Abstract
Savanna occurs in specific locations throughout the Indonesian archipelago, including some high rainfall regions. Little is known about its defining characteristics, such as structure, composition or diversity, and what these characteristics reveal about the origin and age of these savannas. At four locations in eastern Java (Baluran National Park & Alas Purwo National Park), Bali (Bali Barat National Park) and Lombok (Rinjani National Park), we used plots to record the abundance and cover of plant species and to measure local environmental parameters. MODIS burned-area product and field observations were used to obtain information on recent fires. We compared each savanna in terms of dominant species, species diversity and species richness. We also used ANOSIM to analyse the variation in community composition and canonical correspondence analysis to explore relationships between floristic and measured environmental factors. Our results showed there were distinct gradients in elevation (along with related climatic factors such as temperature and precipitation) and fire regime linked to floristic composition across the savannas of Java, Bali and Lombok Islands. Each savanna was characterized by a different set of woody and grass species, with invasive alien species, such as Acacia nilotica (syn. Vachellia nilotica), Lantana camara and Chromolaena odorata, being particularly important in differentiating between savannas. Characteristics of the Baluran savanna suggest that this ecosystem may be of considerable age, whereas the other savannas are likely to be maintained by regular fire. This study is the first study to describe more thoroughly the savanna plant community in the wetter parts of Indonesian archipelago and should serve as a valuable foundation for further studies on the Indonesian savannas and those of other parts of Southeast Asia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remarkably diverse tropical forest communities of Indonesia have been widely recognized for their importance to the world’s biodiversity and ecosystem services (Dossa et al. 2013; Blackie et al. 2014; Boedhihartono 2017). The structural and floristic variation of these Indonesian tropical forests have been extensively studied and related to variation in temperature, precipitation, seasonality of rainfall, edaphic conditions, topography, natural and anthropogenic disturbance, elevation, climate change, and invasive alien species (Harger 1995; van der Kaars and Dam 1997; Brearley et al. 2004; Widyatmoko and Burgman 2006; Sutomo et al. 2015). However, little is known about the savanna communities of Indonesia, and indeed right across Southeast Asia, despite such communities being common across this region (Ratnam et al. 2016). Savannas are tropical grass-dominated ecosystems with sparse to mid-dense woody plants, but throughout much of Indonesia, such vegetation would, based on climate alone, be expected to be forest (Bond and Keeley 2005; Scogings and Sankaran 2020).
Savanna in Indonesia is found in specific locations across the archipelago over a wide range of climate and soils (Whitten et al. 1996; Monk et al. 2000). The most well-known and studied savannas occur on the islands of East Nusa Tenggara (Indonesian: Nusa Tenggara Timur; NTT) in the driest eastern parts of the archipelago which have pronounced seasonal rainfall, such as West Timor, Sumba and Flores (Monk et al. 2000; Fisher et al. 2006; Russell-Smith and Edwards 2006; Tacconi and Ruchiat 2006). Species composition of savanna in NTT was studied by Auffenberg (1981) and he described them in terms of dominant woody species. Borassus flabellifer (Arecaceae/Palmae) dominated the tree layer of the savanna on Komodo Island, Rinca Island and the coast of Flores Island up to an elevation of about 400 m a.s.l. Ziziphus mauritiana was the dominant savanna tree growing from sea level to 500 m a.s.l. Other types of savanna are also commonly found in the eastern part of Indonesia (based on major tree species), namely Eucalyptus savanna in Timor and Casuarina savanna in Sumba and Timor (Goltenboth et al. 2006). However, detailed information on the occurrence and features of savanna in the wetter regions of Indonesia with less strongly seasonal rainfall, such as Java, Bali and Lombok, is scarce. Whitten et al. (1996) mention the existence of savanna in Baluran, East Java and Bali Barat in Bali; however, they did not describe them thoroughly.
Savanna ecosystems in Southeast Asia have long been regarded as anthropogenic, being derived from tropical forests and maintained via ongoing human manipulation, primarily clearing, grazing and/or burning (Stott 1990; Solbrig et al. 1996; Ratnam et al. 2011). This view has certainly been widely reported for Indonesian savannas (Whitten et al. 1996; Goltenboth et al. 2006). However, a recent review by Ratnam et al. (2016) questions this assumption and points to antiquity of some Southeast Asian savannas. Evidence for this comes from: (1) fossil history and phylogenetic data showing existence of savanna species of plants and animals in the region before humans; (2) dominance by species with adaptations to withstand repeated fire and/or grazing; and (3) climatic consistencies with savannas of other continents.
In this paper, we compare and contrast the vegetation characteristics of four savannas in the wetter part of the Indonesian archipelago (Java – Bali – Lombok) to address two important questions: (1) what environmental factors are linked to, and therefore may be driving, differences in floristic composition across these savannas; and (2) what do these vegetation features tell us about the origin, maintenance and age of these savannas? Given the wide geographic spread of study sites, we hypothesized that floristic gradients across these savannas would be primarily linked to elevation, precipitation and related micro-climatic differences, with disturbance type and frequency being of secondary importance.
Methods
Study sites
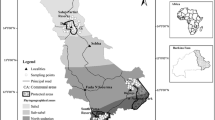
The study was conducted in four Indonesian savannas on Java, Lombok and Bali Islands. These were selected based on all the well-known localities of savanna ecosystems in this region as reported in the literature (Whitten et al. 1996). Two savannas were located in East Java (Baluran National Park and Alas Purwo National Park), one in Bali (Bali Barat National Park) and one in Lombok (Rinjani National Park; Fig. 1). Other savannas reported in the literature were visited but not studied here, as they were no longer savanna (e.g. Pangandaran Nature Reserve in West Java, now largely converted to secondary forest). Typical fire regimes and climate for each savanna studied are described in Table 1. In all of these savannas, the soils are of volcanic origin but differ in age.
Field sampling
Field sampling of vegetation follows standard plot-based approaches commonly used in floristic description and analysis (Kent 2011) and as applied to similar vegetation in the region (e.g. Brearley et al. 2004; Dossa et al. 2013). Between September to November 2014 (dry season), sampling plots (50 × 50 m) were randomly positioned at ten sites in each of the four savanna areas (Java, Bali and Lombok Islands; 40 study sites in total); they were spaced at least 200 m apart to reduce likelihood of spatial auto-correlation. Inside each of the 50 × 50-m plots, we randomly placed four nested subplots of 5 × 5 m. Within the 50 × 50-m plot we identified and measured the cover, height and width of all woody species (trees and shrubs), and recorded the position of all tree species ≥ 10-cm stem diameter at 1.3 m (DBH). In the smaller nested subplots we identified all groundcover species (grasses, ferns and forbs) and estimated their cover (Hardjosuwarno, 1990); average cover across the four subplots was then calculated for each groundcover species. Plant identification was primarily done using the resources of the Hortus Botanicus Baliensis, Bali Botanical Garden, but also the Flora Malesiana (http://floramalesiana.org), PROSEA (Plant Resources of South East Asia; http://proseanet.org) and the Bioportal at Naturalis Biodiversity Centre, the Netherlands (http://bioportal.naturalis.nl). Nomenclature follows the Plant List (http://www.theplantlist.org) maintained by the Royal Botanic Gardens, Kew and Missouri Botanical Garden. We also recorded local environmental data for every plot, at a similar time of day (morning): soil pH and moisture (using portable meters made by Hanna Instruments), local microclimate (light intensity, air temperature, relative humidity, wind velocity and heat stress index, using Lutron light and weather meters) and topography (elevation and slope using a Garmin GPS device and a clinometer). The plot locations were cross-checked with fire-scar maps produced using the MODIS (moderate resolution imaging spectroradiometer) burned-area product (Roy et al. 2008; Sutomo and van Etten 2018) for the years 2000 to 2013 to obtain information on time since fire and fire frequency for each plot. Typical fire regime data for the savanna areas (Table 1) were generalizations based on these fire maps for each location, complemented by interviews with park rangers and field observations.
Data analysis
The importance value index or IVI (Kent 2011) was calculated for each species at each site and then averaged to understand the floristic dominance and plant community composition in each savanna. Species richness and the Shannon–Wiener species diversity and evenness indices (Magurran 2004) were also calculated for each site using the total number of species found within the 50 × 50-m plot (including the 5 × 5-m nested subplots). For the diversity and evenness indices, the relative abundance was based on species cover. Differences in mean values of vegetation parameters between savannas were tested for significance using ANOVA and Tukey’s b post-hoc tests in SPSS (v.23, IBM Corp., New York, USA). The species cover data at each site were square-root transformed prior to constructing a resemblance matrix based on the Bray–Curtis similarity measure (Clarke 1993). A non-metric multidimensional scaling (NMDS) ordination diagram was first generated based on the resemblance matrix. The compositional differences between savannas were then tested for significance using one-way ANOSIM (analysis of similarity; Clarke 1993). SIMPER (similarity percentage) analysis was then used to explore the relative contribution of individual species to the total dissimilarity among the savannas. These multivariate analyses were performed using PRIMER (version 6.0., PRIMER-E Ltd, Plymouth, UK). Correlations between floristic and local environmental gradients were explored using the BEST (Bio-Env+Stepwise) method (Clarke and Ainsworth 1993) in PRIMER, as well as canonical correspondence analysis (CCA) using CANOCO (version 5, Microcomputer Power, Ithaca, New York).
Results
Structure and dominant species
We discovered as many as 43 plant species within 26 families across the four savannas, including one fern, seven grass or grass-like plants, and two forbs (a full list of species, habit and their typical habitats are presented in Appendix 1). Each savanna has structural characteristics and dominant species that differentiate it from the others (Table 2). For instance, Alas Purwo is dominated by the grass Arundinella setosa, the small shrub Desmodium laxiflorum (Fabaceae) and the invasive alien species (IAS) Chromolaena odorata (Asteraceae) in the groundcover layer whereas the tree layer is dominated by Flacourtia rukam (Salicaceae). At Rinjani, the groundcover layer is dominated by the grass Imperata cylindrica and the fern Gleichenia microphylla whereas the tree layer is mainly composed of Engelhardia spicata (Juglandaceae). The ground layer of Bali Barat is also dominated by the Poaceae family (Calamagrostis australis), whilst the tree layer mainly consists of Borassus flabellifer (Arecaceae). In Baluran, the groundcover layer is characterized by Desmodium laxiflorum (Fabaceae), seedlings of Azadirachta indica (Meliaceae) and two grasses, Polytrias indica and Dichanthium caricosum, whereas the tree layer mainly consists of species such as Ziziphus mauritiana (Rhamnaceae) and the IAS Acacia nilotica (syn. Vachellia nilotica; Fabaceae). The presence of invasive alien species is notable in most of the savannas (Appendix 1) and strongly influences their overall structure and composition. Bali Barat and Alas Purwo face similar problems from the obnoxious invasive alien species Chromolaena odorata, while at Baluran, A. nilotica occurs widely in both the ground (i.e. seedlings and small saplings) and tree layers (Table 2).
Diversity measures
There are significant differences between the savannas in terms of mean Shannon–Wiener species diversity and species richness (Table 3). Rinjani has the highest mean species diversity compared to the others, while Alas Purwo has the lowest. There are no differences in species diversity between Baluran and Bali Barat. Significant differences were detected in the mean species richness of savannas, namely between Alas Purwo and Baluran, and between Rinjani and Bali Barat (Table 3). Bali Barat has the highest species richness compared to other savannas, whilst Alas Purwo is lowest in terms of species richness, as well as species diversity. In terms of species evenness, Baluran has the highest evenness score whereas Bali Barat has the lowest (Table 3).
Differences in community composition
The global test of analysis of similarity (ANOSIM) showed there were significant differences in species composition between the savanna sites (Global R = 0.94; P < 0.001) based on all species (both native and alien). The savannas were also floristically different from each other in terms of native plant species (Global R = 0.64; P < 0.001). There is also clear separation of savanna sites in the ordination (Fig. 2). Baluran savanna had the lowest value of average similarity (48.1%), indicating it had the greatest variation in floristic composition between the plots. The reverse is true for Alas Purwo (Fig. 2). SIMPER analysis showed that twenty-two species were primarily responsible for the dissimilarity between the sites (Table 4). From all six combinations of pairwise comparisons of savanna areas, eleven species were important in at least three pairwise comparisons (Acacia nilotica, Albizia chinensis, Borassus flabellifer, Chromolaena odorata, Engelhardia spicata, Flacourtia rukam, Gleichenia microphylla, Imperata cylindrica, Melastoma polyanthum, Polytrias indica, Thespesia lampas), and six species (Antidesma bunius, Calamagrostis australis, Lantana camara, Ocimum tenuiflorum, Passiflora foetida, Streblus asper) occurred in two savanna pairwise comparisons. The species that contributed the most to the dissimilarities among almost all pairs of savanna areas was Desmodium laxiflorum (Table 4). This species was present in all three savanna sites (Baluran, Alas Purwo and Rinjani), but not in the Bali Barat savanna.
Savanna community composition correlation with physical-environmental factors
Canonical correspondence analysis (CCA; Fig. 3) shows that Rinjani (GR) in Lombok floristically separates from the other savannas along elevation and associated climatic gradients whereas Baluran (BA) is clearly separated from other savannas by differences in fire regime (higher frequency and less time since last fire), but also by slope to a lesser degree. According to the BEST (Bio-Env) analysis, elevation and precipitation are the two environmental factors with the highest correlation to species composition across all four of sampled savannas (BEST Global test sample statistic Rho = 0.6; P < 0.001).
Results of canonical correspondence analysis (CCA) showing a biplot of sample sites and environmental variables. Temp – air temperature; Heat Str – heat stress index; Relative – relative humidity; Precip – precipitation; Alt –altitude (elevation); Ph – soil pH; Slope – slope Wind vel – wind velocity; Soil moist – soil moisture; FireFreq – fire frequency; TSLF – time since last fire. Baluran NP (BA), Alas Purwo NP (AP), Bali NP (BB) and Rinjani NP (GR). Together, CCA1 and CCA2 explain 63.2% of the total variation in the floristic-environment relationship
Discussion
In this study we have characterized the tropical savanna plant communities of the wetter regions of Indonesia distributed across three main Islands, namely Java, Bali and Lombok, and their relationships with the physical environment. We found each of the four savanna ecosystems were distinctive in terms of species composition, ecological traits of dominant species, physical environment and physiognomy. We confirmed our main hypothesis that climate and elevation are likely to be the main drivers for savanna plant community differences. However, fire also potentially plays an important role, as we found variation in fire regime was correlated with gradients in floristic composition between the savannas. We also recognize that fire and climatic variables were derived from coarsely mapped data and, consequently, varied little between plots within each savanna. Therefore, the environmental gradients recognized are more likely to explain floristic dissimilarities between savannas, and more work is required to characterize floristic–environment relationships within discrete areas of savanna (i.e. at the landscape scale).
Savanna sites have a defining feature of dominant groundcover, especially grasses and forbs, with sparse tree cover (Frost et al. 1986). All four savanna sites in our study also showed this structure; however, each one of them is characterized by different combinations of tree species. Bali Barat is characterized by the palm species (Borassus flabellifer) as the tree layer. The same species also characterized the savanna in the drier part of the Indonesian archipelago, such as in Kupang, Timor Island in East Nusa Tenggara (Monk et al. 2000). Species of the same genus (Borassus aethiopum) also characterize the humid Lamto savanna of the Cote d’Ivoire in West Africa (Barot et al. 1999), and indeed palm savannas are known in Venezuela (Holbrook and Putz 1996), Namibia (Konstant et al. 1995), as well as islands such as Madagascar and Mauritius (Safford 1997). In Baluran National Park (but not in area sampled), near Bama Beach, one species of palm (Corypha utan) is known to occur in savanna. These palms in Bali Barat, Kupang and Baluran occur in lowland areas, especially near to the coast. In any given situation, plant co-occurrence and abundance may be determined largely by resource availability, heterogeneity of the abiotic environment, and microhabitat specialization. Some palms appear to be adapted to specific edaphic conditions, especially those related to soil drainage and depth (Widyatmoko and Burgman 2006).
In the Baluran savanna, common species in the tree layer included Ziziphus mauritiana, Azadirachta indica and Acacia nilotica. The same species has been reported from the drier eastern parts of Indonesia, such as Komodo, Rinca and Flores Islands, East Nusa Tenggara, from sea level up to 500 m a.s.l.. Ziziphus mauritiana also occurs in savanna areas of India (Pandey and Singh 1991). The same genus (Ziziphus) is common to many savannas of Africa, Arabia and South/Southeast Asia (Hess et al. 1996, Ratnam et al. 2016). In fact, of the four savannas studied, Baluran features typical savanna-type tree species showing adaptations to herbivory (thorns) and drought (small leaves, open architecture) and suggest it may be a relatively old savanna (sensu Ratnam et al. 2016). Even the invasive tree species Acacia nilotica and Azadirachta indica are typical savanna species (Radford et al. 2001; Dhileepan 2009: Swaine et al. 1992). It is not clear if Z. mauritiana (Indian jujube) is exotic or native to Baluran as the species is widely cultivated across Asia (for food and medical products), as well as having an uncertain origin and much disputed taxonomy (Islam and Simmons 2006; Janick and Paull 2008). Other evidence of the relative antiquity of the Baluran savanna are the dominance of C4 grasses, the open vegetation structure and persistence of native forbs, as well as the continued presence of indigenous grazing ungulates (e.g. Bos javanicus). The savanna here occurs in a rain shadow of Mt Baluran and appears to have lower and more strongly seasonal rainfall compared with the other savannas studied.
In terms of groundcover, our savannas share similar common species with those found in other savannas of Southeast Asia, especially in Thailand, where species such as Imperata cylindrica, Lantana camara and Chromolaena odorata are all commonplace (Kurz 1876; Kodandapani 2013; Ratnam et al. 2016; Sutomo et al. 2021). C4 grasses (mostly from the Andropogoneae clade) dominate our savannas, as expected for savannas in general, although Calamagrostis australis, which is widespread in the Bali Barat savanna, appears to be a C3 grass based on studies of other species in the genus (Osborne et al. 2014; Appendix 1). Another species which prominently contributes to the compositional similarities between our savannas is the tropical herbaceous legume Desmodium laxiflorum. Desmodium spp. are native to tropical Southeast Asia and Pacific Islands (Lenne 1981; Woomer et al. 1988) and are known to occur in higher rainfall savannas where they can survive fire and are known to be a palatable species to grazing animals. Although the species is absent in the Bali Barat savanna, it is present in the other savannas studied. Desmodium perhaps requires regular ground disturbance, such as fires, to persist, something which has been largely excluded as part of the management implemented at Bali Barat National Park in recent times.
Although there are some species in common across the savannas, there is very high floristic dissimilarity between them, especially in the tree layer, but also amongst the ground-layer dominants (Table 2). At the family level they are more similar, with certain families common to most of them (e.g. Fabaceae). There are several possible explanatory factors for the dissimilarity at species level, namely geographic, geologic and climatic barriers, as well as management including the use of fire. Baluran in East Java and Bali Barat in Bali, for example, are both national parks and are relatively close to each other (~ 60 km), but they are separated by sea (the Bali Strait) and are climatically different with Baluran having lower and more strongly seasonal precipitation and higher temperatures. This leads to more fire-prone vegetation at Baluran, which also is regularly prescribed to control the domination of woody plant, particularly the invasive alien species Acacia nilotica in the savanna. By constrast, fire is now suppressed in Bali Barat and the savanna is shifting to dry forest or secondary regrowth (Sutomo and van Etten 2021). Also, there were many invasive woody plants and climbing plants in the Bali Barat savanna, which is likely to reflect its proximity to human settlements and activity. Similarly, domination by many invasive alien species at Alas Purwo, especially by Chromolaena odorata, was recorded (Appendix 1). Both Bali Barat and Alas Purwo savannas are in danger of being transformed into a forest structure due to lack of fires and prevalence of invasive species.
Globally, savannas in high rainfall areas typically have some woody vegetation and, in the absence of fire, tend to develop high tree cover that would outcompete and suppress grasses species, thereby disabling further fires and facilitating the transition to a forested ecosystem (Rosleine and Suzuki 2013; Staver et al. 2011, Aleman et al. 2020). Thus, regular fire is important to establish grass-tree coexistence in these areas (Sankaran et al.; Bond and Keeley 2005). Sumardja and Kartawinata (1977) described savanna in Pangandaran Peninsula, West Java and reported that some savanna sites had been abandoned around 1957. These areas have not been experienced any fire since then; subsequently, they have become young secondary forests dominated by Decaspermum fruticosum. By contrast, woody vegetation is mostly in low abundance in savannas of drier sites, whereas grasses are by far the dominant component. Semi-arid savannas have resource limitations (mostly competition for water) and lower biomass/fuels, and, therefore, fire is perhaps less influential for grass-tree coexistence in this type of savanna (Staver et al. 2011). On the island of Java, especially in East Java, recolonization (primary succession) after lava flow and volcanic eruptions is initially dominated by grasses which may then be maintained by regular fire over long periods. Therefore, in this region of Java, which is subject to a more pronounced dry season (less rain and longer period of dry season) than central and western parts of the island, fire is believed to be the major factor that created and then maintained savannas (van Steenis 1972).
Rinjani on the island of Lombok is also very distinct. This savanna is located at higher elevations on the slopes of the volcano Mt Rinjani and has been maintained in savanna form presumably since the early stages of primary succession after the last major eruption and lava flows in the area of study (Sutomo et al. 2021). The influence of soil depth or the depth of the volcanic deposits plays an important role in the succession, as observed in the Mt Merapi primary succession on Java Island (Sutomo 2013). Once grasses recolonized and dominated the area, only small amounts of tree species have developed, perhaps due to high competition for resources with grasses, unsuitable microclimate and/or the role of regular fire which maintains the grass domination (the fire frequency being intermediate between that of Baluran and the other savannas studied; Sutomo et al. 2021). The dominant grass here (Imperata cylindrica) is known for its fire tolerance and is considered a fire-climax species across Southeast Asia (MacDonald 2004). Although the Rinjani savanna studied is not currently shifting to a forest state, of the woody species found in our plots at Rinjani, almost all of them are forest pioneer/edge specialists (Appendix 1), many currently occurring only as seedlings or saplings. This suggests invasion from nearby gallery forests in nearby drainage lines and the potential for conversion to forests in the absence of future fires over a long period. However, the clumps of trees and small forest patches which establish in crevices and drainage lines are likely promoted by the extra moisture and conducive microclimate conditions which encourage dense woody vegetation (Murphy and Bowman 2012). In such landscapes, forests are often constrained to geomorphology/topographic conditions that protected it from fire (Russell-Smith et al. 2012).
Tropical savannas generally have high alpha (local-scale) plant species diversity, particularly when compared with temperate grasslands and dry tropical woodlands (Solbrig et al. 1996). High diversity in savannas, for instance, occurs in the Brazilian Cerrado (Furley 1999). However, savanna diversity can be reduced due to several reasons, such as invasion of exotic species, fire exclusion, herbivore introduction or exclusion, or physical removal of trees and shrubs (Furley 1999, Solbrig et al. 1996). In our study, average species diversity is in the low range for savannas (Magurran 1988). Low species diversity is also found in other Asian savannas, such as in India (Pandey and Singh 1991). In Indonesia, such low diversity is known to occur in the wet-climate savanna at Pangandaran, West Java (Rosleine and Suzuki 2013). This relative paucity of species in these savannas is perhaps due to extensive invasion of exotic species and/or fire exclusion. At Pangandaran, savanna abandonment without recurring fire has resulted in over-dominance of Decaspermum sp. div. (Myrtaceae), which in turn has resulted in a decline in species diversity (Rosleine and Suzuki 2013). Low species evenness may also be the result of the dominance of certain exotic species (both woody and non-woody) in the savannas we studied (i.e. Acacia nilotica in Baluran savanna, Lantana camara in Bali Barat and Chromolaena odorata in Alas Purwo). In their study in Baluran, Caesariantika et al. (2011) found that Acacia nilotica invasion had significant effect on species diversity in Baluran savanna, with values of (Shannon–Wiener) species diversity in the range of 0.46 to 1.34. In our study, we found broadly similar mean Shannon–Wiener species diversity of 0.74 at Baluran. In Baluran, Acacia nilotica, a woody IAS, is fire-tolerant when mature and unpalatable due to considerable thorns (Djufri 2004, FAO 2014). This poses an increased threat of expansion of Acacia nilotica stands in Baluran savanna even with regular fire and high grazing pressure. Evidence of recent expansion of the A. nilotica stand in the Bekol savanna at Baluran is outlined in Sutomo et al. (2016). Fire exclusion at Bali Barat and Alas Purwo may also play a role in the relatively low species diversity of these savannas. Although we sampled our savannas in the dry season, many annual and other short-lived species were still able to be identified as remnants of the flowers and/or leaves were still present on at least some plants. However, we accept that we are likely to have under-estimated plant diversity, especially of annual forbs and geophytes, compared to sampling in the late wet season. It is also possible that some of the more sparsely distributed ground layer species may have been missed due to the subsampling employed.
In conclusion, this study is the first to thoroughly describe savanna plant communities in the wetter parts of the Indonesian archipelago. We have shown that differences in elevation (along with related climatic factors such as temperature and precipitation), as well as differences in fire regime and types of colonizing invasive species, are likely drivers of the distinct floristic differences recorded between the savannas we studied across Java, Bali and Lombok Islands. Therefore, invasive weed management should be considered by these national parks authorities as it is likely to increase native species diversity. Lack of prescribed fire and a range of invasive species threaten to convert savanna at Bali Barat and Alas Purwo into secondary forests or shrubland. The presence of forest pioneer/edge species within the savanna at Rinjani suggests successional change from grassland to forest may occur in the absence of future fires, although the roles of soil, topography and microclimate in maintaining grass dominance are also significant (Sutomo et al. 2021). Compared to the others studied, the savanna in Baluran National Park has characteristics of being relatively old and persistent (Ratnam et al. 2016), rather than one being created and maintained via human conversion of forests. It is recommended that further studies be conducted, including isotopic characterization of soils and organic matter, and manipulative experiments involving fire and grazing as treatments, to more firmly establish the dynamics and age of the savannas studied.
References
Aleman J, Fayolle A, Favier C, Staver A, Dexter K, Ryan C, Azihou A, Bauman D, Te Beest M, Chidumayo E (2020) Floristic evidence for alternative biome states in tropical Africa. Proc Natl Acad Sci USA 117:28183–28190
Auffenberg W (1981) The behavioral ecology of the Komodo monitor. University Press of Florida
Barot S, Gignoux J, Menaut J-C (1999) Demography of a savanna palm tree: predictions from comprehensive spatial pattern analyses. Ecology 80:1987–2005
Blackie R, Baldauf C, Gautier D, Gumbo D, Kassa H, Parthasarathy N, Paumgarten F, Sola P, Pulla S, Waeber P, Sunderland T (2014) Tropical dry forests: the state of global knowledge and recommendations for future research. Bogor, Indonesia: Center for International Forestry Research (CIFOR)
Boedhihartono AK (2017) Can community forests be compatible with biodiversity conservation in Indonesia? Land 6:21
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol Evol 20:387–394
Brearley FQ, Prajadinata S, Kidd PS, Proctor J, Suriantata (2004) Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. Forest Ecol Managem 195:385–397
Caesariantika E, Kondo T, Nakagoshi N (2011) Impact of Acacia nilotica (L.) Willd. ex Del invasion on plant species diversity in the Bekol Savanna, Baluran National Park, East Java, Indonesia. Tropics 20:45–54
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral J Ecol 18:117–143
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Progr Ser 92:205–219
Dhileepan K (2009) Acacia nilotica ssp. indica (L.) Willd. ex Del.(Mimosaceae). In Muniappan R, Reddy GVP, RamanA (eds) Biological control of tropical weeds using arthropods. Cambridge: Cambridge University Press, UK, pp 17–37
Djufri (2004) Acacia nilotica (L.) Willd. ex Del. dan Permasalahannya di Taman Nasional Baluran Jawa Timur. Biodiversitas 5:96–104
Dossa GG, Paudel E, Fujinuma J, Yu H, Chutipong W, Zhang Y, Paz S, Harrison RD (2013) Factors determining forest diversity and biomass on a tropical volcano, Mt. Rinjani, Lombok, Indonesia. PLOS One 8:e67720
FAO (2014) Acacia nilotica: a tree legume out of control. Food and Agriculture Organization, Rome
Fisher R, Bobanuba WE, Rawambaku A, Hill GJ, Russell-Smith J (2006) Remote sensing of fire regimes in semi-arid Nusa Tenggara Timur, eastern Indonesia: current patterns, future prospects. Int J Wildland Fire 15:307–317
Frost PGH, Menaut JC, Walker BH, Medina E, Solbrig OT (1986) Responses of savannas to stress and disturbance. In Scholes RJ, Walker BH (eds) An African savanna: synthesis of the Nylsvley study. Cambridge University Press, Cambridge, pp 2–10
Furley PA (1999) The nature and diversity of neotropical savanna vegetation with particular reference to the Brazilian cerrados. Global Ecol Biogeogr 8:223–241
Goltenboth F, Timotius KH, Milan PP, Margraf J (2006) Ecology of insular Southeast Asia: the Indonesian archipelago. Elsevier, Amsterdam
Hardjosuwarno S (1990) Dasar-Dasar Ekologi Tumbuhan. Fakultas Biologi, UGM. Yogyakarta
Harger JRE (1995) Air-temperature variations and ENSO effects in Indonesia, the Philippines and El-Salvador – ENSO Patterns and Changes from 1866–1993. Atmos Environm 29:1919–1942
Hess T, Stephens W, Thomas G (1996) Modelling NDVI from decadal rainfall data in the North East Arid Zone of Nigeria. J Environm Managem 48:249–261
Holbrook NM, Putz FE (1996) Water relations of epiphytic and terrestrially-rooted strangler figs in a Venezuelan palm savanna. Oecologia 106:424–431
Islam MB,Simmons MP (2006) A thorny dilemma: testing alternative intrageneric classifications within Ziziphus (Rhamnaceae). Syst Bot 31:826–842
Janick J, Paull RE (2008) The encyclopedia of fruit & nuts. CABI Publishing, Oxfordshire
Kent M (2011) Vegetation description and data analysis: a practical approach. John Wiley & Sons, New York
Kodandapani N (2013) Contrasting fire regimes in a seasonally dry tropical forest and a savanna ecosystem in the Western Ghats, India. Fire Ecol 9:102–115
Konstant TL, Sullivan S, Cunningham AB (1995) The effects of utilization by people and livestock on Hyphaene petersiana (Arecaceae) basketry resources in the palm savanna of North-Central Namibia. Econ Bot 49:345–356
Kurz S (1876) Preliminary report on the forest and other vegetation of Pegu. Office of the Superintendent of Government Printing, Calcutta
Lenne JM (1981) Reaction of Desmodium species and other tropical pasture legumes to the root-knot nematode Meloidogyne javanica. Trop Grasslands 15:17
MacDonaldGE (2004) Cogongrass (Imperata cylindrica): biology, ecology, and management. Crit Rev Pl Sci 23:367–380
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, USA
Monk KA, de Fretes Y, Reksodihardjo-Lilley G (2000) Ekologi Nusa Tenggara dan Maluku. Prenhallindo, Jakarta
Murphy B, Bowman DMJS (2012) What controls the distribution of tropical forest and savanna? Ecol Letters 15:748–758
Osborne CP, Salomaa A, Kluyver TA, Visser V, Kellogg EA, Morrone O, Vorontsova MS, Clayton WD, Simpson DA (2014) A global database of C4 photosynthesis in grasses. New Phytol 204:441–446
Pandey CB, Singh JS (1991) Influence of grazing and soil conditions on secondary savanna vegetation in India. J Veg Sci 2:95–102
Radford IJ, Nicholas MD, Brown JR (2001) Impact of prescribed burning on Acacia nilotica seed banks and seedlings in the Astrebla grasslands of northern Australia. J Arid Environm 49:795–807
Ratnam J, Bond WJ, Fensham RJ, Hoffmann WA, Archibald S, Lehmann CE, Anderson MT, Higgins SI, Sankaran M (2011) When is a ‘forest’a savanna, and why does it matter? Global Ecol Biogeogr 20:653–660
Ratnam J, Tomlinson KW, Rasquinha DN, Sankaran M (2016) Savannahs of Asia: antiquity, biogeography, and an uncertain future. Philos Trans Ser B 371:20150305
Rosleine D, Suzuki E (2013) Secondary sucession at abandoned grazing sites, Pangandaran Nature Reserve, West Java, Indonesia. Tropics 21:91–103
Roy DP, Boschetti L, Justice CO, Ju J (2008) The collection 5 MODIS burned area product – global evaluation by comparison with the MODIS active fire product. Remote Sensing Environm 112:3690–3707
Russell-Smith J, Edwards AC (2006) Seasonality and fire severity in savanna landscapes of monsoonal northern Australia. Int J Wildland Fire 15:541–550
Russell-Smith J, Edwards AC, Price OF (2012) Simplifying the savanna: the trajectory of fire-sensitive vegetation mosaics in northern Australia. J Biogeogr 39:1303–1317
Safford RJ (1997) A survey of the occurrence of native vegetation remnants on Mauritius in 1993. Biol Conservation 80:181–188
Sankaran M, Ratnam J, Hanan NP (2003) Tree-grass coexistence in savannas revisited – insights from an examination of assumptions and mechanisms invoked in existing models. Ecol Lettters 7:480-490
Scogings PF, Sankaran M (eds) (2020) Savanna woody plants and large herbivores. John Wiley & Sons
Solbrig OT, Medina E, Silva J (1996) Biodiversity and tropical savanna properties: a global view. In Mooney HA, Cushman JH, Medina E, Sala OE,. Shulze E-D (eds) Functional roles of biodiversity: a global perspective. John Wiley & Sons, New York, pp. 185–211
Staver AC, Archibald S, Levin S (2011) Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92:1063–1072
Stott P (1990) Stability and stress in the savanna forests of South-East Asia. J Biogeogr 17:373–383
Sumardja A, Kartawinata K (1977) Vegetation analysis of the habitat of Banteng (Bos javanicus) at the Pananjung-Pangandaran nature reserve, West Java. Biotrop Bull 15:1–44
Sutomo (2013) Ecological succession on volcanic ecosystem of Mount Merapi Indonesia and its implication for restoration. SEAMEO-BIOTROP, Bogor, Indonesia
Sutomo, van Etten E, Priyadi A (2015) Do water buffalo facilitate dispersal of invasive alien tree species Acacia nilotica in Bekol savanna Baluran National Park? In Damayanti EK, Fernandez JC (eds) Second International Conference on Tropical Biology: Ecological restoration in Southeast Asia: Challenges, gains, and future directions. SEAMEO BIOTROP, Bogor, Indonesia, p 155
Sutomo, van Etten E, Wahab L (2016) Proof of Acacia nilotica stand expansion in Bekol Savanna, Baluran National Park, East Java, Indonesia through remote sensing and field observations. Biodiversitas 17:96–101
Sutomo, van Etten E (2018) Spatial and temporal patterns of fires in tropical savannas of Indonesia. Singapore J Trop Geogr 39:281–299
Sutomo, van Etten E, Iryadi R (2021) Savanna-forest boundary on Mount Rinjani, Lombok Island, West Nusa Tenggara, Indonesia. Biodiversitas 2:726–731
Sutomo, Yulia E, Iryadi R (2021) Kirinyuh (Chromolaena odorata): species distribution modeling and the potential use of fungal pathogens for its eradication. In IOP Conference Series: Earth and Environmental Science, 012023. IOP Publishing, Indonesia
Sutomo, van Etten E (2021) Bali Starling (Leucopsar rothschildi) natural habitat in Bali Barat National Park, Indonesia. Biotropia 28:117–127
Swaine MD, Hawthorne WD, Orgle TK (1992) The effects of fire exclusion on savanna vegetation at Kpong, Ghana. Biotropica 24:166–172
Tacconi L, Ruchiat Y (2006) Livelihoods, fire and policy in eastern Indonesia. Singapore J Trop Geogr 27:67–81
van der Kaars S, Dam R (1997) Vegetation and climate change in West-Java, Indonesia during the last 135,000 years. Quatern Int 37:67–71
van Steenis CGGJ (1972) The mountain flora of Java. E. J. Brill, Leiden
Whitten T, Soeriaatmadja RE, Afiff SA (1996) The ecology of Indonesia series, volume II: The ecology of Java and Bali. Periplus, Hong Kong
Widyatmoko D, Burgman MA (2006) Influences of edaphic factors on the distribution and abundance of a rare palm (Cyrtostachys renda) in a peat swamp forest in eastern Sumatra, Indonesia. Austral Ecol 31:964–974
Woomer P, Singleton PW, Bohlool BB (1988) Ecological indicators of native rhizobia in tropical soils. Appl Environm Microbiol 54:1112–1116
Acknowledgements
Fieldwork was supported by the Rufford Foundation (grant number 15619-B) and the School of Natural Science Edith Cowan University. Plant identification was assisted by Mr Ida Bagus Ketut Arinasa from the Hortus Botanicus Baliensis at the Bali Botanical Garden, Indonesian Institute of Sciences (LIPI). Our thanks go to Ministry of Forestry and Environment of Indonesia for permission to conduct the study in the four National Parks. This research complies with the current laws in Indonesia.
Author information
Authors and Affiliations
Contributions
Conceptual development: Sutomo and Eddie van Etten. Collecting the data: Sutomo. Analysing the data: Sutomo and Eddie van Etten. Writing the paper: primarily Sutomo with support and editing from Eddie van Etten. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there is no conflict of interest among authors of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 154 kb)
Rights and permissions
About this article
Cite this article
Sutomo, van Etten, E. Savanna plant communities in the wetter parts of the Indonesian archipelago. Folia Geobot 56, 193–204 (2021). https://doi.org/10.1007/s12224-021-09401-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-021-09401-y