Abstract
Mineral nutrition of crop plants is one of the major challenges faced by modern agriculture, particularly in arid and semi-arid regions. In alkaline calcareous soils, the availability of phosphorus and zinc is critically less due to their fixation and precipitation as complexes. Farmers use fertilizers to fulfill crop requirements, but their efficacy is less, which increases production costs. Plant growth-promoting rhizobacteria (PGPR) can improve the availability of crop nutrients through solubilizing the insoluble compounds of phosphorus and zinc in soil. In the present study, a total of 40 rhizobacterial isolates were isolated from cotton rhizosphere and screened for improving cotton growth through the solubilization of phosphorus and zinc. Out of these 40 isolates, seven isolates (IA2, IA3, IA6, IA7, IA8, IA13, and IA14) efficiently solubilized insoluble rock phosphate while seven isolates (IA10, IA16, IA20, IA23, IA24, IA28, and IA30) were more efficient in solubilizing insoluble zinc oxide. In liquid media, strain IA7 (2.75 μg/mL) solubilized the highest amount of phosphate while the highest concentration of soluble zinc was observed in the broth inoculated with strain IA20 (3.94 μg/mL). Seven phosphate-solubilizing and seven zinc-solubilizing strains were evaluated using jar trial to improve the growth of cotton seedlings, and the results were quite promising. All the inoculated treatments showed improvement in growth parameters in comparison with control. Best results were shown by the combined application of IA6 and IA16, followed by the combination of strains IA7 and IA20. Based on the jar trial, the selected isolates were further characterized by plant growth-promoting characters such as siderophores production, HCN production, ammonia production, and exopolysaccharides production. These strains were identified through 16S rRNA sequencing as Bacillus subtilis IA6 (accession # MN005922), Paenibacillus polymyxa IA7 (accession # MN005923), Bacillus sp. IA16 (accession # MN005924), and Bacillus aryabhattai IA20 (accession # MN005925). It is hence concluded that the integrated use of phosphate-solubilizing and zinc-solubilizing strains as potential inoculants can be a promising approach for improving cotton growth under semi-arid conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural productivity in the modern era depends heavily on agrochemicals. These are considered to be an integral part of our agricultural systems, but due to climate change, the adoption of diverse biological applications is emerging as a popular strategy to reduce the use of chemical fertilizers. One of the most reliable methods to minimize the use of chemical fertilizers is the introduction of plant growth-promoting rhizobacteria (PGPR) as biofertilizers. These microorganisms live in the rhizosphere, proliferating and competing with other organisms for food (Kloepper and Okon 1994). The PGPR are associated with plant roots and improve crop production through different mechanisms which include direct and indirect influences. These bacteria improve soil fertility as well as nutrient availability and their uptake by crop plants (Saharan and Nehra 2011; Nehra and Choudhary 2015).
PGPR enhance plant growth in different ways (Gouda et al. 2018). They provide nutrients through nitrogen fixation in soil (Islam et al. 2013); control the plant hormone system through the production of phytohormones (Maheshwari et al. 2015), siderophores, enzymes, and organic acids (Ahemad and Kibret 2014; Mumtaz et al. 2019); and improve nutrient availability through the solubilization of insoluble nutrients (Mumtaz et al. 2017; Ahmad et al. 2018). Besides their direct influence on plants, they also enhance plant growth indirectly by acting as biocontrol agents, producing antibiotics and iron-chelating compounds, inducing resistance against pathogens, and synthesizing extracellular enzymes (Beneduzi et al. 2012; Ramadan et al. 2016). These bacteria can also combat stresses and provide a favorable environment for the better development of plants (Nadeem et al. 2014).
Zinc, a micronutrient, is only required in small concentrations but is important for the healthy growth and reproduction of plants. Zinc is essential for photosynthesis, where its main role is in carbonic anhydrase activity, chlorophyll biosynthesis, membrane functioning, hormone synthesis, gene expression, provision of resistance against stresses and pathogens, and improvement of crop growth and yield (Xi-wen et al. 2013; Noulas et al. 2018). Zinc deficiency results in plant health disturbance. Its deficiency symptoms appear as stunted growth and leaf chlorosis (Noulas et al. 2018). More than 70% of the soils in Pakistan are zinc deficient. ZnSO4 is applied to reduce deficiency, but most of the applied ZnSO4 fertilizer becomes unavailable due to fixation. Certain zinc-solubilizing bacteria (ZSB) can solubilize inorganic zinc compounds such as ZnO through the production of organic acids (Mumtaz et al. 2019), thus lowering the pH of the growth media (Mumtaz et al. 2017).
Phosphorus is the second most important macronutrient after nitrogen. It is critical in metabolic activities such as respiration, photosynthesis, nutrient uptake, and biological oxidation. Phosphorus is the structural component of energy-rich compounds, phospho-lipids, phospho-proteins, and nucleic acids (Gouda et al. 2018). Despite the abundance of phosphorus in agricultural soils, most of it remains insoluble and is unavailable to plants (Miller et al. 2010). This is considered a major growth-limiting factor in agricultural systems (Daniels et al. 2009). So, it becomes a requirement to apply soluble forms of this essential nutrient through phosphatic fertilizers, but this may also limit the availability of phosphorus because applied phosphorus gets mixed with other minerals and converted into insoluble forms (Sharma et al. 2013) in high pH alkaline soils (Mendez 2014). Phosphorus-solubilizing bacteria (PSB) can be used to combat the limited availability of phosphorus to plants. These bacteria make phosphorus accessible to plants by producing organic acids that solubilize and mineralize inorganic and organic soil phosphates. Thus, the production of phosphatases lower soil pH and mineralize organic phosphate (Ahmad et al. 2018; Rasul et al. 2019). The PSB increase phosphorus availability and can be utilized as biofertilizers (Li et al. 2017). Integrating the PSB and ZSB as potential biofertilizers can potentially solve the problem of phosphorus and zinc unavailability under alkaline conditions. This paper reports the studies on the screening, characterization, and identification of PSB and ZSB along with multifarious traits for improving cotton growth.
Materials and methods
Isolation of PGPR from cotton rhizosphere
Soil samples were taken from cotton rhizosphere found in cotton fields in the vicinity of Bahawalpur. The rhizobacteria were isolated by following the method of Dworkin and Foster (1958). These colonies were purified by streaking fresh general-purpose agar plates and the plates were incubated at 30 °C for 2 days. The procedure was repeated until pure cultures were obtained in a single colony type. A total of 40 fast-growing isolates were selected and preserved at 4 °C for further experimentation.
Characterization and screening of phosphate-solubilizing and zinc-solubilizing rhizobacteria
The characterization of bacterial isolates was done based on their ability to solubilize zinc and phosphate in their respective medium as described below.
Estimation of phosphate solubilization (qualitative and quantitative)
The phosphate solubilization ability of isolates was confirmed using Pikovskaya agar medium (Pikovskaya 1948). The diameter of halo zones was measured and phosphorus solubilization index (PSI) was calculated using the formula described by Vazquez et al. (2000).
The isolates showing clear halo zone around the colony in Pikovskaya’s agar medium were quantitatively evaluated for phosphorus solubilization by using the yellow color method (Clescerie et al. 1998). The 24-h old fresh cultures of selected strains were inoculated in triplicate to 50 mL of Pikovskaya’s broth and incubated for 48 h. After centrifugation, 5 mL of the supernatant was collected with 5 mL of the reagent (vanadomolybdate). The volume was increased to 25 mL and incubated for color development. The absorbance was measured using UV-visible spectrophotometer (Carry 60, Agilent, USA) at 420-nm wavelength.
Estimation of zinc solubilization (qualitative and quantitative)
Zinc solubilization was determined following the method of Bunt and Rovira (1955), which uses 0.1% ZnO as the insoluble source of zinc. A full loop of bacterial cultures was spot inoculated on Petri plates containing the Bunt and Rovira medium, and the plates were incubated at 28 ± 1 °C for 5 days. After incubation, the development of clear halo zones around colonies indicated the zinc solubilization ability of the bacterial cultures. The diameters of the halo zones were measured, and zinc solubilization index (ZSI) was calculated using the formula described by Vazquez et al. (2000).
Cultures that formed halo zones around colonies were then subjected to quantification assay by growing them in basal broth as described by Bunt and Rovira (1955). For this purpose, inoculated broth flasks with bacterial cultures in triplicate were incubated at 28 ± 1 °C for 15 days. On the 15th day, the medium was centrifuged at 7000 rpm for 15 min. Soluble zinc content in the culture supernatant was determined by using the atomic absorption spectrophotometer (Model: 240FS, Agilent, USA). The solubilized zinc quantity in inoculated samples was compared with uninoculated control and expressed as μg/mL of zinc culture (Saravanan et al. 2003).
Compatibility assay of PSB and ZSB isolates
A compatibility test was conducted to check whether the PSB and ZSB isolates were compatible with each other. For this purpose, phosphate-solubilizing isolates were streaked on general-purpose agar plates in such a way that the zinc-solubilizing isolates were perpendicular to the phosphate-solubilizing isolates, and the inhibition of growth at the point of conjunction was observed as a non-compatible combination after 48 h of incubation at 37 °C (Prasad and Babu 2017).
Screening of effective strains of PSB and ZSB for their separate and combined use to improve cotton growth under axenic conditions
The PSB and ZSB isolates were evaluated for their effectiveness in improving the growth of cotton seedlings in jar trial under axenic conditions; both sole inoculation as well as their compatible combinations were evaluated. Before sowing, the cotton seeds were surface sterilized by using ethanol (95%) and HgCl2 (0.2%) and were then washed with sterilized distilled water. The surface-disinfected seeds were inoculated with their respective rhizobacterial strains by dipping in their respective broths for 5 min. For combined inoculation, an equal volume of respective co-inoculated strains was mixed in a Petri dish, followed by the dipping of seeds for 5 min. In the case of control treatment, seeds were dipped in sterilized broth without bacterial inoculation. The jars were sterilized and filled with sterilized sand, and the inoculated seeds were sown in these glass jars. There were three replications of each treatment, and the experiment was planned completely randomized design (CRD). For the application of nutrients, sterilized Hoagland solution (Hoagland and Arnon 1950) was applied in the jars. The jars were kept in a growth room with controlled temperature (30 °C), light (16 h) and dark (8 h) periods, and 55–65% humidity. The data regarding growth parameters were recorded 25 days after sowing. The strains from the better treatments in the jar trial were selected and characterized in the laboratory studies.
Morphological characterization of selected isolates
The selected bacterial isolates were tested for their morphological characters as described by Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994).
Assays for biochemical and plant growth-promoting characterization of selected strains
The selected strains were characterized for catalase activity by using the methods of MacFaddin (1980). Ammonia production by the strains was determined by using Nessler’s reagent and peptone water, following the protocol of Cappuccino and Sherman (1992). Urease activity was evaluated by adopting the procedure of Shruti et al. (2013). Protease activity test was performed on skim milk agar medium as described by Atlas (1946). The chitinase activity of bacterial strains was determined by using the procedure of Chernin et al. (1998). The siderophore production assay was conducted by using the protocol of Gopalakrishnan et al. (2011). Exopolysaccharides production was determined by using ATCC no. 14 medium as described by Tallgren et al. (1999), and the appearance of thick, slimy mucoid growth in the bacterial cultures was scored as positive for exopolysaccharides production. The Gram staining was done after 48 h of growth and the cell shape of bacteria was observed on general-purpose agar plates (Vincent 1970).
Identification of selected zinc-solubilizing and phosphorus-solubilizing strains
The bacterial strains which showed better results in the jar trial were identified using16S rRNA sequencing as described by Hussain et al. (2011). The resultant sequences were analyzed using the BLAST program on the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). This was followed by muscle alignment in MEGA7 software (Kumar et al. 2016). The phylogenetic tree was constructed by following the neighbor-joining method (Saitou and Nei 1987) while evolutionary distances were computed by using the maximum composite likelihood method (Tamura et al. 2004).
Statistical analysis
The data obtained was subjected to various statistical analyses using Statistix 8.1, and the means were compared using the least significant difference (LSD) at 5% level of probability to see the significance of treatments (Steel et al. 1997).
Results
Isolation and screening of zinc-solubilizing and phosphate-solubilizing rhizobacteria
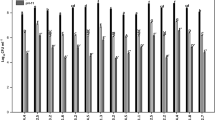
A total of 40 strains were isolated from the cotton rhizosphere and were coded as IA1, IA2, IA3 … … IA40. It was observed from results that seven strains (IA2, IA3, IA6, IA7, IA8, IA13, and IA14) were positive for solubilizing inorganic phosphate compound (rock phosphate) and seven strains (IA10, IA16, IA20, IA23, IA24, IA28, and IA30) effectively solubilized the insoluble zinc compound (ZnO) by forming clear halo zones around colonies (Fig. 2). The results of the phosphate solubilization assay showed that isolates solubilized phosphate within the range of 7.17- to 12.47-mm halo zone diameter. The maximum phosphate solubilization zone (halo zone) was produced by IA6, which was statistically at par with IA3 and IA7. The PSI data showed that the highest index was developed by IA6, followed by IA3 and IA7, and these were statistically similar to each other. These selected isolates were further processed for quantitative estimation of phosphate solubilization. It was found that all the tested isolates showed phosphate solubilization in broth assay. The maximum solubilization in broth media was observed by strain IA7 (2.75 μg/mL), followed by IA8 and IA6 (Table 1).
The results of bioassay of the zinc-solubilizing isolates (IA10, IA16, IA20, IA23, IA24, IA28, and IA30) showed that these isolates were effective in solubilizing the insoluble zinc compound (ZnO) by forming halo zones around the colonies ranging from 6.50 to 13.50 mm. The maximum halo zone was developed by IA20, followed by IA16. Both isolates were statistically significantly better than the other isolates. The ZSI results based on halo zone and colony diameter showed that the highest index was produced by isolate IA20 while the lowest was produced by IA30. These selected zinc-solubilizing isolates were tested for ZnO solubilization in liquid broth. Based on the quantitative data, it was found that solubilized zinc concentration in all inoculated tubes was significantly higher than uninoculated control except IA24, which was non-significant with control. However, the maximum solubilized zinc concentration (3.94 μg/mL) was given by isolate IA20, followed by IA16, IA28, and IA10 (Table 2).
Compatibility assay of PSB and ZSB isolates
The PSB (IA3, IA6, IA7, IA8) and ZSB (IA10, IA16, IA20, IA28) isolates which gave better results in the previous tests were evaluated for their compatibility to be used as co-inoculated combinations, and it was found that all possible co-inoculated combinations were compatible with each other.
Screening of effective strains of PSB and ZSB for their separate and combined use to improve cotton growth under axenic conditions
The eight selected strains—four PSB and four ZSB—were evaluated for their effectiveness in improving the growth of cotton seedlings in jar trial under axenic conditions. Data showed that most of the ZSB and PSB strains alone and in combination significantly enhanced the growth of cotton seedlings.
Data (Table 3) about shoot length showed that all sole and combined applications enhanced the shoot length in comparison to control plants. In general, the co-inoculation of strains showed better results than sole inoculation. Maximum improvement in shoot length (30%) was given by the combination IA7+IA6, followed by the combinations IA6+IA16, IA7+IA20, and IA6+IA20. The results clearly showed that all sole and combined application of isolates significantly enhanced shoot fresh weight in comparison with control. Maximum improvement in shoot fresh weight (28%) was observed by the combination IA7+IA20, followed by the combination IA6+IA16. The results also showed that most of the sole and combined applications of PSB and ZSB strains increased the shoot dry weight more than uninoculated control. Maximum improvement in shoot dry weight (26%) was observed in the jars inoculated with the combination IA7+IA20, followed by the combinations IA6+IA16 and IA6+IA20 and the sole application of strain IA6 (Table 3). However, IA7+IA20 and IA6+IA16 gave more significant results when contrasted with control.
Data (Table 4) regarding root parameters showed that all the treatments enhanced the root growth as compared to control. Results showed that all sole and combined applications significantly improved the root length, except IA10, which was statistically similar to uninoculated control. Maximum improvement in root length (29%) was given by the combination IA6+IA20 while maximum improvement in root fresh weight and root dry weight were observed in the combination IA7+IA16 and IA6+IA16 respectively (Table 4).
Morphological characterization of the strains
Based on the results of the jar trial, selected strains (IA6, IA7, IA16, and IA20) were grown for cell morphological characters. All strains developed circular shape except IA6, which was irregular. All tested strains showed entire margin of colonies while IA6 was undulate. The elevation of IA6 and IA20 was flat while that of IA7 and IA16 were raised and pulvinate, respectively. The colony size of all strains was large except IA7, which was smaller than the other strains. All strains except IA6 had smooth colony texture and were glistening in appearance; IA6 was rough and dull. All strains showed different pigmentation. The optical property of all strains was opaque, except IA7 which was transparent (Table 5).
Biochemical characterization
The selected strains (IA6, IA7, IA16, and IA20) were further characterized for biochemical and plant growth-promoting traits. It was found that all strains were positive in catalase and ammonia production. Urease production was observed in IA6 and IA20. All strains were positive in protease test while chitinase activity was shown by the strains IA7 and IA20. The strains IA16 and IA20 were positive in siderophores production while all strains produced exopolysaccharides during bioassay (Table 6).
Identification of selected zinc-solubilizing and phosphorus-solubilizing strains through 16S rRNA sequencing
Two phosphate-solubilizing isolates (IA6 and IA7) and two zinc-solubilizing isolates (IA16 and IA20) were determined to have several plant growth-promoting characteristics. They also improved the growth of cotton seedlings. These plant growth-promoting isolates were identified by 16S rRNA partial sequencing (Table 7). The 16S rRNA gene sequencing showed that the isolate IA6 exhibited 99.42% similarity with Bacillus subtilis and was submitted in Genbank with the accession number MN005922. The isolate IA7 showed 99.55% similarity with Paenibacillus polymyxa and was deposited in Genbank with the accession number MN005923. The isolate IA16 was identified using primers 785F and 907R, where it showed 91.48% resemblance with Bacillus sp. and was submitted in Genbank under the accession number MN005924. The isolate IA20 was also identified using primers 785F and 907R. It showed 99.27% similarity with Bacillus aryabhattai and was deposited in Genbank with the accession number MN005925. The phylogenic tree showed that these PSB and ZSB isolates resembled members of well-characterized bacterial species which had been identified earlier (Fig. 1).
Discussion
Phosphorus and zinc are essential nutrients for plant growth and development. They perform several key functions in crop growth and physiology (Gouda et al. 2018; Noulas et al. 2018). Despite their natural presence in soil, both of these nutrients become unavailable to plants due to fixation and precipitation in the form of different complexes (Hafeez et al. 2013; Kafle et al. 2019). Thus, it becomes necessary to provide phosphorus and zinc nutrients as fertilizers for healthy plant growth. The solubilization of phosphorus and zinc by rhizobacteria and its potential for enhancing agricultural economy have captured the attention of microbiologists and agricultural researchers. The use of PSB and ZSB as inoculants may prove to be an effective alternative to fulfill plant requirements for essential nutrients.
In this study, the isolated bacteria were characterized based on zinc solubilization and phosphorus solubilization abilities. It was found that out of 40 isolates, seven isolates solubilized phosphate while seven others solubilized zinc in general-purpose agar media. These isolates were further processed for quantitative estimation of phosphorus and zinc solubilization. In a previous study, Mumtaz et al. (2017) observed that thirteen isolates out of seventy showed greater ability for solubilizing zinc. They observed that greater solubilization was shown by Bacillus aryabhattai, Bacillus subtilis (ZM63), and Bacillus sp. (ZM20). Similarly, in another study, it was found that out of 200 isolates, 81 isolates were able to solubilize phosphate more than the other isolates (Tsegaye et al. 2019). The PGPR adopt several mechanisms of zinc and phosphorus solubilization such as decreasing the pH of rhizosphere soil by organic acid production, anion exchange, and chelation (Habib et al. 2016; Dinesh et al. 2018; Mumtaz et al. 2019). The zinc-solubilizing and phosphate-solubilizing isolates which showed greater absorbance were further assessed to check whether these were compatible with each other, and it was found that all strains were compatible and had no antagonistic effect on each other. Previously, Ansari and Ahmad (2019) had found the compatible nature of Pseudomonas fluorescens FAP2 and Bacillus licheniformis B642 under the biofilm mode of growth.
All possible combinations of zinc-solubilizing and phosphate-solubilizing strains were chosen for testing their effectiveness to improve the growth of cotton seedlings under axenic conditions. Data regarding growth parameters showed that all strains enhanced the seedlings’ growth but with different degrees of effectiveness. Co-inoculation of zinc-solubilizing and phosphorus-solubilizing rhizobacteria was better than sole application in most of the cases. There are several reports available that confirm the ability of zinc and phosphorus solubilizers to improve plant growth. Zaheer et al. (2019) isolated Pseudomonas sp. strain AZ5 and Bacillus sp. strain AZ17 and checked their abilities to improve chickpea growth. They suggested that positive results in plant growth may be due to zinc and phosphorus solubilization by these strains. In another study, it was confirmed that maximum plant growth could be linked with ZSB that have several functions, including nutrient availability (nitrogen fixation, zinc and phosphorus solubilization), phytohormones production, siderophores production, and a pathogen-fighting mechanism (Mumtaz et al. 2017). Similarly, increments in plant growth parameters—shoot and root lengths, shoot fresh and dry biomass, root fresh and dry biomass—were obtained by the application of zinc-solubilizing Bacillus sp. (AZ6) (Hussain et al. 2015). The strains B. subtilis and L. fusiformis were found to be positively enhancing plant growth through solubilizing phosphorus. It was reported that Bacillus and Lysinibacillus significantly enhanced plant height and biomass (Rafique et al. 2017). In another study, the maximum plant growth by PSB was observed in terms of root and shoot lengths (Chauhan et al. 2016). Enhancement in plant height were linked with the production of organic substances such as IAA, gibberellin, and cytokinin in the root zone by PSB (Rafique et al. 2017).
In the present study, the selected strains were found to possess a variety of morphological characters. Similar results were obtained by other researchers, where the isolates of Bacillus and Paenibacillus were observed to have colonies that were small to medium in size, glistening in appearance, Gram-positive, and rod-shaped and had smooth texture, different colored pigmentation, and convex elevation (Mumtaz et al. 2017; Ahmad et al. 2018; Suman et al. 2018).
Multifarious plant growth-promoting traits are crucial for utilizing rhizobacteria as biofertilizers. For example, catalase activity is an important trait that enables plants to face adverse conditions (Mumtaz et al. 2017). In this study, all tested strains were found positive for catalase activity. Iqbal et al. (2016) reported that all five PSB isolates were catalase positive as these form gas bubbles on the addition of H2O2 to cells of bacteria. In another study, Ahmad et al. (2018) observed that all seven phosphorus-solubilizing strains were positive for catalase enzyme production. Ammonia production by PGPR influences plant growth indirectly. The bacterial existence in the rhizosphere having the ability to produce ammonia shows the hint of ammonification process (Tsegaye et al. 2019). In this study, it was observed that all selected strains produced ammonia. Another important trait of PGPR is the production of hydrolytic enzymes. The bacteria that produce single or numerous lytic enzymes have a biocontrol mechanism for various plant pathogenic microorganisms, including bacteria and fungi, thus improving crop growth and yield (Tsegaye et al. 2019). Such enzymes (chitinase and proteases) also have a mechanism for the control of plant diseases in the plant vicinity (Tsegaye et al. 2018). In the present study, all of the selected strains produced protease while IA7 and IA20 were positive for chitinase activity. These results are in line with those of Tsegaye et al. (2018), who found that 15.5% of isolates (P. aeruginosa, C. amlonaticus, P. corrugota, P. fuscovaginae, C. gleum, and B. cereus/pseudomycoide) showed positive results for protease activity while 3.5% of isolates (P. fluorescent) were involved in chitin production. Shen et al. (2013) reported the positive effects of siderophore-producing rhizobacteria on plant growth as they enhanced iron availability by reducing the growth and iron acquisition of other pathogenic microorganisms. In our study, two of the selected strains showed positive results for siderophores production. Radhakrishnan et al. (2017) also reported that Bacillus sp. were involved in the production of siderophores and exopolysaccharides. Exopolysaccharides-producing bacteria can maintain plant growth under severe stress conditions. The exopolysaccharides protect plants from drought stress, enhance microbial accumulation and plant-microbe interaction, and are also involved in bioremediation (Naseem et al. 2018). In this study, all the isolates produced exopolysaccharides. Similarly, Tsegaye et al. (2019) reported that 19% of isolates were positive for exopolysaccharide production.
In this study, the selected PSB and ZSB strains IA6, IA7, IA16, and IA20 were identified as Bacillus subtilis IA6, Paenibacillus polymyxa IA7, Bacillus sp. IA16, and Bacillus aryabhattai IA20, respectively. These strains are catalase positive and produce ammonia, urease, hydrolytic enzymes, siderophores, and exopolysaccharides. These identified strains are Gram-positive and rod-shaped and have phosphate-solubilizing and zinc-solubilizing abilities. All these properties are directed towards improving the growth of cotton seedlings in jar trials under axenic conditions. Similar observations were reported in previous studies where the strains Bacillus sp. (Dinesh et al. 2018), Bacillus subtilis and Paenibacillus spp. (Ahmad et al. 2018), and Bacillus arybhattai (Sheirdil et al. 2019) were positive in several traits that favored plant growth (Korir et al. 2017; Ahmad et al. 2018).
Conclusion
The PSB and ZSB strains with multiple plant growth-promoting traits would facilitate greater availability of nutrients (zinc and phosphorus) to crop plants, especially when used in combination with the solubilization of fixed, unavailable zinc and phosphorus. This can lead to better plant growth and yield and can also improve soil fertility through multifunctional properties. In this study, the combined application of selected phosphate-solubilizing and zinc-solubilizing strains proved effective in improving cotton growth under axenic conditions. The best results were shown by the combined application of Bacillus subtilis strain IA6 and Bacillus sp. strain IA16, followed by the combination of Paenibacillus polymyxa strain IA7 and Bacillus aryabhattai strain IA20. These strains have multiple plant growth-promoting traits and can thus prove promising for improving cotton growth under semi-arid conditions.
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Ahmad M, Ahmad I, Hilger TH, Nadeem SM, Akhtar MF, Jamil M, Hussain A, Zahir ZA (2018) Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. Peer J:2–22. https://doi.org/10.7717/peerj.5122
Ansari FA, Ahmad I (2019) Fluorescent pseudomonas-FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-40864-4
Atlas RM (1946) Handbook of microbiological media, 4th edn. CRC Press Taylor and Francis Group, Boca Raton
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051. https://doi.org/10.1590/s1415-47572012000600020
Bunt JS, Rovira AD (1955) Microbiological studies of some subantartic soils. J Soil Sci 6:119–128. https://doi.org/10.1111/j.1365-2389.1955.tb00836.x
Cappuccino JC, Sherman N (1992) Negative staining. In: Cappuccino JC, Sherman N (eds) Microbiology: a laboratory manual. Benjamin/Cummings, Redwood City, pp 125–179
Chauhan AK, Maheshwari DK, Kim K, Bajpai VK (2016) Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can J Microbiol 62:880–892. https://doi.org/10.1139/cjm-2016-0249
Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, Chet I, Williams P, Stewart GSAB (1998) Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J Bacteriol 180:4435–4441. https://doi.org/10.1128/jb.180.17.4435-4441.1998
Clescerie LS, Greenberg AE, Eaton AD (1998) Standard methods for examination of water and wastewater. 20th edn. APHA-AWWA-WEF, Washington, D.C., p 1325
Daniels C, Michan C, Ramos JL (2009) New molecular tools for enhancing methane production, explaining thermodynamically limited lifestyles and other important biotechnological issues. Microb Biotechnol 2:533–536. https://doi.org/10.1111/j.1751-7915.2009.00134.x
Dinesh R, Srinivasan V, Hamza S, Sarathambal C, Gowda SJA, Ganeshamurthy AN, Gupta SB, Nair VA, Subila KP, Lijina A, Divya VC (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186. https://doi.org/10.1016/j.geoderma.2018.02.013
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–603. https://doi.org/10.1128/jb.75.5.592-603.1958
Gopalakrishnan S, Humayun PBK, Kiran IGK, Kannan MS, Vidya K, Deepthi RO (2011) Evaluation of bacteria isolated from rice rhizosphere for biological control of charcoal rot of sorghum caused by Macrophomina phaseolina (Tassi) Goid. World J Microbiol Biotechnol 27:1313–1321. https://doi.org/10.1007/s11274-010-0579-0
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin SH, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Habib SH, Kausar H, Saud HM, Ismail MR, Othman R (2016) Molecular characterization of stress tolerant plant growth promoting rhizobacteria (PGPR) for growth enhancement of rice. Int J Agric Biol 18:1560–8530. https://doi.org/10.17957/IJAB/15.0094
Hafeez B, Khanif YM, Saleem M (2013) Role of zinc in plant nutrition-a review. Am J Exp Agric 3:374–391. https://doi.org/10.9734/AJEA/2013/2746
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Circ Calif Agric Exp Station 347:32
Holt JC, Krieg NR, Sneath PHA, Stanley JY, Williams ST (1994) Subgroup 2: Family vibrionaceae. Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore, pp 190–274
Hussain S, Devers-Lamrani M, El-Azhari N, Martin-Laurent F (2011) Isolation and characterization of an isoproturon mineralizing Sphingomonas sp. strain SH from a French agricultural soil. Biodegradation 22:637–650. https://doi.org/10.1007/s10532-010-9437-x
Hussain A, Arshad M, Zahir ZA, Asghar M (2015) Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak J Agric Sci 52:915–922 http://www.pakjas.com.pk/
Iqbal S, Khan MY, Asghar HN, Akhtar MJ (2016) Combined use of phosphate solubilizing bacteria and poultry manure to enhance the growth and yield of mung bean in calcareous soil. Soil Environ 35:146–154 http://www.sss-pakistan.or
Islam M, Sultana T, Joe MM, Yim W, Cho JC, Sa T (2013) Nitrogen-fixing bacteria with multiple plant growth promoting activities enhances growth of tomato and red pepper. J Basic Microbiol 53:1004–1015. https://doi.org/10.1002/jobm.201200141
Kafle A, Cope KR, Raths R, Yakha JK, Subramanian S, Bücking H, Garcia K (2019) Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Review. Agronomy 9:2–15. https://doi.org/10.3390/agronomy9030127
Kloepper JW, Okon Y (1994) Plant growth-promoting rhizobacteria (other systems). In: Okon Y (ed) Azospirillum/plant associations. CRC Press, Boca Raton, pp 111–118
Korir H, Mungai NW, Thuita M, Hamba Y, Masso C (2017) Co inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci 141:1–10. https://doi.org/10.3389/fpls.2017.00141
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Li Y, Liu X, Hao T, Chen S (2017) Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. Int J Mol Sci 18:1253. https://doi.org/10.3390/ijms18071253
MacFaddin JF (1980) Biochemical tests for identification of medical bacteria, 2nd edn. Williams and Wilkins, Baltimore, pp 51–54
Maheshwari DK, Dheeman S, Agarwal M (2015) Phytohormone-producing PGPR for sustainable agriculture. In: Maheshwari DK (ed) Bacterial metabolites in sustainable agroecosystem. Springer, Cham, pp 159–182
Mendez J (2014) Characterization of phosphate-solubilizing bacteria isolated from the arid soils of a semi-desert region of north-east Mexico. Biol Agric Hortic 30:211–217. https://doi.org/10.1080/01448765.2014.909742
Miller SH, Browne P, Prigent-Cambaret C, Combes-Meynet E, Morrissey JP, Gara OF (2010) Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ Microbiol Rep 2:403–411. https://doi.org/10.1111/j.1758-2229.2009.00105.x
Mumtaz MZ, Ahmad M, Jamila M, Hussain T (2017) Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol Res 202:51–60. https://doi.org/10.1016/j.micres.2017.06.001
Mumtaz MZ, Barry KM, Baker AL, Nichols DS, Ahmad M, Zahir ZA, Britz ML (2019) Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: a possible mechanism for Zn solubilization. Rhizosphere 12:00170. https://doi.org/10.1016/j.rhisph.2019.100170
Nadeem S, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448. https://doi.org/10.1016/j.biotechadv.2013.12.005
Naseem H, Ahsan M, Shahid MA, Khan N (2018) Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol 58:1009–1022. https://doi.org/10.1002/jobm.201800309
Nehra V, Choudhary M (2015) A review on plant growth promoting rhizobacteria acting as bioinoculants and their biological approach towards the production of sustainable agriculture. JANS 7:540–556. https://doi.org/10.31018/jans.v7i1.642
Noulas C, Tziouvalekas M, Karyotis T (2018) Zinc in soils, water and food crops. J Trace Elem Med Biol 49:252–260. https://doi.org/10.1016/j.jtemb.2018.02.009
Pikovskaya RI (1948) Mobilization of phosphorous in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Prasad AA, Babu S (2017) Compatibility of Azospirillum brasilense and Pseudomonas fluorescens in growth promotion of groundnut (Arachis hypogea L.). An Acad Bras Cienc 89:1027–1040. https://doi.org/10.1590/0001-3765201720160617
Radhakrishnan R, Hashem A, Abd-Allah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667. https://doi.org/10.3389/fphys.2017.00667
Rafique M, Sultan T, Ortas I, Chaudhary HJ (2017) Enhancement of maize plant growth with inoculation of phosphate-solubilizing bacteria and biochar amendment in soil. J Soil Sci Plant Nutr 63:460–469. https://doi.org/10.1080/00380768.2017.1373599
Ramadan EM, Abdel-Hafez AA, Hassan EA, Saber FM (2016) Plant growth promoting rhizobacteria and their potential for biocontrol of phytopathogens. Afr J Microbiol Res 10:486–504. https://doi.org/10.5897/AJMR2015.7714
Rasul M, Yasmin S, Suleman S, Zaheer A, Reitz T, Tarkka MT, Islam E, Mirza MS (2019) Glucose dehydrogenase gene containing phosphobacteria for biofortification of phosphorus with growth promotion of rice. Microbiol Res 223–225:1–12. https://doi.org/10.1016/j.micres.2019.03.004
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 1:1–30 https://astonjournals.com/lsmr
Saitou N, Nei M (1987) The neighbor-joining method: a new method for recon- structing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Saravanan SV, Sudalayandy RS, Savariappan (2003) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Braz J Microbiol 34:121–125. https://doi.org/10.1590/S1517-83822004000100020
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2:587. https://doi.org/10.1186/2193-1801-2-587
Sheirdil RA, Hayat R, Zhang X-X, Abbasi NA, Ali S, Ahmed M, Khattak JZK, Ahmad S (2019) Exploring potential soil bacteria for sustainable wheat (Triticum aestivum L.) production. Sustainability 11:3361. https://doi.org/10.3390/su11123361
Shen X, Hu H, Peng H, Wang W, Zhang X (2013) Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genomics 14:271. https://doi.org/10.1186/1471-2164-14-271
Shruti K, Arun K, Yuvneet R (2013) Potential plant growth-promoting activity of rhizobacteria Pseudomonas sp. in Oryza sativa. J Nat Prod Plant Resour 3:38–50 https://www.scholarsresearchlibrary.com/
Steel RGD, Torrie JH, Dicky DA (1997) Principles and procedures of statistics a biometrical approach, 3rd edn. McGraw Hill Book International Co, Singapore, pp 204–227
Suman B, Gopal AV, Reddy RS, Triveni S (2018) Cultural and morphological characterization of native Pseudomonas fluorescens isolates from Telangana. Int J Pure App Biosci 6:592–597. https://doi.org/10.18782/2320-7051.6910
Tallgren AH, Airaksinen U, Von Weissenberg R, Ojamo H, Kuusisto J, Leisola M (1999) Exopolysaccharide producing bacteria from sugar beets. Appl Environ Microbiol 65:862–864. https://doi.org/10.1128/aem.65.2.862-864.1999
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101:11030–11035. https://doi.org/10.1073/pnas.0404206101
Tsegaye Z, Assefa F, Tefera G, Alemu T, Gizaw B (2018) Characterization and identification of Tef (Eragrostis tef) seed endophytic bacterial species and evaluate their effect on plant growth promotion. J Plant Pathol Microbiol 9:438–446. https://doi.org/10.4172/2157-7471.1000438
Tsegaye Z, Gizaw B, Tefera G, Feleke A, Chaniyalew S, Alemu T, Assefa F (2019) Isolation and biochemical characterization of plant growth promoting (PGP) bacteria colonizing the rhizosphere of Tef crop during the seedling stage. Biomed J Sci Tech Res 14:10586–10596. https://doi.org/10.26717/BJSTR.2019.14.002534
Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves growing in a semiarid coastal lagoon. Biol Fertil Soils 30:460–468. https://doi.org/10.1007/s003740050024
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell Scientific, Oxford
Xi-wen Y, Xiao-hong T, Xin-chun L, William G, Yu-xian C (2013) Foliar zinc fertilization improves the zinc nutritional value of wheat (Triticumaestivum L.) grain. Afr J Biotechnol 10:14778–14785. https://doi.org/10.5897/AJB11.780
Zaheer A, Malik A, Sher A, Qaisrani MM, Mehmood A, Khan SU, Ashraf M, Mirza Z, Karim S, Rasool M (2019) Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J Biol Sci 26:1061–1067. https://doi.org/10.1016/j.sjbs.2019.04.004
Acknowledgments
We acknowledge the Department of Soil Science, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Pakistan, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, I., Ahmad, M., Hussain, A. et al. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol 66, 115–125 (2021). https://doi.org/10.1007/s12223-020-00831-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-020-00831-3