Abstract
The aim of the present study was to develop an ion-selective electrode method for the continuous determination of the intracellular pH in Lactobacillus plantarum using a small-scale bioreactor. This method employed a salicylate-selective electrode basing on the distribution of salicylic acid across the cytoplasmic membrane. This developed electrode responded to salicylate concentrations above 20 μmol/L with a Nernstian sensitivity. The energized and concentrated cells were added into a thermostated small-scale bioreactor that contained the salicylate anions dissolved in a 100 mmol/L potassium phosphate buffer at different pH values. The changes in salicylate concentration that occurred in the medium containing bacterial suspension were measured as a voltage change. The cells of Lactobacillus plantarum showed maintenance of pH homeostasis at the studied pH ranging from 4.0 to 7.0, and they kept a neutral intracellular pH up to 5.8. The simplicity of the measuring preparation and the relatively low cellular concentration, as well as the advantages of the small-scale bioreactor, lead us to believe that the described method can facilitate the study of the physicochemical factors on the intracellular pH of lactic acid bacteria using a single pH probe in one method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intracellular or cytoplasmic pH (pHin) is an essential factor for both metabolism and growth parameters due to its significant effect on different cellular processes during fermentation such as enzymatic functions, proton motive force, transport kinetics, and ATP generation (Hansen et al. 2016). In bacteria, the pHin is the relevant factor for most of the cell’s enzymatic machinery, and the pH gradient (ΔpH = pHex − pHin) across the cytoplasm membrane is the major component of the proton motive force in acidic medium (Kashket 1987; Hansen et al. 2016). The different methods used to determine the cytoplasmic pH of bacteria are generally divided into two classes: ion distribution methods such as flow dialysis and ion-selective electrodes and internal pH indicator methods such as 31P nuclear magnetic resonance spectroscopy (NMR) and fluorescent pH probes (Rottenberg 1979; Kashket 1985; Padan and Schuldiner 1986; Zhang et al. 2012; Wu et al. 2014; Bouix and Ghorbal 2015; Hansen et al. 2016; Kudo and Sasaki 2019; Xu et al. 2019). The fluorescent biosensors are generally divided into two groups: fluorescent dyes (probes) that possess several drawbacks including cytotoxic effects, limited cellular uptake, or leakage of the dye from cells and the genetically encoded fluorescent protein (FP)–based pH biosensors, which were developed to overcome some of the problems that are specifically associated with fluorescent dyes (Rupprecht et al. 2017). The radioactivity technique, e.g., accumulation of [14C] radioactive organic acid probe, was also reported for measuring intracellular pH in Lactococcus lactis subsp. cremoris (Mercade et al. 2000) and Leuconostoc oenos (Loubiere et al. 1992). Some of these methods are not available for every researcher because they depend on complex and expensive instruments such as NMR and radioactive and fluorometric methods.
Over the last two decades, the fluorescent probe method was proposed as a new and alternative method to measure the intracellular pH of lactic acid bacteria (Breeuwer et al. 1996; Glaasker et al. 1996; Marty-Teysset et al. 1996; Belguendouz et al. 1997; Cachon et al. 1998; Hache et al. 1999; Rault et al. 2009; Zhang et al. 2012; Wu et al. 2013, 2014; Bouix and Ghorbal 2015; Alwazeer et al. 2018; Kudo and Sasaki 2019). Given that different bacteria can exhibit a wide range of pHin values (typically from 5.6 to 9.0), it is impossible, in the fluorescent probe method, to employ a single pH probe which can cover a wide pH range because this method is limited to the range of pKa of probe ± 1 (Breeuwer et al. 1996; Glaasker et al. 1996; Bouix and Ghorbal 2015; Schäferling 2016), in addition to the dependence of the probe signal upon the esterase activity (Booth 1985; Kudo and Sasaki 2019).
The weak acid or base distribution methods are divided into separation methods by silicon oil centrifugation or filtration and non-separation methods such as flow dialysis and ion-selective electrodes (Rottenberg 1979). These methods are based on the assumption that the neutral lipophilic form of the probe (weak acid) is permeable across the cytoplasmic membrane and the ionized hydrophilic form is impermeable (Rottenberg 1979; Kashket 1985). The probe used must not be metabolized by the cells, neither bind to their components nor be actively transported, and it should be rapidly equilibrated (Rottenberg 1979). In these methods, the weak organic acids, especially the radiolabeled ones, are the most commonly used to measure the delta pH when the pHin is alkaline (Kashket 1985; McDonald et al. 1990). Non-separation of the cells from the external medium is generally recommended because this step might lead to leakage of the probe from the cells (Rottenberg 1979). Only the NMR, flow dialysis, and ion-selective electrode methods allow one to achieve this objective, but the first two methods require a relatively high cell concentration (c.a. 10 mg protein/mL) (Hellingwerf and van Hoorn 1985). Other advantages of the ion-selective electrode method are the simplicity of instruments and uses, instantaneous response time, and continuous monitoring (Rottenberg 1979; Hellingwerf and van Hoorn 1985).
In intracellular pH studies of lactic acid bacteria, the external acidic conditions are most commonly studied because these bacteria often grow in acidic food media. L. plantarum is a lactic acid bacterium that was widely used in the fermentation of vegetables, meat, and dairy products; and it was referenced as a major alteration factor in other food products such as citrus juices (Parish and Higgins 1988). In previous work, our team showed the advantages of ion-selective electrode method over the fluorescent one for the measurement of intracellular pH in Leuconostoc mesonteroides (Alwazeer et al. 2018). In this study, we describe the possibility of using the ion-selective electrode method as a novel application to study continuously the effects of environmental conditions, like medium pH (pHex), on the intracellular pH of living cells in Lactobacillus plantarum.
Materials and methods
Bacterial growth
The strain of L. plantarum used in this study was isolated from orange juice (Parish and Higgins 1988). It was cultivated in a modified MRS broth of pH 6.4 that did not contain tween 80 or acetate, and the cells were harvested in the exponential growth phase (determined spectrophotometrically by measuring the optical density of the growth medium) to avoid the stress conditions that may lead to the synthesis of the multidrug efflux proteins (Paulsen et al. 1996). The cells were washed twice in a 100 mmol/L potassium phosphate buffer (pH 7.0), energized by incubation with 0.5 mol/L glucose at 30 °C for 30 min. The cells were then harvested using a Beckman J2-HC centrifuge (Beckman Instruments, Inc., USA) with a JA-10 rotor for 5 min and 4420 g and concentrated at 18 mg protein/mL (Lowry et al. 1951) in a 100 mmol/L potassium phosphate buffer at appropriate pH. The replications were from independent growth cultures of different days.
Electrode system
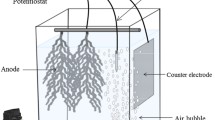
A polyvinyl chloride (PVC) membrane sensitive to salicylate anions was developed in this study. This membrane was constructed as described previously (Setty et al. 1983) with some modifications introduced as follows: A solution (A) was prepared from 297 mg polyvinyl chloride (Sigma Chemical CO., St Louis, MO, USA) that dissolved slowly in a mixture of 0.7 mL of dioctylphtalate (Sigma Chemical CO., St Louis, MO, USA) and 6 mL of tetrahydrofuran (Sigma-Aldrich CO., Gillingham, England). A solution (B) was constituted from 100 mg tetraheptyl ammonium iodide (Merck CO., Schuchardt, Germany) dissolved in 2.4 mL tetrahydrophuran. The solution (B) was added to the solution (A) with vortexing for 20 min and then the mixture was poured into a glass Petri dish (9.5 cm diameter) that was previously washed with ethanol and tetrahydrophuran and dried. The closed Petri dish was placed on a horizontal surface at room temperature for 2 days (for slight evaporation). After the dehydration step, a round piece was punched out of the membrane film and placed on the electrode holder with an elastic ring. A filling solution of 2 mmol/L salicylate dissolved in a 100 mmol/L potassium phosphate buffer (pH 7.0) was added inside the electrode holder where there was a platinum electrode. The salicylate-selective electrode was equipped by a removable salicylate-selective membrane. This membrane was removed and changed before each new assay. The electrode readings were referenced to a standard calomel electrode (Radiometer Analytical S.A., Villeurbanne, France), and the voltage was passed through an electronic interface to the microcomputer (Fig. 1). The software used (Elit 8808, Ficher Bioblock, chemputrix, France) permitted a continuous measurement of salicylate concentration changes during the measuring tests. The medium pH was measured with a combined electrode (Inlab 427, Metler Toledo, S.A.R.L., Paris, France).
Measurement of intracellular pH
A small-scale bioreactor was used to determine the intracellular pH by using the ion-selective electrode method. The applied system contained a measuring vessel (Bioreactor) and an isolation system (to avoid electric interferences). The calibration curve was established to determine the initial concentration and the final concentration of salicylate anion, and the slope (S) was calculated. This procedure was repeated at the end of the experiment to check that the membrane was in working order during the experiment. The energized cells were added to the thermostated and stirred buffer at appropriate pH value, which contained 100 μmol/L of salicylate (the final concentration of cells was 1.8 mg protein/mL). The change in electrode response (ΔE) was recorded. A control assay was performed by adding 1 μmol/L of both ionophores (nigericin and valinomycin) to the concentrated and energized cells, and then the sample was incubated at 30 °C for 60 min before measurement. All the results in this work are the mean values for at least three measurements.
Calculations
The calculations were performed with the assumption that no probe binding occurred between the salicylate anions and the cellular proteins (i.e., binding constant Ki = 0) (Hellingwerf and van Hoorn 1985).
The dissociation constant (pKa) of salicylate equaled to 2.98; so, most of the probe was ionized at the studied pH values ranging from 4.0 to 7.0 and the concentration of the undissociated probe could be neglected (Hellingwerf and van Hoorn 1985). The intracellular pH calculations were performed according to equations reported in Alwazeer et al. (2018).
Results and discussion
Calibration curve
The probe used to measure the intracellular pH of microorganisms by the method of the weak acid–selective electrode must be rigorously chosen because it is known that the inhibitory action of weak acids increases with the decrease of both medium pH and Ka values. In the ion distribution method, benzoic acid (pKa = 4.20) was the probe most commonly used to measure the intracellular pH of acidophilic bacteria (Padan and Schuldiner 1986; Nannen and Hutkins 1991; Wouters et al. 1998). The use of this probe is limited at a value of pHex superior to 5.2 because it is recommended for the alkaline bacteria that the pKa of the probe should be inferior to the value of pHex − 1) (Rottenberg 1979). Booth (1985) reported that the acetic acid (pKa = 4.75), which could be used as a probe in the ion-selective electrode method for internal pH measurement, might be actively transported and might act as a carbon source in Escherichia coli, whereas benzoate acid showed interferences with metabolism. For all these reasons, salicylic acid that has a low pKa (2.98) was selected in the present study to achieve the pHin measurements. For salicylate concentrations ranging from 25 to 800 μmol/L, the electrode described in this work demonstrated a linear response range between 45 and 50 mV per decade change of concentration.
Effect of medium pH on intracellular pH
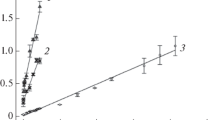
The effect of pH of the medium on the intracellular pH of L. plantarum was demonstrated in Fig. 2 and Table 1. When the medium pH decreases, the intracellular pH decreases, and the cells keep a cytoplasmic pH value higher than the external pH one. The ability of bacteria to maintain pH, i.e., homeostasis by membrane-bound primary proton pumps and solute-proton symport, would contribute to their growth and metabolic activities in acidic environments (Bouix and Ghorbal 2015). In this study, L. plantarum kept a neutral internal pH at acidic medium pH and the delta pH (ΔpH) ranged from 0.55 to 1.8 pH units at external pH ranged from 4.0 to 7.0. On the other hand, results showed that the delta pH was dissipated after adding the nigericin (Fig. 2B). The assumption that no binding occurs between the salicylate anions and the cellular proteins was proved by these observations because the internal salicylate anions left the cytoplasm to external medium after the addition of nigericin.
A Effect of pHex on the pHin in L. plantarum determined by the salicylate-selective electrode. The energized and concentrated cells were added to a 100 mmol/L potassium phosphate buffer at a cell concentration of 1.8 mg/mL protein. Symbols represent means ± standard deviations of three independent experiments. B The action of nigericin on the intracellular pH of L. plantarum suspended in a 100 mmol/L potassium phosphate buffer pH 5.0. The different treatments were effected as follows: a, cell addition at 1.8 mg/mL protein; b, nigericin addition at 1 μmol/L
It is important to indicate that the intracellular pH values of L. plantarum observed in this study were similar to those measured by other sophisticated methods such as radioactive probes and fluorescent probes (Table 1). Many researchers reported that the intracellular pH values measured by the weak acid distribution method were similar to those measured by the NMR method that was generally used to validate this method (weak acid–selective membrane) and also agreed with the results of the flow dialysis method (Hellingwerf and van Hoorn 1985; Kashket 1985). Furthermore, the difference in the bacterial strain can lead also to differences in the pH intercellular values for the same bacteria such the case of L. plantarum (Table 1) (Huang et al. 2016; Kudo and Sasaki 2019).
Conclusion
The important role of lactic acid bacteria in the food industry and food alteration has encouraged many researchers to study the energetic system of these bacteria whose intracellular pH is an important factor. Many types of research have been conducted to study the effect of environmental conditions on the intracellular pH of lactic acid bacteria such as medium pH, pressure, oxygen, and preservatives (Nannen and Hutkins 1991; Tseng et al. 1991; Cook and Russell 1994; Wouters et al. 1998). Many methods have been developed to meet this objective such as pH-sensitive dyes, 31P nuclear magnetic resonance, radioactively labeled weak acids, and fluorescent ratio imaging. The simplicity of the experimental protocol generally required to perform the measurements could make the ion-selective electrode method easily available for researchers compared with the complexity of instrumentation and the costly equipment requirement and time-consuming of the other methods. The application of radioactive salicylic acid as a probe in the determination of intracellular pH of L. plantarum showed the complexity of the procedure, the special precautions concerning the use of radioactive materials, and the high costs of instruments; these disadvantages could be avoided by the present ion-selective electrode method thanks to its simplicity, low costs, and reasonable safety conditions. Thus, this developed method could be of great interest to study the effects of acidic stress conditions on the intracellular pHin of bacteria at an external pH value as low as 4.0. In a previous study, we used the ion-selective electrode method to measure the delta psi (Δψ) of lactic acid bacteria (Hache et al. 1999), and in this study, we described the possibility of using the same system to measure the intracellular pH of one of those bacteria. Thus, now a single system can be used to measure, simultaneously, the effect of environmental conditions on both components of the proton motive force (Δψ) in bacteria.
References
Alwazeer D, Riondet C, Cachon R (2018) Comparison between fluorescent probe and ion-selective electrode methods for intracellular pH determination in Leuconostoc mesenteroides. Curr Microbiol 75:1493–1497. https://doi.org/10.1007/s00284-018-1550-9
Belguendouz T, Cachon R, Diviès C (1997) pH homeostasis and citric acid utilization: differences between Leuconostoc mesenteroides and Lactococcus lactis. Curr Microbiol 35:233–236
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49:359–378
Bouix M, Ghorbal S (2015) Rapid assessment of Oenococcus oeni activity by measuring intracellular pH and membrane potential by flow cytometry, and its application to the more effective control of malolactic fermentation. Int J Food Microbiol 193:139–146
Breeuwer P, Drocourt JL, Rombouts FM, Abee T (1996) A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-) -carboxyfluorescein succinimidyl ester. Appl Environ Microbiol 62:178–183
Cachon R, Antérieux P, Diviès C (1998) The comparative behavior of Lactococcus lactis in free and immobilized culture processes. J Biotechnol 63:211–218
Cook GM, Russell JB (1994) The effect of extracellular pH and lactic acid on pH homeostasis in Lactococcus lactis and Streptococcus bovis. Curr Microbiol 28:165–168
Glaasker E, Konings WN, Poolman B (1996) The application of pH-sensitive fluorescent dyes in lactic acid bacteria reveals distinct extrusion systems for unmodified and conjugated dyes. Mol Membr Biol 13:173–181
Hache C, Cachon R, Wache Y et al (1999) Influence of lactose-citrate co-metabolism on the differences of growth and energetics in Leuconostoc lactis, Leuconostoc mesenteroides ssp. mesenteroides and Leuconostoc mesenteroides ssp. cremoris. Syst Appl Microbiol 22:507–513
Hansen G, Johansen CL, Marten G, Wilmes J, Jespersen L, Arneborg N (2016) Influence of extracellular pH on growth, viability, cell size, acidification activity, and intracellular pH of Lactococcus lactis in batch fermentations. Appl Microbiol Biotechnol 100:5965–5976
Hellingwerf KJ, van Hoorn P (1985) A polyvinylchloride-membrane based anion selective electrode for continuous registration of delta pH (interior alkaline) with salicylate as the indicator probe. J Biochem Biophys Methods 11:83–93
Huang R, Pan M, Wan C, Shah NP, Tao X, Wei H (2016) Physiological and transcriptional responses and cross protection of Lactobacillus plantarum ZDY2013 under acid stress. J Dairy Sci 99:1002–1010. https://doi.org/10.3168/jds.2015-9993
Kashket ER (1987) Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol Rev 46:233–244
Kashket ER (1985) The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol 39:219–242
Kudo H, Sasaki Y (2019) Intracellular pH determination for the study of acid tolerance of lactic acid bacteria. In: Lactic Acid Bacteria. Humana Press, New York, pp 33–41
Loubiere P, Salou P, Leroy MJ, Lindley ND, Pareilleux A (1992) Electrogenic malate uptake and improved growth energetics of the malolactic bacterium Leuconostoc oenos grown on glucose-malate mixtures. J Bacteriol 174:5302–5308
Lowry OH, Rosebrough NJ, Farr AL, Randell RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marty-Teysset C, Posthuma C, Lolkema JS, Schmitt P, Divies C, Konings WN (1996) Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J Bacteriol 178:2178–2185
McDonald LC, Fleming HP, Hassan HM (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56:2120–2124
Mercade M, Lindley ND, Loubiere P (2000) Metabolism of Lactococcus lactis subsp. cremoris MG 1363 in acid stress conditions. Int J Food Microbiol 55:161–165
Molina-Gutierrez A, Stippl V, Delgado A, Gänzle MG, Vogel RF (2002) In situ determination of the intracellular pH of Lactococcus lactis and Lactobacillus plantarum during pressure treatment. Appl Environ Microbiol 68:4399–4406
Nannen NL, Hutkins RW (1991) Proton-translocating adenosine triphosphatase activity in lactic acid bacteria. J Dairy Sci 74:747–751
Padan E, Schuldiner S (1986) Intracellular pH regulation in bacterial cells. Methods Enzymol 125:337–352
Parish ME, Higgins D (1988) Isolation and identification of lactic bacteria from samples of citrus molasses and unpasteurized orange juice. J Food Sci 53:645–646
Paulsen IT, Brown MH, Skurray RA (1996) Proton-dependent multidrug efflux systems. Microbiol Rev 60:575–608
Rault A, Bouix M, Béal C (2009) Fermentation pH influences the physiological-state dynamics of Lactobacillus bulgaricus CFL1 during pH-controlled culture. Appl Environ Microbiol 75:4374–4381
Rottenberg H (1979) The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol 55:547–569
Rupprecht C, Wingen M, Potzkei J, Gensch T, Jaeger KE, Drepper T (2017) A novel FbFP-based biosensor toolbox for sensitive in vivo determination of intracellular pH. J Biotechnol 258:25–32
Schäferling M (2016) Nanoparticle-based luminescent probes for intracellular sensing and imaging of pH. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8:378–413
Setty OH, Hendler RW, Shrager RI (1983) Simultaneous measurements of proton motive force, delta pH, membrane potential, and H+/O ratios in intact Escherichia coli. Biophys J 43:371–381
Tseng CP, Tsau JL, Montville TJ (1991) Bioenergetic consequences of catabolic shifts by Lactobacillus plantarum in response to shifts in environmental oxygen and pH in chemostat cultures. J Bacteriol 173:4411–4416
Wouters PC, Glaasker E, Smelt JPPM (1998) Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl Environ Microbiol 64:509–514
Wu C, He G, Zhang J (2014) Physiological and proteomic analysis of Lactobacillus casei in response to acid adaptation. J Ind Microbiol Biotechnol 41:1533–1540
Wu C, Zhang J, Du G, Chen J (2013) Aspartate protects Lactobacillus casei against acid stress. Appl Microbiol Biotechnol 97:4083–4093
Xu J, Koyanagi Y, Isogai E, Nakamura S (2019) Effects of fermentation products of the commensal bacterium Clostridium ramosum on motility, intracellular pH, and flagellar synthesis of enterohemorrhagic Escherichia coli. Arch Microbiol 201:841–846
Zhang J, Wu C, Du G, Chen J (2012) Enhanced acid tolerance in Lactobacillus casei by adaptive evolution and compared stress response during acid stress. Biotechnol Bioprocess Eng 17:283–289. https://doi.org/10.1007/s12257-011-0346-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alwazeer, D., Cachon, R. Ion-selective electrode integrated in small-scale bioreactor for continuous intracellular pH determination in Lactobacillus plantarum. Folia Microbiol 65, 467–473 (2020). https://doi.org/10.1007/s12223-019-00749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00749-5