relationships between the key traits affecting the entrance to, and persistence in, the alpine seed bank and evolutionary origins of various responses to alpine environments. Furthermore, research in many more alpine regions other than central Asia is needed. Phylogenetic explanators of seed bank membership, if they exist, would allow more insightful predictions of responses to environmental change where species composition of the above- and below-ground communities is known, but responses of seed germination under warming conditions is untested.
-
(6)
A critical understanding of the germination requirements of local and invading species is essential. Many species probably survive changing climate by migrating to the locations where the originally adapted-for conditions still prevail (Hughes, 2000; Walther et al., 2009). Climate change has now surpassed a threshold, inexorably forcing us to get a glimpse of types of plants that would survive in the future climate and in so doing, more meaningful conservation strategies can be devised. There is persuasive evidence that global warming will result in migration of low-altitude plants to alpine environments. The lower likelihood of alpine seeds reaching new environments highlight the need to recognize the interaction of encroached species with the endemic alpine seeds germinate from soil seed banks. In the future climate, these interactions essentially determine the species composition in alpine environment.
Insights into these areas will essentially help reveal the selective forces behind the seed adaptation/selection in alpine environment. Such results also would provide more understanding on how warming climate affects alpine seed banks.
Literature Cited
Acharya, S. N. 1989. Germination response of two alpine grasses from the Rocky Mountains of Alberta. Canadian Journal of Plant Science 69: 1165–1177.
Adzhiev, R., V. Onipchenko & D. Tekeev. 2011. Viability of buried plant seeds from alpine plant communities (Northwest Caucasus): results of a five year experiment. Zhurnal obshchei biologii 73: 453–458. Abstract in English.
Allen, P. S. & S. E. Meyer. 1998. Ecological aspects of seed dormancy loss. Seed Science Research 8: 183–192.
Amen, R. D. 1965. Seed dormancy in the alpine rush, Luzula Spicata L. Ecology 46: 361–364.
——— 1966. The extent and role of seed dormancy in alpine plants. Q. Rev. Biol. 41: 271–281.
——— & E. K. Bonde. 1964. Dormancy and germination in alpine Carex from the Colorado front range. Ecology 45: 881–884.
Arroyo, M. T. K., L. A. Cavieres, C. Castor & A. M. Humaña. 1999. Persistent soil seed bank and standing vegetation at a high alpine site in the central Chilean Andes. Oecologia 119: 126–132.
———, ——— & A. M. Humaña. 2004. Experimental evidence of potential for persistent seed bank formation at a subantarctic alpine site in Tierra del Fuego, Chile. Annals of the Missouri Botanical Garden 91: 357–365.
Baker, H. G. 1972. Seed weight in relation to environmental conditions in California. Ecology 53: 997–1010.
Bakker, J., P. Poschlod, R. Strykstra, R. Bekker & K. Thompson. 1996. Seed banks and seed dispersal: important topics in restoration ecology. Acta Botanica Neerlandica 45: 461–490.
Baskin, C. C. & J. M. Baskin. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Pr.
———, O. Zackrisson & J. M. Baskin. 2002. Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp. American Journal of Botany 89: 486–493.
Baskin, J. M. & C. C. Baskin. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16.
Bekker, R., J. Bakker, U. Grandin, R. Kalamees, P. Milberg, P. Poschlod, K. Thompson & J. Willems. 1998. Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Functional Ecology 12: 834–842.
Bell, K. L. & L. Bliss. 1980. Plant reproduction in a high arctic environment. Arctic and Alpine Research 12: 1–10.
Bewley, J. D., K. . Bradford, H. W. Hilhorst, & H. Nonogaki 2013. Environmental Regulation of Dormancy and Germination. (3rd edition), Springer.
Billings, W. 1974. Adaptations and origins of alpine plants. Arctic and Alpine Research 6: 129–142.
——— & L. Bliss. 1959. An alpine snowbank environment and its effects on vegetation, plant development, and productivity. Ecology 40: 388–397.
——— & H. A. Mooney. 1968. The ecology of arctic and alpine plants. Biological Reviews 43: 481–529.
Blionis, G. J. & D. Vokou. 2005. Reproductive attributes of Campanula populations from Mt. Olympos, Greece. Plant Ecology 178: 77–88.
Bliss, L. 1958. Seed germination in arctic and alpine species. Arctic 11: 180–188.
Brändel, M. & W. Schütz. 2005. Temperature effects on dormancy levels and germination in temperate forest sedges (Carex). Plant Ecology 176: 245–261.
Bruun, H. H., R. Lundgren & M. Philipp. 2008. Enhancement of local species richness in tundra by seed dispersal through guts of muskox and barnacle goose. Oecologia 155: 101–110.
Bu, H., X. Chen, Y. Wang, X. Xu, K. Liu & G. Du. 2007a. Germination time, other plant traits and phylogeny in an alpine meadow on the eastern Qinghai-Tibet Plateau. Community Ecology 8: 221–227.
———, ———, X. Xu, K. Liu, P. Jia & G. Du. 2007b. Seed mass and germination in an alpine meadow on the eastern Tsinghai–Tibet plateau. Plant Ecology 191: 127–149.
———, G. Du, X. Chen, X. Xu, K. Liu & S. Wen. 2008. Community-wide germination strategies in an alpine meadow on the eastern Qinghai-Tibet plateau: phylogenetic and life-history correlates. Plant Ecology 195: 87–98.
———, G. Z. Du, X. L. Chen, Y. Wang, X. L. Xu & K. Liu. 2009. The evolutionary significance of seed germinability in an alpine meadow on the eastern Qinghai-Tibet Plateau. Arctic, Antarctic, and Alpine Research 41: 97–102.
Bullock, J., B. C. Hill, J. Silvertown & M. Sutton. 1995. Gap colonization as a source of grassland community change: effects of gap size and grazing on the rate and mode of colonization by different species. Oikos 72: 273–282.
Cain, M. L., B. G. Milligan & A. E. Strand. 2000. Long-distance seed dispersal in plant populations. American Journal of Botany 87: 1217–1227.
Carasso, V., A. Fusconi, F. R. Hay, S. Dho, B. Gallino & M. Mucciarelli. 2012. A threatened alpine species, Fritillaria tubiformis subsp moggridgei: Seed morphology and temperature regulation of embryo growth. Plant Biosystems 146: 74–83.
Cavieres, L. A. 1999. Persistent seed banks: delayed seed germination models and their application to alpine environments. Revista Chilena De Historia Natural 72: 457–466.
——— & M. T. Arroyo. 2000. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae)–altitudinal variation in the Mediterranean Andes of central Chile. Plant Ecology 149: 1–8.
——— & M. T. K. Arroyo. 2001. Persistent soil seed banks in Phacelia secunda (Hydrophyllaceae): experimental detection of variation along an altitudinal gradient in the Andes of central Chile (33 degrees S). Journal of Ecology 89: 31–39.
Celedon-Neghme, C., L. A. San Martin, P. F. Victoriano & L. A. Cavieres. 2008. Legitimate seed dispersal by lizards in an alpine habitat: The case of Berberis empetrifolia (Berberidaceae) dispersed by Liolaemus belii (Tropiduridae). Acta oecologica 33: 265–271.
Cerabolini, B., R. M. Ceriani, M. Caccianiga, R. D. Andreis & B. Raimondi. 2003. Seed size, shape and persistence in soil: a test on Italian flora from Alps to Mediterranean coasts. Seed Science Research 13: 75–85.
Chambers, J. C., J. A. MacMahon & J. H. Haefner. 1991. Seed entrapment in alpine ecosystems: effects of soil particle size and diaspore morphology. Ecology 72: 1668–1677.
——— & ———. 1994. A day in the life of a seed: movements and fates of seeds and their implications for natural and managed systems. Annual Review of Ecology and Systematics 25: 263–292.
——— 1995. Disturbance, life history strategies, and seed fates in alpine herbfield communities. American Journal of Botany 82: 421–433.
——— 2000. Seed movements and seedling fates in disturbed sagebrush steppe ecosystems: implications for restoration. Ecological Applications 10: 1400–1413.
Chu, C. J., Y. S. Wang, G. Z. Du, F. T. Maestre, Y.-J. Luo & G. Wang. 2007. On the balance between niche and neutral processes as drivers of community structure along a successional gradient: insights from alpine and sub-alpine meadow communities. Annals of Botany 100: 807–812.
Clebsch, E. E. & W. Billings. 1976. Seed germination and vivipary from a latitudinal series of populations of the arctic-alpine grass Trisetum spicatum. Arctic and Alpine Research 8: 255–262.
Coomes, D. A., M. Rees, P. J. Grubb & L. Turnbull. 2002. Are differences in seed mass among species important in structuring plant communities? Evidence from analyses of spatial and temporal variation in dune‐annual populations. Oikos 96: 421–432.
Dainese, M. & T. Sitzia. 2013. Assessing the influence of environmental gradients on seed mass variation in mountain grasslands using a spatial phylogenetic filtering approach. Perspectives in Plant Ecology, Evolution and Systematics 15: 12–19.
Danvind, M. & C. Nilsson. 1997. Seed floating ability and distribution of alpine plants along a northern Swedish river. Journal of Vegetation Science 8: 271–276.
Dar, A. R., Z. Reshi & G. H. Dar. 2009. Germination studies on three critically endangered endemic angiosperm species of the Kashmir Himalaya, India. Plant Ecology 200: 105–115.
Densmore, R. & J. Zasada. 1983. Seed dispersal and dormancy patterns in northern willows: ecological and evolutionary significance. Canadian Journal of Botany 61: 3207–3216.
——— 1997. Effect of day length on germination of seeds collected in Alaska. American Journal of Botany 84: 274–274.
Dorne, A. J. 1981. Variation in seed germination inhibition of Chenopodium bonus-henricus in relation to altitude of plant growth. Canadian Journal of Botany 59: 1893–1901.
Elkington, T. 1971. Biological flora of the British Isles: Dryas Octopetala L. Journal of Ecology 59: 887–905.
Fenner, M. & K. Thompson. 2005. The ecology of seeds. Cambridge Univ Pr.
Fernandez-Pascual, E., B. Jimenez-Alfaro, A. I. Garcia-Torrico, F. Perez-Garcia & T. E. Diaz. 2012. Germination ecology of the perennial Centaurium somedanum, a specialist species of mountain springs. Seed Science Research 22: 199–205.
Finch-Savage, W. E. & G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Physiologist 171: 501–523.
Forbis, T. A. 2003. Seedling demography in an alpine ecosystem. American Journal of Botany 90: 1197–1206.
——— & P. K. Diggle. 2001. Subnivean embryo development in the alpine herb Caltha leptosepala (Ranunculaceae). Canadian Journal of Botany 79: 635–642.
Frei, E. S., J. F. Scheepens & J. Stoecklin. 2012. Dispersal and microsite limitation of a rare alpine plant. Plant Ecology 213: 395–406.
Funes, G., S. Basconcelo, S. Díaz & M. Cabido. 1999. Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Science Research 9: 341–345.
Garnock‐Jones, P. J. & D. G. Lloyd. 2004. A taxonomic revision of Parahebe (Plantaginaceae) in New Zealand. New Zealand Journal of Botany 42: 181–232.
Gilbert, B., W. F. Laurance, E. G. Leigh Jr & H. E. Nascimento 2006. Can Neutral Theory Predict the Responses of Amazonian Tree Communities to Forest Fragmentation?. The American Naturalist 168: 304–317.
Giménez-Benavides, L., A. Escudero & F. Pérez-García. 2005. Seed germination of high mountain Mediterranean species: altitudinal, interpopulation and interannual variability. Ecological Research 20: 433–444.
Glaser, P. H. 1981. Transport and deposition of leaves and seeds on tundra: a late-glacial analog. Arctic and Alpine Research 13: 173–182.
Gottfried, M., H. Pauli, K. Reiter & G. Grabherr. 2001. A fine‐scaled predictive model for changes in species distribution patterns of high mountain plants induced by climate warming. Diversity and Distributions 5: 241–251.
———, ———, A. Futschik, M. Akhalkatsi, P. Barancok, J. L. Benito Alonso, G. Coldea, J. Dick, B. Erschbamer, M. R. Fernandez Calzado, G. Kazakis, J. Krajci, P. Larsson, M. Mallaun, O. Michelsen, D. Moiseev, P. Moiseev, U. Molau, A. Merzouki, L. Nagy, G. Nakhutsrishvili, B. Pedersen, G. Pelino, M. Puscas, G. Rossi, A. Stanisci, J.-P. Theurillat, M. Tomaselli, L. Villar, P. Vittoz, I. Vogiatzakis & G. Grabherr. 2012. Continent-wide response of mountain vegetation to climate change. Nature Clim. Change 2: 111–115.
Graae, B. J., I. G. Alsos & R. Ejrnaes. 2008. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecology 198: 275–284.
Greene, D. & E. Johnson. 1997. Secondary dispersal of tree seeds on snow. Journal of Ecology 85: 329–340.
Güleryüz, G., S. Kırmızı, H. Arslan & F. S. Sakar. 2011. Dormancy and germination in Stachys germanica L. subsp. bithynica (Boiss.) Bhattacharjee seeds: Effects of short-time moist chilling and plant growth regulators. Flora-Morphology, Distribution, Functional Ecology of Plants 206: 943–948.
Guo, H., S. J. Mazer & G. Du. 2010. Geographic variation in seed mass within and among nine species of Pedicularis (Orobanchaceae): effects of elevation, plant size and seed number per fruit. Journal of Ecology 98: 1232–1242.
Haggas, L., R. W. Brown & R. S. Johnston. 1987. Light requirement for seed germination of Payson sedge. Journal of range management 40: 180–184.
Heide, O. M. & Y. Gauslaa. 1999. Developmental strategies of Koenigia islandica, a high-arctic annual plant. Ecography 22: 637–642.
Hölzel, N. & A. Otte. 2004. Ecological significance of seed germination characteristics in flood-meadow species. Flora-Morphology, Distribution, Functional Ecology of Plants 199: 12–24.
Hu, X., T. Li, J. Wang, Y. Wang, C. C. Baskin & J. M. Baskin. 2013. Seed dormancy in four Tibetan Plateau Vicia species and characterization of physiological changes in response of seeds to environmental factors. Seed Science Research 23: 133–140.
Hubbell, S. P. 2001. The unified neutral theory of biodiversity and biogeography (MPB-32). Princeton University Press.
Hughes, L. 2000. Biological consequences of global warming: is the signal already apparent? Trends in ecology & evolution 15: 56–61.
Jakobsson, A. & O. Eriksson. 2000. A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos 88: 494–502.
Jankowska-Blaszczuk, M. & M. Daws. 2007. Impact of red: far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Functional Ecology 21: 1055–1062.
Jurado, E. & J. Flores. 2005. Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16: 559–564.
Kaye, T. N. 1997. Seed dormancy in high elevation plants: implications for ecology and restoration. Pp 115–120. In: T. N. Kaye, A. Liston, R. Love, D. Luoma, R. Meinke, & M. Wilson (eds). Conservation and management of native Plants and Fungi. Native Plant Society of Oregon, Corvallis.
Kibe, T. & T. Masuzawa. 1994. Seed germination and seedling growth of Carex doenitzii growing on alpine zone of Mt. Fuji. Journal of Plant Research 107: 23–27.
Klug-Pümpel, B. & G. Scharfetter-Lehrl. 2008. Soil diaspore reserves above the timberline in the Austrian Alps. Flora-Morphology, Distribution, Functional Ecology of Plants 203: 292–303.
Komatsu, T., T. Itino & S. Ueda. 2014. First report of seed dispersal by ants in Dicentra peregrina (Papaveraceae), an alpine plant in the Japanese Alps. Entomological Science. doi:10.1111/ens.12110.
Körner, C. 1999. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, New York.
——— 2003. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer Verlag
Larsen, H. & K. C. Burns. 2012. Seed dispersal effectiveness increases with body size in New Zealand alpine scree weta (Deinacrida connectens). Austral Ecology 37: 800–806.
Leishman, M. & M. Westoby. 1998. Seed size and shape are not related to persistence in soil in Australia in the same way as in Britain. Functional Ecology 12: 480–485.
———, M. Westoby & E. Jurado. 1995. Correlates of seed size variation: a comparison among five temperate floras. Journal of Ecology 83: 517–530.
———, I. J. Wright, A. T. Moles & Westoby, M. 2000 The evolutionary ecology of seed size. pp 31–57 in Fenner, M. (Ed) Seeds: the ecology of regeneration in plant communities, CAB International.
——— & B. R. Murray. 2001. The relationship between seed size and abundance in plant communities: model predictions and observed patterns. Oikos 94: 151–161.
Liebst, B. & J. Schneller. 2008. Seed dormancy and germination behaviour in two Euphrasia species (Orobanchaceae) occurring in the Swiss Alps. Botanical Journal of the Linnean Society 156: 649–656.
Lindgren, Å., O. Eriksson & J. Moen. 2007. The impact of disturbance and seed availability on germination of alpine vegetation in the Scandinavian mountains. Arctic, Antarctic, and Alpine Research 39: 449–454.
Liu, K., J. M. Baskin, C. C. Baskin, H. Bu, M. Liu, W. Liu & G. Du. 2011. Effect of storage conditions on germination of seeds of 489 species from high elevation grasslands of the eastern Tibet Plateau and some implications for climate change. American Journal of Botany 98: 12–19.
———, ———, ———, ———, G. Du & M. Ma. 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the Eastern Tibet Plateau. PloS one 8: e69364.
Lorite, J., A. M. Ruiz-girel & J. Castro. 2007. Patterns of seed germination in Mediterranean mountains: study on 37 endemic or rare species from Sierra Nevada, SE Spain. Candollea 62(1): 1–12.
Lucas-Borja, M. E., T. F. Fonseca, J. L. Lousada, P. Silva-Santos, E. Martinez Garcia & M. Andres Abellan. 2012. Natural regeneration of Spanish black pine Pinus nigra Arn. ssp salzmannii (Dunal) Franco at contrasting altitudes in a Mediterranean mountain area. Ecological Research 27: 913–921.
Lütz, C. 2011. Plants in Alpine Regions: Cell Physiology of Adaption and Survival Strategies. Springer.
Ma, M., X. Zhou & G. Du. 2010a. Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora-Morphology, Distribution, Functional Ecology of Plants 205: 128–134.
———, ———, G. Wang, Z. Ma & G. Du. 2010b. Seasonal dynamics in alpine meadow seed banksalong an altitudinal gradient on the Tibetan Plateau. Plant and soil 336: 291–302.
———, ——— & G. Du. 2011. Soil seed bank dynamics in alpine wetland succession on the Tibetan Plateau. Plant and soil 346: 1–10.
———, ———, W. Qi, K. Liu, P. Jia & G. Du. 2013. Seasonal dynamics of the plant community and soil seed bank along a successional gradient in a subalpine meadow on the Tibetan Plateau. PloS one 8: e80220.
Marcante, S., A. Sierra‐Almeida, J. P. Spindelböck, B. Erschbamer & G. Neuner. 2012. Frost as a limiting factor for recruitment and establishment of early development stages in an alpine glacier foreland? Journal of Vegetation Science 23: 858–868.
Marchand, P. J. & D. A. Roach. 1980. Reproductive strategies of pioneering alpine species: seed production, dispersal, and germination. Arctic and Alpine Research 12: 137–146.
Mariko, S., H. Koizumi, J.-I. Suzuki & A. Furukawa. 1993. Altitudinal variations in germination and growth responses of Reynoutria japonica populations on Mt Fuji to a controlled thermal environment. Ecological Research 8: 27–34.
Matlack, G. 1989. Secondary dispersal of seed across snow in Betula lenta, a gap-colonizing tree species. The Journal of Ecology 77: 853–869.
Mazer, S. J. 1989. Ecological, taxonomic and life history correlates of seed mass among Indiana dune angiosperms. Ecological Monograph 59: 153–175.
McDonough, W. T. 1970. Germination of 21 species collected from a high-elevation rangeland in Utah. American Midland Naturalist 84: 551–554.
McGill, B. J. 2003. A test of the unified neutral theory of biodiversity. Nature 422: 881–885.
McGraw, J., M. Vavrek & C. Bennington. 1991. Ecological genetic variation in seed banks I. Establishment of a time transect. The Journal of Ecology 79: 617–625.
——— & M. C. Vavrek 1989. The Role of Buried Viable Seeds in Arctic and Alpine plant communities in Leck, M.A., Parker, V.T. and Simpson, R.L. (Eds) Ecology of soil seed banks, Academic Press.
Meyer, S., S. Kitchen & S. Carlson. 1995. Seed germination timing patterns in intermountain Penstemon (Scrophulariaceae). American Journal of Botany 82: 377–389.
Meyer, S. E. & S. B. Monsen. 1991. Habitat-correlated variation in mountain big sagebrush (Artemisia tridentata ssp. vaseyana) seed germination patterns. Ecology 72: 739–742.
——— & S. G. Kitchen. 1994a. Life history variation in blue flax (Linum perenne: Linaceae): seed germination phenology. American Journal of Botany 81: 528–535.
——— & ———. 1994b. Habitat-correlated variation in seed germination response to chilling in Penstemon Section Glabri (Scrophulariaceae). American Midland Naturalist 132: 349–365.
Milberg, P., L. Andersson & K. Thompson. 2000. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Science Research 10: 99–104.
Molau, U. & E. L. Larsson. 2000. Seed rain and seed bank along an alpine altitudinal gradient in Swedish Lapland. Canadian Journal of Botany 78: 728–747.
Moles, A. T., D. W. Hodson & C. J. Webb. 2000. Seed size and shape and persistence in the soil in the New Zealand flora. Oikos 89: 541–545.
——— & M. Westoby. 2004. Seedling survival and seed size: a synthesis of the literature. Journal of Ecology 92: 372–383.
Mondoni, A., M. I. Daws, J. Belotti & G. Rossi. 2009. Germination requirements of the alpine endemic Silene elisabethae Jan: effects of cold stratification, light and GA(3). Seed Science and technology 37: 79–87.
———, G. Rossi, S. Orsenigo & R. J. Probert. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–164.
Mooney, H. A. & W. Billings. 1961. Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecological Monographs 31: 1–29.
Muñoz, A. A. & M. T. K. Arroyo. 2002. Postdispersal seed predation on Sisyrinchium arenarium (Iridaceae) at two elevations in the central Chilean Andes. Arctic, Antarctic, and Alpine Research 34: 178–184.
——— & L. A. Cavieres. 2006. A multi-species assessment of post-dispersal seed predation in the central Chilean Andes. Annals of Botany 98: 193–201.
Nishitani, S. & T. Masuzawa. 1996. Germination characteristics of two species of Polygonum in relation to their altitudinal distribution on Mt. Fuji, Japan. Arctic and Alpine Research 28: 104–110.
Norden, N., M. I. Daws, C. Antoine, M. A. Gonzalez, N. C. Garwood & J. Chave. 2009. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Functional Ecology 23: 203–210.
Novak, J., C. Wawrosch, C. Schmiderer, C. M. Franz & B. Kopp. 2011. Germination responses of Peucedanum ostruthium (Apiaceae) to genotype, light, temperature and gibberellic acid. Seed Science and technology 39: 552–558.
Ozinga, W. A., R. M. Bekker, J. H. J. Schaminee & J. M. Van Groenendael. 2004. Dispersal potential in plant communities depends on environmental conditions. Journal of Ecology 92: 767–777.
Parolo, G. & G. Rossi. 2008. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology 9: 100–107.
Pauli, H., M. Gottfried & G. Grabherr. 1996. Effects of climate change on mountain ecosystems–upward shifting of alpine plants. World Resource Review 8: 382–390.
———, ——— & ——— (eds). 2001. High summits of the Alps in a changing climate. Kluwer Academic/Plenum Publ, New York.
Peco, B., J. Traba, C. Levassor, A. M. Sánchez & F. M. Azcárate. 2003. Seed size, shape and persistence in dry Mediterranean grass and scrublands. Seed Science Research 13: 87–95.
Pluess, A., W. Schütz & J. Stöcklin. 2005. Seed weight increases with altitude in the Swiss Alps between related species but not among populations of individual species. Oecologia 144: 55–61.
Pons, T. L. & M. Fenner 2000 Seed responses to light. pp 237–260 in Fenner, M. (Ed) Seeds: the ecology of regeneration in plant communities, CABI
Pufal, G., K. G. Ryan & P. Garnock-Jones. 2010. Hygrochastic capsule dehiscence in New Zealand alpine veronica (Plantaginaceae). American Journal of Botany 97: 1413–1423.
Rees, M. & M. J. Long. 1992. Germination biology and the ecology of annual plants. American Naturalist 139: 484–508.
——— 1996. Evolutionary ecology of seed dormancy and seed size. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 351: 1299–1308.
Reynolds, D. N. 1984. Alpine annual plants: phenology, germination, photosynthesis, and growth of three Rocky Mountain species. Ecology 65: 759–766.
Royal Botanic Gardens Kew Seed Information Database (SID). Seed Information Database (SID). Version 7.1. Available from: http://data.kew.org/sid/ (March 2014).
Sayers, R. L. & R. T. Ward. 1966. Germination responses in alpine species. Botanical Gazette 127: 11–16.
Scherff, E., C. Galen & M. Stanton. 1994. Seed dispersal, seedling survival and habitat affinity in a snowbed plant: limits to the distribution of the snow buttercup, Ranunculus adoneus. Oikos 69: 405–413.
Schütz, W. & G. Rave. 1999. The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecology 144: 215–230.
——— 2002. Dormancy characteristics and germination timing in two alpine Carex species. Basic and Applied Ecology 3: 125–134.
Schwienbacher, E., S. Marcante & B. Erschbamer. 2010. Alpine species seed longevity in the soil in relation to seed size and shape-A 5-year burial experiment in the Central Alps. Flora-Morphology, Distribution, Functional Ecology of Plants 205: 19–25.
———, J. A. Navarro-Cano, G. Neuner & B. Erschbamer. 2011. Seed dormancy in alpine species. Flora-Morphology, Distribution, Functional Ecology of Plants 206: 845–856.
Shimono, Y. & G. Kudo. 2003. Intraspecific variations in seedling emergence and survival of Potentilla matsumurae (Rosaceae) between alpine fellfield and snowbed habitats. Annals of Botany 91: 21–29.
——— & ———. 2005. Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecological Research 20: 189–197.
Sommerville, K. D., A. J. Martyn & C. A. Offord. 2013. Can seed characteristics or species distribution be used to predict the stratification requirements of herbs in the Australian Alps? Botanical Journal of the Linnean Society 172: 187–204.
Stinson, K. A. 2004. Natural selection favors rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. American Journal of Botany 91: 531–539.
Stöcklin, J. & E. Bäumler. 1996. Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. Journal of Vegetation Science 7: 45–56.
Tackenberg, O., P. Poschlod & S. Bonn. 2003. Assessment of wind dispersal potential in plant species. Ecological Monographs 73: 191–205.
——— & J. Stöcklin. 2008. Wind dispersal of alpine plant species: a comparison with lowland species. Journal of Vegetation Science 19: 109–118.
Takahashi, K. & Y. Miyajima. 2010. Effects of roads on alpine and subalpine plant species distribution along an altitudinal gradient on Mount Norikura, central Japan. Journal of Plant Research 123: 741–749.
Thompson, K. 1987. Seeds and seed banks. New Phytologist 106: 23–34.
———, S. Band & J. Hodgson. 1993. Seed size and shape predict persistence in soil. Functional Ecology 7: 236–241.
———, J. P. Bakker & R.M. Bekker. 1997. The soil seed banks of North West Europe: methodology, density and longevity. Cambridge Univ Pr.
——— 2000 The functional ecology of soil seed banks. pp 215–235 in Fenner, M. (Ed) Seeds: the ecology of regeneration in plant communities, CABI
———, A. Jalili, J. G. Hodgson, B. Hamzeh’ee, Y. Asri, S. Shaw, A. Shirvany, S. Yazdani, M. Khoshnevis & F. Zarrinkamar. 2001. Seed size, shape and persistence in the soil in an Iranian flora. Seed Science Research 11: 345–356.
———, R. M. Ceriani, J. P. Bakker & R. M. Bekker. 2003. Are seed dormancy and persistence in soil related? Seed Science Research 13: 97–100.
Van der Pijl, L. 1982. Principles of seed dispersal in higher plants. Principles of seed dispersal in higher plants.
Vera, M. 1997. Effects of altitude and seed size on germination and seedling survival of heathland plants in north Spain. Plant Ecology 133: 101–106.
Vleeshouwers, L., H. Bouwmeester & C. Karssen. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037.
Wagner, I. & A. M. Simons. 2008. Intraspecific divergence in seed germination traits between high- and low-latitude populations of the arctic-alpine annual Koenigia islandica. Arctic Antarctic and Alpine Research 40: 233–239.
——— & ———. 2009. Divergence in germination traits among arctic and alpine populations of Koenigia islandica: light requirements. Plant Ecology 204: 145–153.
Wagner, J. & E. Mitterhofer. 1998. Phenology, seed development, and reproductive success of an alpine population of Gentianella germanica in climatically varying years. Botanica Acta 111: 159–166.
Walther, G. R., E. Post, P. Convey, A. Menzel, C. Parmesan, T. J. C. Beebee, J. M. Fromentin, O. Hoegh-Guldberg & F. Bairlein. 2002. Ecological responses to recent climate change. Nature 416: 389–395.
———, S. Beißner & C. A. Burga. 2009. Trends in the upward shift of alpine plants. Journal of Vegetation Science 16: 541–548.
Wang, B., G. Wang & J. Chen. 2012. Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecology 213: 1329–1336.
Ware, C., D. M. Bergstrom, E. Muller & I. G. Alsos. 2012. Humans introduce viable seeds to the Arctic on footwear. Biological Invasions 14: 567–577.
Weiblen, G. D. & J. D. Thomson. 1995. Seed dispersal in Erythronium grandiflorum (Liliaceae). Oecologia 102: 211–219.
Welling, P. & K. Laine. 2000. Characteristics of the seedling flora in alpine vegetation, subarctic Finland, I. Seedling densities in 15 plant communities. Helsinki: Societas Biologica Fennica 37: 69–76.
———, A. Tolvanen & K. Laine. 2004. The alpine soil seed bank in relation to field seedlings and standing vegetation in subarctic Finland. Arctic, Antarctic, and Alpine Research 36: 229–238.
Whinam, J. & N. Chilcott. 1999. Impacts of trampling on alpine environments in central tasmania. Journal of Environmental Management 12: 205–220.
Williams, R. 1987. Patterns of air temperature and accumulation of snow in subalpine heathlands and grasslands on the Bogong High Plains, Victoria. Australian journal of ecology 12: 153–163.
Willson, M. F., B. Rice & M. Westoby. 1990. Seed dispersal spectra: a comparison of temperate plant communities. Journal of Vegetation Science 1: 547–562.
Wu, G.-L., G.-Z. Du & Z.-H. Shi. 2013. Germination strategies of 20 alpine species with varying seed mass and light availability. Australian Journal of Botany 61: 404–411.
Young, L. M., D. Kelly & X. J. Nelson. 2012. Alpine flora may depend on declining frugivorous parrot for seed dispersal. Biological Conservation 147: 133–142.
Xu, J., W. Li, C. Zhang, W. Liu & G. Du. 2014. Variation in seed germination of 134 common species on the Eastern Tibetan plateau: phylogenetic, life history and environmental correlates. Plos one 9: e98601.
Yu, S., M. Sternberg, P. Kutiel & H. Chen. 2007. Seed mass, shape, and persistence in the soil seed bank of Israeli coastal sand dune flora. Evolutionary Ecology Research 9: 325–340.
Yu, X., C. Xu, F. Wang, Z. Shang & R. Long. 2012. Recovery and germinability of seeds ingested by yaks and Tibetan sheep could have important effects on the population dynamics of alpine meadow plants on the Qinghai-Tibetan Plateau. Rangeland Journal 34: 249–255.
Zhang, H., B. Gilbert, X. Zhang & S. Zhou. 2012. Community assembly along a successional gradient in sub-alpine meadows of the Qinghai-Tibetan Plateau, China. Oikos 122: 952–960.
Zhang, S. T., G. Z. Du & J. K. Chen. 2004. Seed size in relation to phylogeny, growth form and longevity in a subalpine meadow on the East of the Tibetan plateau, China. Folia Geobotanica 39: 129–142.
Zhao, L. P., G. L. Wu & J. M. Cheng. 2011. Seed mass and shape are related to persistence in a sandy soil in northern China. Seed Science Research 21: 47–53.
Acknowledgments
We thank Prof. Carol C. Baskin, Prof. Mary A. Leck and two anonymous reviewers for critically reading and suggesting numerous improvements on an earlier version of this manuscript. It is a pleasure to thank Xiao Qun for her help in preparing Table 4 and verifying plant names throughout. We are indeed thankful to the financial support by NSFC (Grant No.51076108) and the program for Professor of Eastern Scholar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaganathan, G.K., Dalrymple, S.E. & Liu, B. Towards an Understanding of Factors Controlling Seed Bank Composition and Longevity in the Alpine Environment. Bot. Rev. 81, 70–103 (2015). https://doi.org/10.1007/s12229-014-9150-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-014-9150-2