Abstract
The use of local therapy with antibiotics in a suitable carrier is essential in the treatment and prevention of infections in orthopedic surgery and traumatology. In our orthopedic surgery department, a synthetic calcium sulfate hemihydrate (CaSO4·½H2O) is used as an antibiotic carrier, enabling the application of most types of intravenous antibiotics in the form of powder and liquid. This type of carrier with antibiotics is prepared in the theater during the procedure. During a surgical procedure, a small dead space is created (hand and foot area), which must be filled with an antibiotic carrier, and the situations arise where a large amount of the carrier is not used and thrown away. Therefore, we verified the efficacy of vancomycin in the pre-prepared carrier by an orientation microbiological method and by measuring the concentrations of the vancomycin released in active form and its two crystalline degradation products. Based on the agar diffusion test, we did not measure any difference in the effectiveness of the antibiotic in the carrier after its 12-day storage. Although vancomycin concentrations decreased by approximately 32% at the end of 12 days of storage, the concentrations of the released active form of vancomycin are many times higher than the minimum inhibitory concentrations for resistant strains of Staphylococcus aureus. Thus, the calcium sulfate carrier with vancomycin can be prepared several days in advance before its application, certainly up to 12 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An early and sufficiently radical surgical approach is essential for managing infectious complications in orthopedics and traumatology (Jahoda et al. 2006; 2008; Melicherčík 2011; Metsemakers et al. 2020). It includes targeted intravenous antimicrobial therapy. Achieving high levels of antibiotics directly at the site of the infectious complication is crucial (Jahoda et al. 2006, 2008; Kurmis 2021; Marson et al. 2018; Meani et al. 2007; Winkler et al. 2008). The availability of antibiotics administered intravenously is usually insufficient at the site of ongoing osteomyelitis, especially in joint replacement surgery. The application of local antibiotics is beneficial especially in arthroplasty and necessary if implants are utilized to prevent a rapid biofilm prevention (Flierl et al 2017; Jahoda et al. 2006, 2008; Meani et al. 2007; Melicherčík 2011; Doub et al. 2021; Witso et al. 2000).
The most frequently used proven method of antibiotic application to the sites of musculoskeletal and joint replacement infections is local carriers. Local carriers not only deliver antibiotics in high concentrations directly to the site of infection, but may also fill the dead space created by surgery. There are several types of local antibiotic carrier systems used in orthopedics. They can be divided according to the composition of the material into synthetic polymers (polymethyl methacrylate), natural polymers (collagen), ceramics (hydroxyapatite, calcium sulfate, calcium phosphate), composites (materials composed of ceramics and polymers), and bone grafts. Furthermore, local carriers can be divided according to the properties of the material (biodegradation, osteoinduction, and osteoconduction).
Clinical practice has shown that the most suitable materials used as local carriers of antibiotics in orthopedics appear to be polymers with the property of biodegradation and osteoconduction. These properties are most often fulfilled by ceramic material carriers (Meani et al. 2007; Melicherčík 2011). Perhaps most importantly is the release of antibiotics from the carrier in sufficiently high concentrations at the site of infection long enough to eradicate the infection (for 2–3 weeks) (Howlin et al. 2015; Meani et al. 2007; Jiang et al. 2021).
One of the most promising carrier materials for effective local antibiotic therapy is synthetic calcium sulfate hemihydrates (CaSO4·½H2O) with the product name Stimulan®. An advantage of Stimulan® is the possibility of applying intravenous antibiotics with this material, both in powder and liquid form or in a combination of two types of antibiotics or antifungals (Kanellakopoulou et al. 2009; Kurmis 2021; Noor et al. 2018; Biocomposites 2020). The use of calcium sulfate in orthopedics has been increasing, both as a bone void filler and also as a delivery agent for antibiotics in arthroplasty, chronic osteomyelitis, open fractures, and combat injuries (Abosala et al. 2020; Somasundaram et al. 2013). This is especially advantageous in cases where we already know the cause of the infection. Stimulan® is a fully absorbable material and allows predictable release of antibiotics at sufficiently high local levels (Aiken et al. 2015; Cooper 2004; Kallala et al. 2018; Lei et al. 2012; McPherson et al. 2013; Biocomposites 2020), does not cause damage to surrounding tissues and joint surfaces, and thus is useful for its application both to joints and around joint replacements (Cowie et al. 2016; Kallala et al. 2013; Lazarou et al. 2011; Lewicki et al. 2017). It does not act as a nidus for the development of infection (Howlin et al. 2015; Lazarou et al., 2011; Lei et al. 2007, 2012) and its drainage losses are minimal (Borrelli et al. 2003; Gauland 2011; Helgeson et al. 2009; McKee et al. 2002; McPherson et al. 2013; Morley et al. 2016).
After application of this calcium sulfate carrier to the area of joint replacements during reimplantation, no increased incidence of heterotopic ossification was observed (Kallala et al. 2018). Antibiotics (gentamicin, tobramycin, and vancomycin) are released from this carrier in concentrations exceeding the minimum inhibitory concentration (MIC) for at least 42 days (Howlin et al. 2015). The preparation of the Stimulan® carrier is performed in the operating theater during the operation. It can be applied either in the form of a paste with a syringe or in the form of beads. Beads can be prepared using a silicone bead mat in different sizes with diameter 3.0, 4.8, and 6.0 mm (Biocomposites 2020). Stimulan kit is available in sizes of 5 cm3 and 10 cm3 (Biocomposites 2020). The package size of the Stimulan® carrier is sufficient when it is used during the operation where there is relatively large dead space; sometimes it is even necessary to apply several packages at once. However, in some cases, especially in the leg and hand, the dead space after sequestrotomy is minimal. Therefore, only a small part of the prepared carrier is used, and the rest of the material is discarded. As a possible solution to reduce unnecessary waste of the local carrier and antibiotics, would be to prepare Stimulan® beads with antibiotics and store them in the necessary amount for their subsequent use for various patients within a few days of carrier preparation.
Studies working on the elution of antibiotics from Stimulan® have been performed in model situations only as carrier preparation during surgery (Howlin et al. 2015; Aiken et al. 2015; Morley et al. 2016). The data do not report whether there is a change in the elution curve and the potency of the antibiotic from the calcium sulfate carrier after several days of storage of the prepared beads already containing the antibiotic at room temperature (approx. 22 °C).
The aim of our study was (a) to measure the exact concentrations of the active form of vancomycin and its two crystalline degradation products (CDP-M and CDP-m) after release from Stimulan® beads by HPLC and to determine the antibacterial activity in vitro to find out whether the concentrations are higher than MIC for resistant bacterial strains (Klapkova et al. 2020) and (b) to verify the activity and stability of vancomycin by both methods after storage of this carrier at room temperature for 12 days.
Ethics
This study was conducted according to the guidelines laid down in the 1964 Declaration of Helsinki and its later amendments.
Material and methods
Preparation of the materials
Under sterile conditions, we prepared Stimulan® beads (synthetic semi-hydrated form of calcium sulfate, Biocomposites, Ltd., Keele, UK) with vancomycin (diameter 3.0 and 6.0 mm) according to the manufacturer’s recommended procedure. We added 1 g of vancomycin (Vancomycin Mylan 1 g) to 20 g of pharmaceutical-grade calcium sulfate hemihydrate powder (10cm3 kit, Stimulan®). The powder was mixed, and then after curing, i.e., after dehydration of the support, we prepared samples so that in one there was a carrier weighing 0.5 g, which corresponds to three 6.0-mm beads and one 4.8-mm beads.
Microbiological method
An agar diffusion test (an orientation bacteriological method) was used to verify the stability of vancomycin in the synthetic semi-hydrated form of calcium sulfate as an antibiotic carrier. One antibiotic bead (6 mm diameter) prepared by the method described above was immersed in a solid medium (Mueller–Hinton agar from Oxoid). Then the agar surface was overlaid with a suspension of the reference strain Staphylococcus aureus CCM 4223 (ATCC 29,213) in cell concentration with a turbidity of 0.5 according to the McFarland standard. The diameter of the inhibition zone formed around the test sample was evaluated for six samples after 18 h of incubation at a temperature of 37 °C. The same test was performed with another six samples of the same size after storage for 12 days at laboratory temperature (22 °C).
Beads weighing 0.5 g were put into 72 sterile tubes and stored in the dark at laboratory temperature for different time intervals (0, 24, 48, 72, 96, 120, 144, 168, 192, 216, 240, 264, and 288 h). After each defined interval, 10 mL of phosphate buffer (95 mL of 0.2 mol/L NaH2PO4·H2O, 405 mL of 0.2 mol/L Na2HPO4·7 H2O, and 500 mL of water corrected to pH 7.4) was added to the sample in the test tube. Six samples were prepared each time. The test tubes were then placed in an incubator controlled by a thermostat. After 6 h at controlled temperature (37 °C), 200 μL of phosphate buffer from each test tube was taken for determination of vancomycin concentration.
HPLC analysis
The concentrations of vancomycin and its crystalline degradation products were measured by using an Agilent 1260 system equipped with a diode array detector, quaternary pump system, column thermostat, and an auto sampler (Agilent Technologies, USA). Separation was performed by column Zorbax Sb-Ag (4.6 × 250 mm, particle size 5 μm) (Agilent Technologies, USA). The column was maintained at a temperature 22 °C. A gradient of mobile phases A and B was used. Mobile phase A consisted of 910 mL of phosphate buffer (2.14 g K2HPO4 and 11.94 g KH2PO4 dissolved in 2 L of distilled water, pH 7.4), 50 mL of acetonitrile, and 40 mL of methanol. Mobile phase B consisted of 840 mL of the same phosphate buffer, 80 mL of acetonitrile, and 80 mL of methanol. Flow rate was set at 1.5 mL/min and resulted in a pressure of 23 MPa. Total run time per sample was 28 min. Retention times of vancomycin, CDP-m, CDP-M, and cefazolin were 11 min, 5.0 min, 7.0 min, and 17 min, respectively (Fig. 1). Quantification was based on the peak area ratio of each analyte to the internal standard. Detector wavelength was set at 210 nm and injected volume of sample was 50 μL. The cefazolin was selected as an internal standard in concentration 100 mg/L. The chromatograms were processed by OpenLab (Agilent Technologies). The recovery of SPE columns was 79–87%. The calibration curves of vancomycin and its crystalline degradation products were constructed by measuring eight calibration standards: for vancomycin—1, 10, 30, 60, 100, 200, 400, and 600 mg/L; for CDP-M—1.08, 10.8, 32.3, 64.6, 100, 200, 400, and 769 mg/L; and for CDP-m—0.3, 3.2, 9.7, 19.4, 30, 60, 120.1, 231 mg/L. The assays were linear across the whole range of concentrations with mean correlation coefficients R2 of 0.9999 for all calibrated analytes. Two levels of vancomycin control samples were prepared at concentrations of 15 and 100 mg/L, and two crystalline degradation product control samples were prepared at concentrations of CDP-M—50 and 100 mg/L and CDP-m—15 and 30 mg/L. The entire HPLC method and its validation parameters are described in a paper written by Klapkova et al. (2020).
Statistics
The statistical evaluation was done using GraphPad Prism 8.0.1. The data obtained were tested using the Games-Howell’s multiple comparisons test. All the variance analysis was performed at a 95% confidence level. Value of p < 0.05 was considered to be significant.
Results
Verification of the stability of vancomycin in the calcium sulfate carrier by an orientation bacteriological method (agar diffusion test) is shown in Fig. 2. The diameter of the inhibition zones in the vancomycin samples was the same in all cases, i.e., 27 mm. This suggests that vancomycin activity remained unchanged over the times. As a control, the antibiotic-free Stimulan® bead showed no antibacterial activity.
Orientation bacteriological method, agar diffusion test; the diameter of the inhibition zone after 18 h of incubation in a thermostat at 37 °C. A Vancomycin carrier bead applied immediately after processing; diameter = 27 mm. B Vancomycin carrier bead applied 12 days after its preparation; diameter = 27 mm. C Control sample, Stimulan® bead without antibiotic; no zone
During the first measurement of six samples, we measured the average concentration of vancomycin 710.5 mg/L, CDP-M 20.5 mg/L, and CDP-m 9.4 mg/L. In other time intervals with increasing storage time of the carrier, we noticed, with the exception of small oscillations during the first five measurements, a decrease in the average concentrations of both the active form of vancomycin and its two crystalline degradation products. The highest average concentrations of vancomycin and its thermodegradation products (780.9 mg/L, 20.4 mg/L, and 8.0 mg/L, respectively) were reached after 120 h of storage while the lowest concentrations of vancomycin, CDP-M, and CDP-m were measured after 12 days of storage (479.9 mg/L, 13.1 mg/L, and 5.6 mg/L, respectively). Statistical evaluation of the data showed no statistically significant differences by comparing the following two concentrations (Table 1). However, if we compare the data to the first value, which is the concentration of freshly prepared carrier released to the phosphate buffer, a statistically significant decrease is observed for vancomycin after 174 h of storage (day 7, p = 0.0427). A more significant decrease was evaluated after 246 h of storage (day 10, p = 0.002). No statistically significant difference for CDP-M was observed until the tenth day of storage, for CDP-m until the end of experiment.
Discussion
Local antibiotic therapy using carriers is now commonly used in orthopedics and traumatology for the successful eradication of infections. The benefits of synthetic calcium sulfate hemihydrate antibiotic carriers are already mentioned in the “Introduction” section. This type of material has also been shown to be effective in the treatment of small defects and osteomyelitis in the foot area in diabetics (Morley et al. 2016). For these reasons, we focused in this work on commercially available calcium sulfate carrier with the product name Stimulan®.
We chose vancomycin as the antibiotic. Vancomycin is very often used in combination with other antibiotics. In case that we already know the cause of the infection, we use calcium sulfate mixed with vancomycin only, especially for the treatment of multidrug-resistant infections caused by Gram-positive bacteria (MRSA, MRSE) (Mühlberg et al. 2020). The second reason why we decided to test the stability of vancomycin in the prepared material was the fact that vancomycin breaks down over time to form crystalline degradation products at a temperature of 20–25 °C. Furthermore, we did not want to effect the release kinetics of vancomycin by another antibiotic. In addition, local vancomycin concentrations up to 1000 mg/L have a minimal negative effect on bone cells (Edin et al. 1996).
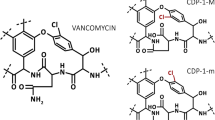
For verification of the stability, i.e., the efficacy of vancomycin in the calcium phosphate carrier after its storage for 12 days at laboratory temperature, a diffusion agar test was used. This microbiological method is considered indicative, but is fully sufficient for this model. Based on the measurements, there was no difference in the effectiveness of vancomycin applied as a carrier to the agar immediately after preparation compared to the application of the carrier with the antibiotic after its 12-day storage at laboratory temperature. However, it is important to realize that vancomycin undergoes crystalline thermal degradation even at body temperature. This results in the formation of crystalline degradation products (CDP-1), which consists of two conformational isomers—major CDP-M and minor CDP-m. These products are formed by hydrolytic cleavage of ammonia. Although they are structurally similar to vancomycin, they lack antibiotic efficacy. This is mainly the case with the accumulation of vancomycin in the body, frequently occurring in patients with renal insufficiency. These degradation products can then accumulate in the body and contribute to treatment failure and to the development of resistance. In addition, in most immunochemical assays, it is impossible to distinguish these products from vancomycin, and therefore, the assay has falsely elevated results (Backes et al. 1998; Ghassempour et al. 2005; Klapkova et al. 2020; Melicherčík 2011; Melicherčík et al. 2012, 2014; Somerville et al. 1999). For this reason, we measured the concentration of released vancomycin and its two degradation products by HPLC.
The concentrations of antibiotic released from the carrier into the phosphate buffer were measured after 6 h at a thermostatically controlled temperature of 37 °C. Our previous measurements and data from the literature showed that the maximum vancomycin concentrations were reached on the first or second day after its application (Aiken et al. 2015; Klapkova et al. 2020; Melicherčík 2011; Melicherčík et al. 2012, 2014). This demonstrates that a sufficiently high concentrations of vancomycin suitable for comparison of storage time will be achieved after 6 h of incubation. From the total amount of vancomycin contained in the beads, we can conclude that approximately 30% of the antibiotic retained in the material. Aiken et al. reported that in the case of the calcium sulfate carrier, vancomycin concentrations are sufficiently high even after 42 days (Aiken et al. 2015). However, our work was not focused on the elution curve of vancomycin from the carrier used.
We observed a decrease of both vancomycin and crystalline degradation products average concentrations. Due to the fact that all measured forms of vancomycin changed approximately proportionally in the same way, it can be said that during storage for 12 days in sterile test tubes at a laboratory temperature and in the dark there was no increase in crystalline thermal degradation of vancomycin caused by storage time. Although vancomycin concentrations decreased by approximately 32% at the end of 12 days of storage, concentrations of the released active form of vancomycin are many times higher than the minimum inhibitory concentrations for resistant strains of Staphylococcus aureus (MIC ≥ 16 mg/L) (Appelbaum 2007) without increasing the toxic risk for surrounding tissues (local levels of vancomycin ≤ 1000 mg/L have little or no effect on osteoblast replication) (Cowie et al. 2016). Vancomycin could be converted into its crystalline degradation products but we should also take into account that other low molecular by-products are created from vancomycin. Cao et al. (2018) studied metabolism and degradation of vancomycin in vitro in four systems. They identified four novel degradation products, where three of them were found in the phosphate buffer system and two of them in the pure water system (Cao et al. 2018).
Conclusions
Based on our results, maintaining the correct procedure according to the carrier manufacturer (Stimulan®, Biocomposites) and ensuring sterile conditions during preparation, it could be recommended to prepare a calcium sulfate carrier with vancomycin a few days before its application, certainly at least within 12 days. Thanks to this procedure, it is possible to reduce the waste of the carrier and the antibiotic in cases where this antibiotic carrier is used in smaller cavities, for example, dead spaces of hand and foot. This procedure will lead to the optimization of ecological and economic impacts. Furthermore, in this way, it is also possible to save time during the operation, which eliminates the preparation of the carrier in the operating room and thus reduces the risks for the patient resulting from a longer period of anesthesia.
References
Abosala A, Ali M (2020) The use of calcium sulphate beads in periprosthetic joint infection, a systematic review. J Bone Jt Infect 5(1):43–49
Aiken SS, Cooper JJ, Florance H, Robinson MT, Michell S (2015) Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect (larchmt) 16(1):54–61
Appelbaum PC (2007) Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 30:398–408
Backes DW, Aboleneen HI, Simpson JA (1998) Quantitation of vancomycin ant its crystalline degradation products (CDP-1) in human serum by high performance liquid chromatography. J Pharm Biomed Anal 16(8):1281–1287
Borrelli J Jr, Prickett WD, Ricci WM (2003) Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res 411:245–254
Cao M, Feng Y, Kang W, Lian K, Ai L (2018) Studies on the metabolism and degradation of vancomycin in simulated in vitro and aquatic environment by UHPLC-Triple-TOF-MS/MS. Sci Rep 8:15471
Cooper JJ (2004) Method of producing surgical grade calcium sulfate; United States Patent, No: US 6,780,391 B1
Cowie RM, Carbone S, Aiken S, Cooper JJ, Jennings JM (2016) Influence of third-body particles originating from bone void fillers on the wear of ultra-high-molecular-weight polyethylene. Proc Inst Mech Eng H 230(8):775–783
Doub JB, Ng VY, Wilson E, Corsini L, Chan BK (2021) Successful treatment of a recalcitrant Staphylococcus epidermis prosthetic knee infection with intraoperative bacteriophage therapy. Pharmaceuticals 14(3):231
Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE (1996) Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop 333:245–251
Flierl MA, Culp BM, Okroj KT, Springer BD, Levine BR, Della Valle CJ (2017) Poor outcomes of irrigation and debridement in acute periprosthetic joint infection with antibiotic-impregnated calcium sulfate beads. J Arthroplasty 32(8):2505–2507
Gauland C (2011) The use of antibiotic impregnated, implanted synthetic calcium sulfate tablets in the treatment of soft tissue, vancomycin resistant, enterococcus infections. Wound Repair and Regeneration 19(2):A23
Ghassempour A, Abdollahpour A, Tabar-Heydar K, Nabid MR, Mansouri S, Aboul-Enein HY (2005) Crystalline degradation products of vancomycin as a new clinical stationary phase for liquid chromatography. Chromatographia Tehran 61:151–155
Helgeson MD, Potter BK, Tucker CJ, Frisch HM, Shawen SB (2009) Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Orthopedics 32(5):323
Howlin RP, Webb MJ, Cooper JJ, Aiken SS, Stoodley P (2015) Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 59(1):111–120
Jahoda D, Nyč O, Pokorný D, Landor I, Sosna A (2006) Antibiotic treatment for prevention of infectious complications in joint replacement. Acta Chirurgiae Orthopaedicae Et Traumatologiae Čechosl 73:108–114
Jahoda D, Sosna A, Nyč O et al (2008) Infectious complications in joint replacement (Infekční komplikace kloubních náhrad). Triton, Praha
Jiang N, Dusane DH, Brooks JR, Delury CP, Aiken SS, Laycock PA, Stoodley P (2021) Antibiotic loaded β-tricalcium phosphate/calcium sulfate for antimicrobial potency, prevention and killing efficacy of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Sci Rep 11:1446–1454
Kallala R, Nizam I, Haddad FS (2013) Outcomes following use of antibiotic-eluting, absorbable, calcium sulfate beads in revision hip and knee surgery for periprosthetic infection. Orthopaedic Proceedings 95-B(34):364
Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E (2018) Use of Stimulan absorbable calcium sulfate beads in revision lower limb arthroplasty: safety profile and complication rates. Bone Joint Res 7(10):570–579
Kanellakopoulou K, Galanopoulos I, Soranoglou V, Tsaganos T, Tziortzioti V, Maris I, Papalois A, Giamarellou H, Giamarellos-Bourboulis EJ (2009) Treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with a synthetic carrier of calcium sulfate (Stimulan) releasing moxifloxacin. Int J Antimicrob Agents 33(4):354–359
Klapkova E, Nescakova M, Melichercik P, Jahoda D, Dunovska K, Cepova J, Prusa R (2020) Vancomycin and its crystalline degradation products released from bone grafts and different types of bone cement. Folia Microbiol 65(3):475–482
Kurmis AP (2021) Eradicating fungal periprosthetic TKA ‘super infection’: review of the contemporary literature and consideration of antibiotic-impregnated dissolving calcium sulfate beads as a novel PJI treatment adjunct. Arthroplasty Today 8:163–170
Lazarou SA, Contodimos GB, Gkegkes D (2011) Correction of alveolar cleft with calcium-based bone substitutes. J Craniofac Surg 22(3):854–857
Lei D, Zhanzhong M, Huaikuo Y, Lei X, Gongbo Y (2012) Treatment of distal radius bone defects with injectable calcium sulfate cement. In Tech bone grafting, China, pp 125–134
Lei D, Zhanzhong M, Jing X (2007) Treatment of bone defect with injectable calcium sulfate powder in distal fractures of radius. Chinese Journal of Bone Tumor and Bone Disease 5:274–277
Lewicki K, Prioreschi B, Koenig K, Keeney B, Bartelstein M, Moucha C, Citters DV (2017) The effect of absorbable calcium sulfate on wear rates in ultra-high-molecular-weight polyethylene: potential implications for its use in treating arthroplasty infections. J Am Acad Orthop Surg 25(6):114–120
Marson BA, Deshmukh SR, Grindlay DJC, Ollivere BJ, Scammell BE (2018) A systematic review of local antibiotic devices used to improve wound healing following the surgical management of foot infections in diabetics. Bone Joint J 100-B(11):1409–1415
McKee MD, Wild LM, Schemitsch EH, Waddell JP (2002) The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma 16(9):622–627
McPherson EJ, Dipane MV, Sherif SM (2013) Dissolvable antibiotic beads in treatment of periprosthetic joint infection and revision arthroplasty. The use of synthetic pure calcium sulfate (Stimulan®) impregnated with vancomycin & tobramycin. Reconstruct Rev 3(1):32–43
Metsemakers WJ, Fragomen AT, Moriarty TF, Morgenstern M, Egol KA, Zalavras Ch, Obremskey WT, Raschke M, McNally MA, Infection F-R, consensus group, (2020) Evidence-based recommendations for local antimicrobial strategies and dead space management in fracture-related infection. J Orthop Trauma 34(1):18–29
Meani E, Romanò C, Crosby L, Hofmann G (2007) Infection and local treatment in orthopedic surgery. Springer, US
Melicherčík P (2011) Utilization of local carriers of antibiotics for treatment of infection of locomotive apparatus. Dissertation work, 1st Department of Orthopaedics, 1st Faculty of Medicine, Charles University in Prague and Motol University Hospital
Melicherčík P, Klapková E, Landor I, Judl T, Síbek M, Jahoda D (2014) The effect of vancomycin degradation products in the topical treatment of osteomyelitis. Bratislava Medical Journal 115(12):796–799
Melicherčík P, Jahoda D, Nyč O, Klapková E, Barták V, Landor I, Pokorný D, Judl T, Sosna A (2012) Bone grafts as a vancomycine carrier for local therapy of resistant infections. Folia Microbiol 57:459–462
Morley R, Lopez F, Webb F (2016) Calcium sulfate as a drug delivery system in a deep diabetic foot infection. Foot (edinb) 27:36–40
Mühlberg E, Umstätter F, Kleist Ch, Domhan C, Mier W, Uhl P (2020) Renaissance of vancomycin: approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can J Microbiol 66:11–16
Noor S, Bridgeman P, David M, Humm G, Bose D (2018) The use of Osteoset-T in the treatment of chronic osteomyelitis of the tibia following exogenous trauma: a review of 22 patients at a regional trauma centre. Bone & Joint Journal Orthopaedic Proceedings Supplement 95-B (SUPP 23):4
Somasundaram K, Huber CP, Babu V, Zadeh H (2013) Proximal humeral fractures: the role of calcium sulfate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury 44(4):481–487
Somerville AL, Wright DH, Rotschafer JC (1999) Implications of vancomycin degradation products on therapeutic drug monitoring in patients with end-stage renal disease. Pharmacotherapy 19:702–707
Winkler H, Stobier A, Kaudela K, Winter F, Menschik F (2008) One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br 90:1580–1584
Witso E, Persen L, Loseth K, Benum P, Bergh K (2000) Cancellous bone as an antibiotic carrier. Acta Orthop Scand 71:80–84
Biocomposites (2020) https://www.biocomposites.com/our-products/stimulan/
Acknowledgements
Supported by Ministry of Health, Czech Republic—conceptual development of research organization, University Hospital Motol, Prague, Czech Republic 00064203. Special thanks to Dr. John Wilson for English language revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melicherčík, P., Klapková, E., Nyč, O. et al. Antimicrobial efficacy and activity perseverance in arthroplasty of calcium sulfate beads containing vancomycin prepared ahead of time and stored in ready-to-use formula. Folia Microbiol 67, 63–69 (2022). https://doi.org/10.1007/s12223-021-00916-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-021-00916-7