Abstract
Halophytic vegetation on fossil salt deposits and salt springs is an extreme habitat occurring in isolated continental areas of temperate Europe. It has a relatively rich representation in the Transylvanian Basin of Romania; however, comprehensive research into this highly specialized vegetation is lacking. We provide the first phytosociological survey of recent data of inland salt habitats in Cluj County, where they are widely distributed. Cluster analysis distinguished eight euhalophytic plant communities occupying several zones depending on the micro-topography of the salt-affected area: Scorzonero parviflorae-Juncetum gerardii (including Astero tripolio-Triglochinetum maritime), Plantagineto cornuti-Agrostetum stoloniferae, Artemisio-Festucetum pseudovinae, Artemisio-Petrosimonietum triandrae, Limonio gmelinii-Artemisietum monogynae, Puccinellietum limosae (each allocated to the class Festuco-Puccinellietea), then Suaedetum maritimae and Salicornietum prostratae, belonging to the class Therosalicornietea. We revised the nomenclature of these associations, characterized their species composition and ecological preferences based on indicator values for salinity, pH, moisture and nutrients in the soil. We broadly discussed our findings in light of the historical data and documented the current habitat conditions of the sites. Most of them were found to be degraded due to increasing tourism exploiting the curative effects of salt springs. Beyond recognizing the high conservation value of the Transylvanian salt habitats, it is also important to highlight their biogeographical significance, as they fill the gap between the inland salt marshes of Central Europe and the continental salt habitats of Eurasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saline habitats develop on salt-affected soils with a fluctuating water regime. They are largely distributed in coastal areas across the globe, except in Antarctica (Chapman 1960), in non-coastal regions in zones with an arid climate (Szabolcs 1974). Due to differences in soil properties, topography and climate, their salinity level varies (e.g. Leuschner and Ellenberg 2017). These specific edaphic conditions enabled the formation of several saline habitats with a high number of plant specialists (halophytes) tolerating the abiotic stress.
Romania is an important hotspot of saline areas. Coastal saline habitats occur in the Black Sea region and inland saline vegetation is found throughout the country: in the Wallachian Plain, Moldova, the Transylvanian Basin and the Pannonian Lowland (Grigore and Cojocariu 2020). These are territories in which the negative water balance (evaporation) in the summer causes the rising of the groundwater to the surface, carrying the soluble salts up to the soil (Réti et al. 2016). In other regions of Europe, for example in Poland, they are also related to fossil salt deposits and springs influenced by saline water (Piernik 2020).
Knowledge regarding the saline vegetation of Romania is incomplete compared to other regions of temperate Europe, e.g. Germany (Brandes 1999), Poland (Piernik 2020), Serbia (Zlatković et al. 2019), Hungary, Austria, Slovakia or Bulgaria (Eliáš et al. 2013). Limnological research about the salt lakes is up to date (e.g. Alexe et al. 2018), but few studies on the terrestrial vegetation of salt-affected areas have been published. With the exception of several detailed historical studies on a local scale, for example Ţopa (1939) and Doltu et al. (1977), the saline vegetation is listed in the newest national syntaxonomical survey (Sanda et al. 2008), but data on the distribution of the vegetation types and their ecological conditions are not included there.
Our contribution focuses exclusively on a discrete region of Romania, the Transylvania basin. It is a remarkable area with frequent occurrences of salt deposits originating in the Miocene period (Podar et al. 2019). Inland saline habitats occupying these natural salt domes in the deforested landscape have been attracting the attention of field biologists for centuries. The first floristic records date back to the end of the eighteenth century from Turda (Schur 1780 sec. in Todor 1947), and Csűrös (1947) published numerous floristic notes and herbarium collections from the area in the 19th and 20th centuries. Among more recent studies are those carried out by Nyárády (1941) with detailed characteristics of salt ponds around Cluj, and even more recently by Alec (2010) and Diodiu (2010). Several vegetation studies were published in the first half of the twentieth century, notably by Soó (1927, 1933, 1947), Ţopa (1939), Todor (1947, 1948), and later by Csűrös-Káptalan (1965), Csűrös-Káptalan and Péterfi (1966), Pop and Hodişan (1980), and Pop et al. (1983, 2002). The most frequently mentioned halophytic plant communities are the class Therosalicornietea, order Camphorosmo-Salicornietalia and the class Festuco-Puccinellietea, orders Scorzonero-Juncetalia gerardi and Puccinellietalia. So far, no comprehensive work has been published dealing with these communities in the area in question. To fill this gap, the classification of plant communities needs to be re-analysed, the nomenclature of the syntaxa needs to be corrected, and degradation stages of the recent sites should be described.
In our study we analyse phytosociological data recorded in Cluj County, the region with the highest concentration of inland salt habitats in the Transylvanian Basin. Our aim was to perform a comprehensive syntaxonomical vegetation survey of the strictly halophytic vegetation which includes: (i) characterization of the ecological preferences of the vegetation types based on indicator values, (ii) broad syntaxonomical discussion of the results using historical and other relevant literature, and (iii) documentation of the recent stage of all known sites in the area with regard to the pressure of tourism in relation to nature conservation concerns.
Methods
Study Area
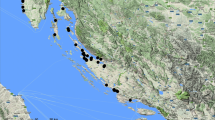
The most frequent occurrence of inland saline vegetation is situated in Cluj County (Județul Cluj) in the Transylvanian Basin (Bazinul Transilvaniei), in north-central Romania (Fig. 1). They are concentrated in the wider vicinity of the towns of Cluj-Napoca and Turda, in the catchment area of the rivers Someşul Mic and Mureş, at elevations of 250–380 m a.s.l. The climate is temperate continental, with warm summers contrasted by very cold winters. The area sees annual precipitation of 520–650 mm with a peak (June, July, August), and has a mean annual temperature of 8.4–9.7°C (Kun et al. 2004). It is a deforested landscape called as Transylvanian Plain (in Rom. Câmpia Transilvaniei, in Hung. Mezőség), where among the salt vegetation within the vast agricultural lands very diverse semi-natural dry grasslands have been preserved (Ruprecht 2005).
Important salt areas of Transylvania outside of Cluj County were not investigated: they are either heavily degraded (e.g. Ocna Mureş and Ocna Sibiului) or they are geographical outliers (e.g. salt springs in Harghita County). Sixteen sites with common geology and land history were visited.
Inland saline environments, including salt ponds and salt marshes, are characteristic features of the Transylvanian Basin landscape. They are evolutionarily associated with massive Middle Miocene evaporite (halite and gypsum) deposits originally up to 300 m thick (Krézsek and Bally 2006). Evaporite successions were covered by post-salt sediments (up to 2,000 m thick in the northeastern part of the basin), most of which were eroded due to Pliocene regional uplift. The present distribution and thickness of salt layers is controlled mainly by Mio-Pliocene salt tectonics (Krézsek and Filipescu 2005; Krézsek and Bally 2006). The salt outcrops follow roughly north-south oriented linear structures that include salt-cored anticlines in the basin centre and two diapiric alignments near the eastern and western parts of the present Transylvanian Basin depression. Due to vertical migration of plastic salt, numerous salt diapirs and extrusions reached the surface. The appearance of salt led to mining and/or evolution of surface pseudokarst, resulting in numerous artificial (anthroposaline) or natural (karstosaline) lakes (Alexe et al. 2018). All saline lakes presently found in the Transylvanian Basin (Sovata, Ocna Sibiului, Ocna Mureş, Turda, Cojocna, Sic, Ocna Dej, Jabeniţa) were formed by salt dissolution of underground halite deposits (Har et al. 2010).
The massive salt accumulation was promoted by salt mining, beginning in the Middle Ages (Jakab et al. 2019), the most important salt mines were in Sic and Turda. Near the salt efflorescences, old mines and salt lakes, the underground water can easily come into contact with soluble salt deposits and emerge as salt springs. The surface erosion created wide flat meadows accompanied by salt springs and sapropelic mud plateaus, both inhabited by highly specialized halophytic vegetation (Podar et al. 2019).
Vegetation Data Sampling
Phytosociological relevés of vascular plants were sampled between 2015 and 2018 on sixteen sites with euhalophytic vegetation of the classes Therosalicornietea and Festuco-Puccinellietea. The plot selection was subjective, on the investigated sites we recorded each vegetation type which were easily recognized by their different physiognomy along the salt-affected habitat. Contact vegetation zones only marginally affected by salinization and a fluctuating water regime (e.g. dry grasslands on foothills, aquatic communities in salt ponds) were not sampled. The recording followed the Zürich-Montpellier approach using the adapted nine-point Braun-Blanquet cover-abundance scale (Barkmann et al. 1964). All of the relevés were plotted on a unified area (4 × 4 m), which is the standard plot size of recording non-forest vegetation (Chytrý and Otýpková 2003). We surveyed 109 relevés, the complete table is in Electronic supplementary material 4.
Plant nomenclature follows Euro+Med (2018), and the names of syntaxa up to alliance level are according to Mucina et al. (2016). Association names were used according to Sanda et al. (2008). If the syntaxon is not published there, we include it with the authors' abbreviations and the year of description.
Data Processing
The dataset of 109 phytosociological relevés were stored in a TURBOVEG database (Hennekens and Schaminée 2001) and exported for classification in the JUICE program (Tichý 2002). As a first step, we distinguished the alliances and the associations using hierarchical clustering (log-transformed percentage abundances, correlation distance measure and Ward’s group linkage method) in PC-ORD 5.0 software (McCune and Mefford 2006). Coherence of clusters was checked using non-metric multidimensional scaling (NMDS; log-transformed percentage abundances, Bray-Curtis dissimilarity measure) with the ‘Spiderplot’ visualization function from the R package ‘Vegan’ (Oksanen et al. 2013), and their cluster were evaluated analysing diagnostic (Dg), constant (Cs) and dominant (Dm) species in JUICE. As a fidelity measure, the phi coefficient was calculated; the size of all relevé groups was standardized to equal size. Species were excluded when the occurrence concentration in the target clusters was not significant in Fisher’s exact test at P < 0.01 (Chytrý et al. 2002). Diagnostic species in the combined synoptic table were based on two criteria: (1) fidelity greater than 0.25 and (2) species frequency higher than 50%. Threshold frequency value for constant species was 50 and threshold value of cover for dominant species was 20 with frequency greater than 30%.
The indicator values for moisture, nutrients, soil reaction and salt concentration of the soil are based on Borhidi (1995) and were displayed by boxplots. The indicator values were weighted by species cover. Normality was graphically checked by normal probability plots with correlation coefficients equal or more than 095 for all four indicators. Statistically significant differences among groups displayed in boxplots were tested using ANOVA and Tukey’s HSD for unequal N test (alpha = 0.05) in STATISTICA 7 software (StatSoft 2004).
The map of the study area was created using the program QGIS, version 3.2 (QGIS Development Team 2018) with the QuickMapServices plugin and a terrain background layer from Stamen Design with data by OpenStreetMap.
Results
During the sampling of the euhalophytic vegetation in the Transylvanian Basin, we recorded a total of 59 species of vascular plants on sixteen different sites within Cluj County. About twenty of them formed the main vegetation composition, while 27 species were recorded only sporadically with little cover range (Electronic supplementary material 4). In spite of low species richness, the plant communities were well distinguished by their various species composition and edaphic conditions driven by the micro-elevation of the terrain, typical for halophytic habitats (e.g. Deák et al. 2014). The most pronounced variables were salt content and moisture. Analysis of phytocoenological relevés detected three main groups (Fig. 2) with eight clearly distinguished clusters (Fig. 3, Table 1, Electronic supplementary material 2). The main axis (X) represents the salt content decreasing to the right, while axis Y reflects decreasing moisture moving upwards. Analysed indicator values (preferences for soil salinity, soil reaction, moisture and nutrients, Fig. 4a–d) of plant communities fit the description of syntaxonomical groups in the literature and were in accordance with our field observations.
Relationship between the distinguished alliances in ordination space of NMDS with “Spiderplot” visualisation. Numerals denote centroids of clusters formed by relevés classified to the same alliance. Group 1 – Juncion gerardi, group 2 – Festucion pseudovinae, group 3 – Puccinellion limosae and Salicornion prostratae. Stress value 0.142. Relationship between the distinguished alliances in ordination space of NMDS with “Spiderplot” visualisation. Numerals denote centroids of clusters formed by relevés classified to the same alliance. Group 1 – Juncion gerardi, group 2 – Festucion pseudovinae, group 3 – Puccinellion limosae and Salicornion prostratae. Stress value 0.142
Relationship between the distinguished associations in ordination space of NMDS with “Spiderplot” visualisation. Numerals denote centroids of clusters formed by relevés classified to the same association. Cluster 1 – Scorzonero parviflorae-Juncetum gerardi and Astero tripolio-Triglochinetum maritimi, cluster 2 – Plantagineto cornuti-Agrostetum stoloniferae, cluster 3 – Artemisio-Festucetum pseudovinae, cluster 4 – Artemisio-Petrosimonietum triandrae, cluster 5 – Limonio gmelinii-Artemisietum monogynae, cluster 6 – Puccinellietum limosae, cluster 7 – Suaedetum maritimae, cluster 8 – Salicornietum prostratae. Stress value 0.142.Fig. 3. Relationship between the distinguished associations in ordination space of NMDS with “Spiderplot” visualisation. Numerals denote centroids of clusters formed by relevés classified to the same association. Cluster 1 – Scorzonero parviflorae-Juncetum gerardi and Astero tripolio-Triglochinetum maritimi, cluster 2 – Plantagineto cornuti-Agrostetum stoloniferae, cluster 3 – Artemisio-Festucetum pseudovinae, cluster 4 – Artemisio-Petrosimonietum triandrae, cluster 5 – Limonio gmelinii-Artemisietum monogynae, cluster 6 – Puccinellietum limosae, cluster 7 – Suaedetum maritimae, cluster 8 – Salicornietum prostratae. Stress value 0.142
Differences in selected Borhidi indicator values between the eight distinguished associations (clusters) for salt concentration of the soils (a), soil reaction (b), moisture (c) and nutrients (d). Boxplots show median (red squares), interquartile range (boxes), non-outlier range (whiskers), outliers (empty circles) and extreme values (plus). Statistical significance by ANOVA.The upper letters (a, b, c, d, e) indicate homogeneous groups according to Tukey's post-hoc test (α = 0.05).Differences in selected Borhidi indicator values between the eight distinguished associations (clusters) for salt concentration of the soils (a), soil reaction (b), moisture (c) and nutrients (d). Boxplots show median (red squares), interquartile range (boxes), non-outlier range (whiskers), outliers (empty circles) and extreme values (plus). Statistical significance by ANOVA.The upper letters (a, b, c, d, e) indicate homogeneous groups according to Tukey's post-hoc test (α = 0.05)
Description and Allocation of the Syntaxonomic Units, Ecological Preferences
Group 1
Class Festuco-Puccinellietea, order Scorzonero-Juncetalia gerardi, alliance Juncion gerardi (Fig. 2)
Here belong salt marshes and salt meadows occupying the zone of moderately salinized soils and lower moisture (Fig. 4a–c) that form the outer belt of the salt-affected area. The hemicryptophytic vegetation of the Scorzonero-Juncetalia order occurs in contiguous zones or in scattered islands depending on the micro-elevation of the terrain. They had the highest number of vascular species among the studied vegetation types (average 7, maximum 15). Two clusters were identified here (No. 1 and 2; Fig. 3, Table 1).
Cluster 1: association Scorzonero parviflorae-Juncetum gerardii (Wenzl 1933) Wendelberger 1943, incl. Astero tripolio-Triglochinetum maritimi Soó 1937, Ţopa 1939. (relevés No. 1–26 in Electronic supplementary material 4)
Dg, Cs and Dm species: Tripolium pannonicum, Scorzonera parviflora, Triglochin maritima
Dg and Cs species: Agrostis stolonifera, Bolboschoenus maritimus agg., Juncus geradi
Vegetation of moderately saline soils (Fig. 3) and the lowest nutrient content (Fig. 4d). In addition to the frequent occurrence of Cluster 1 in the peripheral zone, the community was recorded also in wet depressions saturated with mineral waters throughout the salt-affected area. Except typical stands of Scorzonero parviflorae-Juncetum gerardii (relevés 10–16 in Table 1), we identified vegetation with a strong dominance of Triglochin maritima (relevés 1–9) which can be regarded as association Astero tripolio-Triglochinetum maritimi. Relevés with high cover of Bolboschoenus maritimus agg. (No. 17–21) were also assigned to Cluster 1.
Cluster 2: association Plantagineto cornuti-Agrostetum stoloniferae Soó et Csűrös 1947, 1973. (relevés No. 22–32 in Electronic supplementary material 4).
Dg, Cs and Dm species: Juncus geradi, Plantago cornuti
Dg and Cs species: Lotus tenuis
Cs species: Tripolium pannonicum, Triglochin maritima, Puccinellia distans agg.
Relevés with similar species composition and moisture preferences as Cluster 1 belong here, with significant dominance of Plantago cornuti. The stands occurred in narrow strips on the outer belt of the salt-affected area, crossing into mesic meadow communities. It had the lowest preferences for salt content and soil reaction (Fig. 4a, b). The dominance of Plantago cornuti alternated in some cases with Juncus geradi (relevés No. 28–32 in Table 1). A strong disturbance was observed here, reflected by higher values of nutrients caused by trampling and heavy grazing (Fig. 4d). This type of vegetation occurred rarely, usually in small areas; we recorded it in only three locations (Sic, Bonţ, Turda, see Electronic supplementary material 1).
Group 2
Class Festuco-Puccinellietea, order Puccinellietalia, alliance Festucion pseudovinae (Fig. 2, Table 1). Three clusters belong here (No. 3, 4, 5).
This group includes short-grass/herb vegetation on soils with high salt content and lower moisture (Fig. 4a, c). It formed the middle zone of salt habitats and occupied slightly inclined lands with significant desiccation of the surface in the second half of the growing season.
Cluster 3: association Artemisio-Festucetum pseudovinae (Magyar 1928) Soó (1933) 1945. (relevés No. 33–43 in Electronic supplementary material 4).
Dg, Cs and Dm species: Artemisia santonicum, Festuca valesiaca subsp. parviflora
Cs species: Limonium hungaricum, Puccinellia distans agg.
This closed, relatively homogeneous short-herb vegetation brings to mind the Pannonic salt steppes of Central-Eastern Europe in the large salt-affected areas (e.g. localities Gădălin, Jucu de Mijloc). In most cases, however, this vegetation is restricted to smaller areas (several tens of m2) overtopping from the flat terrain. It is also typically seen on the slopes of salt domes, where it can blend into dry grasslands. In intensively grazed localities Limonum gmelinii was highly represented. Average species number was 6, which in the case of salt vegetation is relatively high.
Cluster 4: association Artemisio-Petrosimonietum triandrae Soó (1927) 1947. (relevés No. 44–51 in Electronic supplementary material 4).
Dg, Cs and Dm species: Artemisia santonicum, Petrosimonia triandra
Vegetation with the highest preferences for soil salinity (Fig. 4a) and lowest moisture (Fig. 4c) within group 2. The initial sparse stands inhabited gentle slopes or small hummocks rising above the level of the longer inundated parts. It primarily occurred on smaller patches and was typical also of secondary habitats: edges of field roads, canal banks and other disturbed places. The open, species-poor vegetation (average species number 4.5) is characterized by the dominance of the annual Petrosimonia triandra. In the case of heterogeneous microrelief caused by the irregular soaking of the saline groundwater, the salt-demanding taxon Salicornia perennans penetrated into the gaps.
Cluster 5: association Limonio gmelinii-Artemisietum monogynae Ţopa 1939. (relevés No. 52–57 in Electronic supplementary material 4).
Dg, Cs and Dm species: Limonium hungaricum
Constant species: Puccinellia distans agg.
This vegetation occupied the bottoms of flat, intensively grazed salt steppes. Compared to other associations of the Festucion pseudovinae alliance it also requires saline soils, but differences in the water regime are less extreme and the summer drying is less pronounced as in the case of Artemisio-Petrosimonietum triandrae and Artemisio-Festucetum pseudovinae. It was also confirmed by the indicator value analysis as having the highest demands on moisture within group 2 (Fig. 4d). It forms a transition to the Puccinellion limosae alliance (cluster 6 in group 3, see below), which is revealed by the position of the cluster in the ordination space (Fig. 3). We consider this vegetation to be an initial variant of Limonio gmelinii-Artemisietum monogynae. Average species number was 4.5.
Group 3
Based on the position of clusters in the NMDS ordinations (Figs. 2, 3), we allocated into this group two alliances from two different classes: Puccinellion limosae (class Festuco-Puccinellietea) and Salicornion prostratae (class Therosalicornietea). These extremely species-poor vegetation groups formed spatially closely related patterns, as a mosaic of several different communities (cluster 6, 7 and 8) in the centre of the salt-affected area, usually in the close vicinity of emerging salt springs in the mudflat zone.
Vegetation preferring high soil salinity, pH and higher moisture with a moderately fluctuating water regime (Fig. 4a–c) was assigned to class Festuco-Puccinellietea, order: Puccinellietalia, alliance Puccinellion limosae (Fig. 2, Table 1). Cluster 6 belongs here.
Cluster 6: association Puccinellietum limosae Rapaics ex Soó 1933, 1936. (relevés No. 58–76 in Electronic supplementary material 4).
Dg, Cs and Dm species: Tripolium pannonicum
Cs and Dm species: Puccinellia distans agg.
Cs species: Salicornia perennans
These rather hemicryptophytic stands usually formed the zone near the salt springs, under a strong influence of fluctuating saline groundwater. They preferred desiccating places and lower moisture (Fig. 4d), and had the lowest soil salinity and pH preferences among group 3 (Fig. 4a, b). The species-poor stands are represented by the three above-mentioned Dg, Cs and Dm species (Electronic supplementary material 4, relevés No. 58–69). Species richness was low (average: 4). Rarely, in drier and less saline habitats with lower nutrient content, Tripolium pannonicum was co-dominant, and Salicornia perennans was absent (Electronic supplementary material 4, relevés No. 70–76). Stands of Cluster 6 were identified with the association Puccinellietum limosae, however, they formed a transitional vegetation to the therophytic alliance Salicornion prostratae. Vegetation of cluster 6 in our study was therefore allocated to group 3.
Vegetation preferring the highest soil salinity, pH and nutrients (Fig. 4a–d) within the entire vegetation under study was assigned to the class Therosalicornietea, order Camphorosmo-Salicornietalia, Salicornion prostratae (Fig. 2, Table 1). Its characteristic species greatly differ in both life form (annual succulents) and the physiognomy of the stands. Clusters 7 and 8 belong here; their habitat preferences largely overlap (Fig. 3).
Cluster 7: association Suaedetum maritimae (Soó 1927, 1957) Wendelberger 1943. (relevés No. 77-86 in Electronic supplementary material 4).
Dg, Cs and Dm species: Suaeda prostrata
Dg and Cs species: Atriplex littoralis, Salicornia perennans
Cs and Dm species: Puccinellia distans agg.
In these stands, Suaeda prostrata, Puccinellia distans agg. and Salicornia perennans occurred with similar frequency, while Atriplex littoralis had a lower cover. Cluster 7 had high preferences for salt content in the soil, pH and moisture (Fig. 4a–c). We found this vegetation near the emerging saline groundwater as a transition zone into the stands of Salicornietum prostratae, and on disturbed soils (trampled or excavated) of the salt-affected area. The average species number was 5.
Cluster 8: association Salicornietum prostratae Soó 1964. (relevés No. 87–109 in Electronic supplementary material 4).
Dg and Dm species: Salicornia perennans
Cs species: Puccinellia distans agg.
Mono-dominant initial vegetation occupied the most extreme habitats in the immediate vicinity of salt springs. Crusts of salt crystals were typical. It had the narrowest ecological amplitude within the studied vegetation, with the highest demands on salinity, soil reaction, moisture (stable water regime) and nutrient content (Fig. 4a–d). The stands were formed by Salicornia perennans, accompanied by Puccinellia distans agg. (Electronic supplementary material 4, cluster 8, relevés 105–107). The vegetation was easily identified by the reddish colour of Salicornia specimens, and was observed in large areas of the lowest lying places of the larger salt-affected localities (e.g. Gădălin, Sic, Turda, Bonţ), or was scattered in areas where salt springs discharge. The average species number was 2.
Discussion
Inland Salt Marshes
All associations within the Juncion gerardi alliance in the study area are very similar in terms of species composition and habitat requirements. They differ mainly in dominants, which also determine their physiognomy.
Cluster 1
Several associations of Juncion geradi have been published from the Transylvanian Basin. The first, the community Triglochinetum maritimae, was reported by Soó (1927) from Someşeni and Apahida. Later, Soó (1947) used the name Astereto-Triglochinetum and identified it with Scorzonero parviflorae-Juncetum gerardi, described by Wendelberger (1943) from Lake Neusiedl in the Pannonian Lowland. Csűrös (1947) documented the vegetation Triglochin maritima – Aster tripolium with one phytosociological relevé. The following studies reported Scorzonero parviflorae-Juncetum gerardi under the synonym Juncetum geradi Wendelbg. 1950: from Cojocna, Pop and Hodişan (1980), from Ocna Dej, Pop et al. (1983), with the most up-to-date contribution being from Sic and Cojocna (Dítě et al. 2015a). The association is relatively widespread in the study area and its species composition does not differ from the stands in other parts of Central Europe (e.g. Saxony Anhalt in Germany) and the Pannonian Lowland. All recorded stands of Scorzonero parviflorae-Juncetum gerardi are regarded as the variant Tripolium pannonicum (see Dítě et al. l.c.).
Ţopa (1939) considered the stands with Triglochin maritima and Aster tripolium to be a special case and extended its area to NE Romania and the Black Sea coast. He used the name Astero tripolio-Triglochinetum maritimi and included it in the coastal alliance Juncion maritimae as did Doltu et al. (1977). Later, Pop et al. (2002) included the association under the older name Triglochino maritimae-Asteretum pannonici (Soó 1927) Ţopa 1939 in the alliance Juncion geradi. They mention several sites of the community in the Transylvanian Basin (Someşeni, Apahida, Bonțida, Cojocna, Salina Turda, Valea Florilor). Finally, in the newest national vegetation survey (Sanda et al. 2008) it was published as Astero tripolio-Triglochinetum maritimi Soó 1937, Ţopa 1939 in the alliance Juncion gerardi. The association described from the Transylvanian Basin with a very similar species composition to Scorzonero parviflorae-Juncetum gerardii is known only from the territory of Romania, and has not been noticed in the Pannonian Lowland (e.g. Vicherek 1973; Mucina 1993; Šumberová et al. 2007; Borhidi et al. 2012; Melečková et al. 2014; Dítě et al. 2015a). We propose not to distinguish these two communities (Astero tripolio-Triglochinetum maritime and Triglochino maritimae-Asteretum pannonici), as their ecological and floristical differences are negligible.
Cluster 2
Similarly, the wet saline meadow association Plantagineto cornuti-Agrostetum stoloniferae is reported only from Romania. It was first noted from Apahida, Someşeni, Sic and Coasta by Soó (1947), as a subassociation of the Triglochinetum maritimae-Asteretum tripolii. Pop et al. (2002) and Sanda et al. (2008) regard it as a discrete association named Plantagineto cornuti-Agrostetum stoloniferae. The species composition is identical to the above described associations of the Juncion gerardi alliance, the single difference being the strong dominance of Plantago cornuti. Regarding abiotic conditions, the vegetation is not under the direct influence of the core saline area (Bădărău 2005 sec. in Diodiu 2010), similarly to Scorzonero parviflorae-Juncetum gerardii. Plantagineto cornuti-Agrostetum stoloniferae preferred a little lower salinity concentrations according to the indicator value analyses (Fig. 4a). The species composition found is similar to the one found by Soó (1947). Analogous vegetation described by Golub et al. 2003 (Plantagini cornuti-Juncetum gerardii) occurs in wet solonchak soils of river floodplains of the forest-steppes of Eastern Ukraine. This clearly shows the continuity of these salt meadows. The sites of Transylvania are the westernmost occurrence of the association in Eurasia.
Inland Salt Steppes (Clusters 3, 4, 5)
Regarding the alliance Festucion pseudovinae in the Transylvanian Basin, Sanda et al. (2008) include four associations: 1. Achilleo-Festucetum pseudovinae (Magyar 1928) Soó (1933) 1945, 2. Artemisio-Festucetum pseudovinae, 3. Artemisio-Petrosimonietum triandrae and 4. Limonio gmelinii-Artemisietum monogynae. The first two are widespread and well documented from all over Pannonia (Vicherek 1973; Mucina 1993; Šumberová et al. 2007; Borhidi et al. 2012; Dítě et al. 2014), while information about the latter two is scarce.
Artemisio-Petrosimonietum triandrae, described from the Transylvanian Basin, is of a great phytogeographical interest in the study area. Petrosimonia triandra, the co-dominant taxon of the community, is a continental species with core occurrence in the steppe and forest steppe zones of Central Asia. To the west it extends through Ukraine and Moldova to Romania with an isolated location in Eastern Hungary (Jalas and Suominen 1980). The association is mentioned only in the Romanian literature, where it has been recorded in the Wallachian Plain (e.g. Mititelu et al. 1969). In Ukraine, the association Puccinellio distantis-Petrosimonietum triandrae (alliance Puccinellion limosae) has recently been described from the NW coast of the Black Sea near Odessa (Dubyna et al. 2017).
The association Limonio gmelinii-Artemisietum monogynae was also described in Romania under the name Staticeto-Artemisietum monogynae Ţopa 1939 and was assigned to the alliance Puccinellio-Staticion gmelinii (note: no other authors used this syntaxon name; it is usually regarded as Puccinellion limosae). The association constitutes the boundary between the alliances Festucion pseudovinae and Puccinellion limosae. Sanda et al. (2008) assigned the community to Festucion pseudovinae, which we confirmed in our analyses. Borhidi et al. (2012) allocated it into the Puccinellion limosae. He considers the association to be a continental community typical of the Transylvanian Basin, extending only marginally to the southeastern part of Pannonia. This community has been erroneously reported from northern Pannonia and from Slovakia (Vicherek 1973), which represents the northernmost occurrence of its main species Limonium hungaricum. Those stands are, however, the typical association of Artemisio-Festucetum pseudovinae (Dítě et al. 2014). Limonio gmelinii-Artemisietum monogynae can be regarded as secondary vegetation indicating disturbance (overgrazing).
The salt steppe vegetation occupied a rather small area in each surveyed locality. The most widespread stands were Artemisio-Festucetum pseudovinae. In the vegetation of the intensively grazed or drained sites (e.g. Juc de Mijloc), Elytrigia repens had high abundance. Together with Poa pratensis agg. They indicated vegetation degradation, as its central distribution was reported to be in the Pannonian Lowland (Dítě et al. 2013; 2021).
Mudflat Vegetation of the Most Salinized Soils (Clusters 6, 7, 8)
The association Puccinellietum limosae was first mentioned by Csűrös (1947) and was subsequently recorded in each study concerning the Transylvania Basin. Regarding the wider range of its habitat preferences (particularly with respect to moisture) the species composition is various; Sanda et al. (2008) distinguishes seven subassociations. The special feature of stands in the Transylvanian Basin is the high abundance of Salicornia perennans. This species in the neighbouring Pannonian Lowland is rare, growing mainly on the exposed bottoms of salt lakes (Dítě et al. 2017).
From the Black Sea coast, Popescu et al. (1987) recorded vegetation with a broad variety of abundance of Salicornia europaea. In addition, Puccinellia limosa, Tripolim pannonicum and Spergularia media were constantly present with several cover ranges, as seen in the Transylvanian salt marshes. The stands are distinguished by the coastal species Suaeda maritima, Aeluropus littoralis, Frankenia hirsuta and Halimione pedunculata and are recognized as association Puccinellio-Salicornietum of the class Therosalicornietea.
The annual succulent vegetation with euhalophytic species (Cluster 7) in the Transylvanian Basin is emphasized by Csűrös (1947). Pop and Hodişan (1980) noted both associations from Cojocna, and Pop et al. (1983) recorded them from Sic and Ocna Dej.
Pop et al. (2002) provided numerous data on the associations Salicornietum prostratae and Suaedetum maritimae from the localities Someşeni, Cojocna, Valea Florilor, Băile Sărate Turda, Ocna Dej and Sic. Both associations are identical in species composition (see p. 161, Table 35 in Pop et al. 2002), the only difference being the proportion of Salicornia europea and Suaeda maritima. In contrast to the published material, we did not record stands with a dominance of S. prostrata, although it figured as a characteristic species with up to 40% cover (see Electronic supplementary material 4). This corresponds to our findings in the Pannonian Lowland, where S. prostrata (unlike Suaeda pannonica) does not form dominant stands but constitutes a characteristic species in the association Puccinellion limosae (Dítě et al. 2015b; 2017). In this study we use the syntaxon name Suaedetum maritimae, under which the association was published in the Romanian vegetation survey of Sanda et al. (2008), despite the fact that Suaeda maritima does not occur in Transylvania. Since the name of Suaeda prostrata was not properly used and it was often named as S. maritima in the older literature (e.g. Soó 1927), the confusion was transferred into the syntaxonomy. We propose Suaedetum prostratae as the name for the vegetation with S. prostrata in the Transylvanian Basin. The name Suaedetum prostratae J. M. Gehu 1975 was published by Hobohm and Pott (1992) for stands of S. prostrata from the Wadden Sea in N Germany. Using the name Spergulario marginatae-Suaedetum prostratae Vicherek in Moravec et al. 1995 is also appropriate; however, this community, described from the westernmost part of Pannonia (South Moravia in the Czech republic), has become extinct due to destruction of localities in the 1960s (Šumberová et al. 2007).
In contrast to our records, several published relevés of the mentioned studies had a significant dominance of Salicornia perennans and are more species-rich. This may be related to the size of the relevés, as in the past they often comprised areas of 100 to 200 m2. Borhidi et al. (2012) pointed out the peculiarity of salt marshes in the Transylvanian Basin through their extremely low species number (2–3 species) in the Salicornia stands. Similar vegetation was observed in the Harghita County east of our study area (Praid and Băile Chirului; Dítě Z. and Dítě D. 2015, ined.). Outside Romania, analogous vegetation and origin occurs in central eastern Germany, where saline vegetation develops secondarily on mining heaps (Garve and Garve 2000), or in the Polish region of Kujawy around soda factories (Piernik 2005).
Although Salicornia perennans is able to grow in several salt habitats (Puccinellietum, etc.), its association Salicornietum prostratae has a very narrow ecological amplitude (see Fig. 4). Its demand for high salt content and a fluctuating water regime is limited due to the recent exploitation of existing sites by human activities.
Băile Someşeni – a Unique Salt Marsh Site Differing from the Others
As part of the sampling of the salt habitats in the study area, we would like to especially highlight the locality of Băile Someşeni near the city of Cluj. The site is known for its characteristic fen vegetation on mineral salt waters with high salt content and it is one of the few existing localities in the Transylvania Basin of two brackish habitat specialists, Glaux maritima and Cladium mariscus. Since the origin and the stage of the vegetation differ from the other studied localities, we did not include data from this site in our analyses. We document the recent stage of the vegetation with the occurrence of the two mentioned species by the following relevé, recorded on the most preserved part of the area:
Cluj, Băile Someşeni, the edge of saline, low-herb vegetation near the trampled path surrounded by stands of Phragmites australis, 46°46′44.3″ N, 23°39′13.2″ E, 16 m2, 21 July 2018
E1: Glaux maritima 3, Agrostis stolonifera 2a, Phragmites australis 2a, Plantago maritima 2a, Carex distans 1, Cladium mariscus 1, Eleocharis uniglumis 1, Scorzonera parviflora 1, Sonchus palustris 1, Triglochin maritima 1, Mentha arvenis +, Odontites vulgaris +, Schoenoplectus tabernaemontani +.
Recently, the whole site has suffered from significant degradation. More than 90% of the area was occupied by Phragmites australis and there is a massive expansion of shrubs. The remnants of the low herb vegetation are preserved on the edges of reed beds and were regarded as the Juncion gerardi alliance, in particular the Scorzonero parviflorae-Juncetum gerardii association. We have found one micro-site with vital populations of Glaux maritima. There are no management activities on the locality, therefore, the long-term persistence of the species is threatened by the secondary succession.
Current Conservation Status of Inland Salt Marshes and Salt Steppes of Transylvania
Inland halophytic vegetation of salt springs belongs to the group of highly threatened habitats throughout Europe with a high number of endangered plant and animal species (CORINE 1991). They are classified on the European Red List of habitats (Janssen et al. 2016), and in the newest European Nature Information System (EUNIS) as E6.3 Temperate inland salt marsh (Chytrý et al. 2020). They are also listed as Annex 1 habitats within the Natura 2000 network (https://eunis.eea.europa.eu/habitats/10029).
The unique physico-chemical composition of salt waters and the curative effects of organic-rich sediments are the most relevant features of Transylvanian salt areas that led to early economic exploitation and scientific interest (Alexe et al. 2018). Their hypersaline and heliothermal water alongside sapropelic muds have been exploited since the sixteenth century for therapeutic purposes. Increasing tourism promotes the development of the local economy (e.g. in the city of Turda, partially in Cojocna), but the high vulnerability of salt lakes due to their closed basins fed only by precipitation is particularly prone to negative alteration and fragmentation of existing sites (e.g. large influx of swimmers, modifications of the catchments). The recently built vast parking areas decreased the area of salt vegetation; this and the capture of springs for water extraction, and increasing waste and euthrophication are serious threats. The sites Buneşti (near Gherla) or Apahida (near Cluj) are threatened by infrastructure development, such as road construction and rubbish dumping (see the photographical documentation of the different landscapes and disturbance regimes in Electronic supplementary material 3). Each site has a disturbed water regime, and drainage ditches are being built continuously. Although many localities were declared to be special protected areas, the destruction leads to irreversible negative processes. Controlled grazing is an appropriate management measure for preserving the habitat in good condition, but its intensification leads to high degradation (e.g. on the localities Turda and Pata).
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Alec A (2010) The interrelation between the geological substratum and the populations of some rare halophytic species from the Transylvanian basin. PhD. Thesis, Babeş-Bolyai University, Cluj-Napoca
Alexe M, Șerban G, Baricz A, Andrei A, Cristea A, Battes K, Cîmpean M, Momeu L, Muntean V, Porav S, Banciu H (2018) Limnology and plankton diversity of salt lakes from Transylvanian Basin (Romania): a review. J Limnol 77:17–34
Barkmann JJ, Doing H, Segal S (1964) Kritische Bemerkungen und Vorschläge zur quantitativen Vegetationsanalyse. Acta Bot Neerl 13:394–419
Borhidi A (1995) Social behaviour types, their naturalness and relative ecological indicator values of the higher plants of the Hungarian flora. Acta Bot Hung 39:97–182
Borhidi A, Kevey B, Lendvai G (2012) Plant communities of Hungary. Akadémiai Kiadó, Budapest
Brandes D (1999) Auswahlbibliographie zur binnenländischen Halophytenvegetation in Deutschland [Selected bibliography on inland halophyte vegetation in Germany] – In: Brandes D. (Hrsg.): Vegetation salzbeeinflußter Habitate im Binnenland [Vegetation of salt-influenced habitats in the mainland] Braunschweiger Geobotanische Arbeiten 6:259–270 [in German]
Chapman VJ (1960) Salt marshes and salt deserts of the world. Leonard Hill (Books) Ltd., London
Chytrý M, Otýpková Z (2003) Plot sizes used for phytosociological sampling of European vegetation. J Veg Sci 14:563–570
Chytrý M, Tichý L, Holt J, Botta-Dukát J (2002) Determination of diagnostic species with statistical fidelity measures. J Veg Sci 13:79–90
Commision of the European Communities (1991) CORINE Biotopes manual, Habitats of the European Community EUR 12587/3. Office for Official Publications of the European Communities, Luxembourg
Csűrös ŞT (1947) Contribuţiuni la cunoaşterea vegetaţiei sărăturilor din împrejurimile Clujului [Contributions to the knowledge of the salt vegetation in the surroundings of Cluj]. Bul Grăd Bot Univ Cluj 27:80–84 [in Romanian]
Csűrös-Káptalan M (1965) Vegetaţia halofilă din Valea Aitonului [Halophytic vegetation of the Aiton Valley]. Contr Bot Univ “Babeş-Bolyai” Cluj Grăd Bot 221–229 [in Romanian]
Csűrös-Káptalan M, Péterfi LS (1966) Vegetația lacului de la Ceanu Mic (raion Turda) [Vegetation of the lake Ceanu Mic (Turda district)]. Contr Bot Univ “Babeş-Bolyai” Cluj Grăd Bot 43–48 [in Romanian]
Deák B, Valkó O, Alexander C, Mücke W, Kania A, Tamás J, Heilmeier H (2014) Fine-scale vertical position as an indicator of vegetation in alkali grasslands – case study based on remotely sensed data. Flora 209:693–697
Diodiu R (2010) Plantago cornuti Gouan, a rare halophyte: sites from Transylvanian Depression (Romania) ELBA Bioflux 2:58–63
Dítě D, Eliáš P jun, Melečková Z (2013) Artemisia santonicum subsp. patens in Slovakia: the sad story of obligate halophyte on the Northern edge of its distribution range. Hacquetia 12:5–16
Dítě D, Melečková Z, Eliáš P jun (2014) Vegetácia vnútrozemskej slanej stepi (Festuco-Puccinellietea) [Vegetation of inland salt steppe] In Hegedüšová Vantarová K, Škodová I (eds) Rastlinné spoločenstvá Slovenska 5. Travinno-bylinná vegetácia [Plant communities of Slovakia 5. Grassland vegetation]. Veda, Bratislava pp 483–510 [in Slovak]
Dítě D, Melečková Z, Šuvada R, Píš V, Eliáš P jun (2015a) The phytosociology and ecology of saline vegetation with Scorzonera parviflora in the Pannonian-Western Balkan gradient. Phytocenologia 45:33–47
Dítě D, Perić R, Melečková Z, Eliáš P jun (2015b) Distribution and communities of Suaeda pannonica in Serbia. Bull Nat Hist Mus Belgrade 7:101–117
Dítě D, Eliáš P jun, Dítětová Z, Píš R, Šuvada R (2017) Vegetation classification and ecology of Pannonian salt lake beds. Phytocoenologia 47:329–344
Dítě Z, Šuvada R, Tóth T, Eliáš P jun, Píš V, Dítě D (2021) Current condition of Pannonic salt steppes at their distribution limit: What do indicator species reveal about habitat quality? Plants 10:530
Doltu MI, Sanda V, Popescu A (1977) Vegetaţia solurilor saline şi alcalice din România [Vegetation of saline and alkaline soils in Romania]. Stud Com Muz Şt Nat Brukenthal Sibiu 23:197–217 [in Romanian]
Dubyna DV, Ennan AA, Dziuba TP, Vakarenko LP, Shykhaleeva HM (2017) Syntaxonomy of halophytic vegetation of Kuialnyk Estuary. Ukrain Bot J 74:562–573
Eliáš P jun, Sopotlieva D, Dítě D, Hájková P, Apostolova I, Senko D, Melečková Z, Hájek M (2013) Vegetation diversity of salt-rich grasslands in the south-east Europe. Appl Veg Sci 16:521–537
Euro+Med (2018) Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity. Available at ww2.bgbm.org/EuroPlusMed [accessed February 2020]
Garve E, Garve V (2000) Halophyten an Kalihalden in Deutschland und Frankreich (Eisass). Tuexenia 20:375–417
Golub VB, Karpov DN, Lysenko TM, Bazhanova NB (2003) Conspectus of communities of the class Scorzonero-Juncetea gerardii Golub et al. 2001 on the territory of the Commonwealth of Independent States and Mongolia. Bull “Der Samarer Bogen” Samara 13:88–140
Grigore MN, Cojocariu A (2020) A tentative list of Romanian halophytes: taxonomy, distribution, and ecology. In Grigore MN (ed) Handbook of halophytes. Springer, Cham
Har N, Rusz O, Codrea V, Barbu O (2010) New data on the mineralogy of the salt deposit from Sovata (Mureş county, Romania). Carpathian J Earth Environm Sci 5:127–135
Hennekens SM, Schaminée JHJ (2001) TURBOVEG, a comprehensive database management system for vegetation data. J Veg Sci 12:589–591
Hobohm C, Pott R (1992) Das Suaedetum prostratae: eine bislang übersehene Salzwiesenassoziation im Wattenmeerbereich und Vorschläge zur Gliederung der Klasse Thero-Salicornietea. [The Suaedetum prostratae: a previously overlooked salt marsh association in the Wadden Sea and suggestions for the subdivision of the class Thero-Salicornietea]. Ber Reinhold-Tüxen-Ges 4:123–133 [in German]
Jakab G, Silye L, Sümegi P, Tóth A, Sümegi B, Pál I, Benkő E (2019) Relict anthropogenic ecosystem from the middle ages: history of a salt marsh from transylvania (Sic, N Romania). Environm Archaeol 25:96–113
Jalas J, Suominen J (eds) (1980) Atlas Florae Europaeae. Distribution of vascular plants in Europe. 5. Chenopodiaceae to Basellaceae. The Committee for Mapping the Flora of Europe and Soc Biol Fenn Vanamo, Helsinki, pp. 119
Janssen JAM, Rodwell JS, Garcia Criado M, Gubbay S, Haynes T, Nieto A, Sanders N, Landucci F, Loidi J, Ssymank A et al (2016) European red list of habitats. Part 2. Terrestrial and freshwater habitats. Luxembourg: Publications Office of the European Union
Krézsek C, Bally AW (2006) The Transylvanian Basin (Romania) and its relation to the Carpathian fold and thrust belt: Insights in gravitational salt tectonics. Mar Petrol Geol 23:405–442
Krézsek C, Filipescu S (2005) Middle to Late Miocene sequence stratigraphy of the Transylvanian Basin (Romania). Tectonophysics 410:437–463
Kun A, Ruprecht E, Szabó A (2004) Az Erdélyi-medence bioklimatológiai jellemzése [The bioclimatological characteristics of the Transylvanian Basin (Romania)]. Múz Füz 13:63–81 [in Hungarian]
Leuschner C, Ellenberg H (2017) Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats. Vegetation Ecology of Central Europe [Vol. II]. Springer, Cham, CH
McCune B, Mefford MJ (2006) PC-ORD. Multivariate analysis of ecological data. Ver. 5.31 MjM software. Gleneden Beach, Oregon
Melečková Z, Dítě D, Eliáš P jun (2014) Vegetácia kontinentálních slaných lúk (Scorzonero-Juncetea gerardii) [Vegetation of continental salt meadows]. In Hegedüšová Vantarová K, Škodová I (eds) Rastlinné spoločenstvá Slovenska 5. Travinno-bylinná vegetácia [Plant communities of Slovakia 5. Grassland vegetation]. Veda, Bratislava pp 513–532 [in Slovak]
Mititelu D, Gociu Z, Pătraşcu A, Gheorghiu E (1969) Caracterul florei şi vegetaţia din câmpia Galaţilor şi Brăilei [The character of the flora and the vegetation from the Galaţi and Brăila plain]. Comun Bot 10:191–200 [in Romanian]
Mucina L (1993) Puccinellio-Salicornietea. In Mucina L, Grabherr G, Ellmauer T (eds) Die Pflanzengesellschaften Österreichs. Teil 1, Anthropogene Vegetation. [Plant communities of Austria, Part 1, Anthropogenic vegetation]. Gustav Fischer Verlag, Jena, pp 522–549 [in German]
Mucina L, Bültmann H, Dierßen K, Theurillat JP, Raus T, Čarni A, Šumberová K, Willner W, Dengler J, Gavilán García R. et al (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19(Suppl.1):3–264
Nyárády E Gy (1941) Kolozsvár környékének mocsárvilága [Wetlands of the vicinity of Cluj Napoca] Erdélyi Tudományos Füzetek 125:1–30 [in Hungarian]
Oksanen J, Guillaume-Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Vegan: community ecology package. R package version 2.0-10. Available at http://CRAN.R-project.org/package=vegan [accessed September 2017]
Piernik A (2005) Vegetation-environment relations on inland saline habitats in Central Poland. Phytocoenologia 35:19–37
Piernik A (2020) An ecological overview of halophytes in inland areas of Central Europe. In Grigore MN (ed) Handbook of halophytes. Springer, Cham
Podar D, Macalik K, Réti K, Martonos I, Carpa R, Török E, Csiszár J, Szekely Gy (2019) Morphological, physiological and biochemical aspects of salt tolerance of halophyte Petrosimonia triandra grown in natural habitat. Physiol Molec Biol Pl 25:1335–1347
Popescu A, Sanda V, Fişteag G (1987) Cercetări fitocenotice în zona grindurilor Letea şi Stipoc (Delta Dunării) [Phytocenological research in the area of Letea and Stipoc ridges (Danube Delta)] Studii şi de Cercetări Biologie. Seria Biologie Vegetală 39(1):25–33 [in Romanian]
Pop I, Hodişan I (1980) Analiza cormoflorei şi a vegetaţiei de la Băile Cojocna (jud. Cluj) [Analysis of cormoflora and vegetation from Băile Cojocna (Cluj distr.)]. Contr Bot 69–87 [in Romanian]
Pop I, Cristea V, Hodişan I, Raţiu O (1983) Studii biologice asupra florei şi vegetaţiei din zona lacurilor de la Ocna Dej şi Sic (jud. Cluj) [Biological studies on the flora and vegetation of lakes in Ocna Dej and Sic (Cluj distr.)]. Contr Bot 1983:45–63 [in Romanian]
Pop I, Cristea V, Hodişan I. 2002. Vegetaţia judeţului Cluj. (Studiu fitocenologic, ecologic, bioeconomic şi eco-protectiv) [The vegetation of Cluj district. Phytosociological studies] Contr Bot 35:5–254 [in Romanian]
QGIS Development Team (2018) QGIS geographic information system. Open Source Geospatial Foundation. Available at http://qgis.osgeo.org
Réti KO, Macalik K, Carpa R, Kis E, Székely G (2016) Physico-chemical properties of soils populated with wild halophytes in some Romanian areas. Stud Univ “Babes-Bolyai” Biol 61:107–116
Ruprecht E (2005) Secondary succession in old-fields in the Transylvanian Lowland. Preslia 77:145–157
Sanda V, Öllerer K, Burescu P (2008) Fitocenozele din România. Sintaxonomie, structură, dinamică și evoluţie [Phytocoenosis in Romania. Syntaxonomy, structure, dynamics and evolution]. Editura Ars Docendi, București [in Romanian]
Soó R (1927) Geobotanische Monographie von Kolozsvár (Klausenburg) [Geobotanical monography of Cluj]. Tisza István Tud Társ Honism Bizott Kiadv J 4:1–151 [in German]
Soó R (1933) A Hortobágy növénytakarója [Vegetation of the Hortobágy]. A Debreceni Szemle kölönszáma, Debrecen: Városi Nyomda, pp 26 [in Hungarian]
Soó R (1947) Des groupements vegetaux dans les Bassins Carpathiques. I. Les associations halophiles [Plant communities of the Carpathian Basin. I. Halophilic associations]. Institut. Botanique de l’Universite a Debrecen, Debrecen, pp. 60 [in French]
StatSoft, Inc (2004) STATISTICA (data analysis software system), version 7. www.statsoft.com
Šumberová K, Novák J, Sádlo J (2007) Slaniskové trávníky (Festuco-Puccinellietea). [Saline grasslands]. In Chytrý M (ed) Vegetace České republiky 1. [Vegetation of the Czech Republic 1]. Academia, Praha, pp 150–164 [in Czech]
Szabolcs I (1974) Salt-affected soils in Europe. Martinus Nijhoff , Hague
Tichý L (2002) JUICE, software for vegetation classification. J Veg Sci 13:451–453
Todor I (1947) Flora şi vegetaţia de la Băile Sărate-Turda I [Flora and vegetation from Băile Sărate-Turda I]. Bul Grăd Bot Univ Cluj 27:1–64 [in Romanian]
Todor I (1948) Flora şi vegetaţia de la Băile Sărate-Turda II. [Flora and vegetation from Băile Sărate-Turda]. Bul Grăd Bot Univ Cluj 28:21–174 [in Romanian]
Ţopa E (1939) Vegetaţia halofitelor din nordul României în legătură cu cea din restul ţării. [Halophytic vegetation of northern Romania in connection with the rest of the country]. Bul Fac Şt Cernăuţi 13:1–79 [in Romanian]
Vicherek J (1973) Die Pflanzengsellschaften der Halophyten und Subhalophytenvegetation der Tschechoslowakei [The plant communities of halophytes and subhalophytic vegetation of Czechoslovakia]. Vegetace ČSSR, ser. A, Praha 5:1–200 [in German]
Wendelberger G (1943) Die Salzpflanzengesellschaften des Neusiedler Sees [Salt vegetation of the Lake Neusiedl]. Wiener Bot Zeitung 3:124–144 [in German]
Zlatković ID, Jenačković DD, Ranđelović VD (2019) Inland salt areas of Southeast Serbia: ecological preferences of certain representatives of flora. Biologia 74: 1425–1440
Acknowledgements
We thank Scott Burgess for the language revision, Anna Szabó (Babes Bolyai University, Cluj). The study was financially supported by the VEGA Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic, project No. 2/0001/20, title ‘Islands of continental saline vegetation in temperate Europe – what they have in common and in what they differ?’.
Author information
Authors and Affiliations
Contributions
DD and ZD conceived of the research idea, collected data in the field and wrote the manuscript; R.Š. performed statistical analyses; all authors discussed the results and commented on the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dítě, D., Šuvada, R. & Dítě, Z. Habitat shaped by ancient salt: vegetation of the classes Therosalicornietea and Festuco-Puccinellietea in the Transylvanian Basin (Romania). Folia Geobot 56, 109–123 (2021). https://doi.org/10.1007/s12224-021-09396-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-021-09396-6